Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7372
Peer-review started: April 22, 2021
First decision: May 24, 2021
Revised: June 28, 2021
Accepted: July 28, 2021
Article in press: July 28, 2021
Published online: September 6, 2021
Processing time: 130 Days and 22.1 Hours
Necrotizing enterocolitis (NEC) of the newborn is a frequently occurring clinical disease in infants. The mortality rate of NEC in premature infants is as high as 50%, and the morbidity rate is on the rise. NEC has already caused serious impacts on newborn survival and poses serious threats to both children and families.
To investigate the expression and significance of mucin 1 (MUC1) and interleukin-11 (IL-11) in the intestinal mucosa of infants with neonatal NEC after surgery.
Forty-eight postoperative intestinal mucosal specimens from children with NEC (NEC group) and twenty-two intestinal mucosal specimens from children with congenital intestinal atresia (control group) were collected in our hospital. Immunohistochemical staining and Western blot analysis were used to examine the protein expression of MUC-1 and IL-11 in the two groups. The serum levels of tumor necrosis factor-α (TNF-α) and IL-1β in the two groups were measured by enzyme-linked immunosorbent assay, and the relationship between MUC-1 and IL-11 protein expression and serum TNF-α and IL-1β levels was analyzed by the linear correlation method.
The protein expression of MUC-1 and IL-11 in the NEC group was significantly lower than that in the control group, and the difference was statistically significant (P < 0.05). The levels of serum TNF-α and IL-1β in the NEC group were significantly higher than those in the control group (P < 0.05). The protein expression of MUC-1 and IL-11 in the NEC group negatively correlated with serum TNF-α and IL-1β levels (P < 0.05). There was a significant negative correlation between the protein expression of MUC-1 and IL-11 and the levels of serum TNF-α and IL-1β in the NEC group.
The protein expression of MUC1 and IL-11 in the intestinal mucosa of children with NEC is significantly downregulated after surgery. This downregulation may be involved in the pathogenesis of this disease and has a certain correlation with inflammatory response factors in children with NEC.
Core Tip: This study analyzed the expression changes of mucin 1 and interleukin-11 in intestinal mucosa after necrotizing enterocolitis operation in neonatal necrotizing enterocolitis. It may be related to the pathogenesis of the disease, and providing clinical guidance and basis.
- Citation: Pan HX, Zhang CS, Lin CH, Chen MM, Zhang XZ, Yu N. Mucin 1 and interleukin-11 protein expression and inflammatory reactions in the intestinal mucosa of necrotizing enterocolitis children after surgery. World J Clin Cases 2021; 9(25): 7372-7380
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7372.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7372
In the clinic, neonatal necrotizing enterocolitis (NEC) is a frequently occurring pediatric intestinal inflammatory disease. The mortality of premature infants with NEC is as high as 50%, and the incidence is increasing, which has serious impacts on the quality of life of these children[1,2].
At present, the etiology and specific pathogenesis of neonatal NEC are not completely known. Thus, it is critical to identify reliable and sensitive molecules or genes to reduce the morbidity and mortality of NEC[3]. At present, intestinal mucus is considered an important component of the intestinal mucosal barrier, and mucin is the main macromolecular substance in intestinal mucus, which is critical in ensuring the stability of bacteria and the intestinal mucosal barrier, but there have been few clinical studies on mucin. Interleukin-11 (IL-11) has good mucosal protective effects against intestinal epithelial cell injury due to several factors. Moreover, IL-11 is involved in signal transduction and mediating inflammatory reactions during the pathogenesis of NEC. This study analyzed changes in the expression of mucin 1 (MUC1) and IL-11 in the intestinal mucosa in infants with neonatal NEC after surgery to provide clinical guidance and a basis for treatment.
Intestinal mucosal specimens from 48 children with NEC (NEC group) and 22 patients with congenital intestinal atresia (control group) were collected in our hospital from June 2015 to January 2019. The inclusion criteria were as follows: (1) the NEC diagnosis and grading criteria refer to the indexes in Practical Pediatrics by Zhu Futang; (2) the gestational age of the child was 29-36 wk; (3) the child was delivered in our hospital and was 7-35 days old; and (4) the relevant regulations of the Medical Ethics Committee were followed, and informed consent was obtained from the parents of the children. The exclusion criteria were as follows: (1) the presence of intestinal tumors; (2) chromosomal genetic defects; (3) severe infections (septicemia, meningitis), and (4) major diseases associated with other systems.
The gestational age was 29 to 36 wk in the NEC group, with an average of 32.5 ± 1.8 wk, and the group included 25 males and 23 females; the body mass was 2630.4 ± 225.0 g, and the mean age was 17.9 ± 4.2 d, ranging from 7 to 35 d. The gestational age was 29 to 36 wk in the control group, with an average of 33.0 ± 2.0 wk, and the group included 10 males and 12 females; the body mass was 2604.1 ± 203.9 g, and the age was 735 d, with an average of 19.0 ± 6.2 d. There was no significant difference in gestational age, sex, body mass or age between the two groups (P > 0.05).
The diseased intestinal tissue was embedded in paraffin. Continuous sections (4-μm-thick) were obtained, routine hematoxylin and eosin staining was performed, and routine dewaxing and hydration treatment was conducted. In addition, the sections were washed twice with phosphate-buffered saline (PBS) for 5 min each. PBS was used to prepare fresh 3% H2O2, and the sections were sealed at room temperature for 5-10 min. Moreover, we heated the 0.01 mol/L sodium citrate buffer solution in a water bath to approximately 95 ℃; the human tissue slices were heated for 10-15 min, cooled to room temperature with tap water, and washed for 5 min with PBS. Then, 5% BSA blocking solution was added and incubated for 15 min, and the primary antibody (1:50 dilution) was added dropwise. After an overnight incubation at 4°C, the sections were washed 3 times at room temperature and incubated for 45 min. The secondary antibody was added and incubated for 1 h and washed with 3 times with PBS at room temperature for 2 min each. When the target signal of the DAB chromogenic kit was dark and the background was light, the reaction was terminated with distilled water. Hematoxylin was added and incubated for 20 s, and the sections were washed twice with distilled water for 2 min, dehydrated, and placed into 100% dimethylbenzene for 10 min to create a transparent seal. MUC1-positive cells were yellow, brown, and yellowish brown and were located in the cell membrane.
According to the immunohistochemistry results, MUC1 and IL-11 proteins were stained yellow, brown and yellowish brown and were located in the cell membrane. (1) According to the degree of staining, the samples were assessed as unstained (0 points), light yellow staining (1 point), brown staining (2 points), and brown and black staining (3 points). And (2) The percentages of stained cells were assessed as follows: ≤ 10%:1 point; 11%-50%: 2 points; 51%-75%: 3 points; and > 75%: 4 points. The product of the staining degree and positive cell score was calculated, and < 3 points was negative, while ≥ 3 points was positive.
Fasting venous blood (3 mL) was extracted and centrifuged at 3000 rpm for 5 min. Forty microliters of sample diluent was added to the sample wells of the enzyme-coated plate, and 10 μL of the sample was added. The plate was gently shaken and mixed, sealed, and incubated with 30× concentrated washing solution at 37°C for 30 min. The solution was diluted 30 times with distilled water, the sealing film was removed, the liquid was discarded, and the sample was shaken dry. Each well was filled with washing solution and rested for 30 s. This process was repeated 5 times, and 50 μL of enzyme-labeled reagent was added. After the sealing plate was incubated at 37°C for 30 min, we removed the sealing film, discarded the liquid, dried the plate, filled each well with washing solution, rested the plate for 30 s, and repeated the process 5 times. Chromogenic agent A (50 μL) was added, and then chromogenic agent B (50 μL) was added. The plate was gently shaken and mixed in the dark at 37°C for 15 min, after which 50 μL of termination solution was added to stop the reaction, and the absorbance (OD value) of each well was measured at a wavelength of 450 nm. The corresponding concentration was determined from the standard curve and was multiplied by the dilution factor to determine the actual concentration of the sample according to the OD value.
SPSS 21.0 software was used for statistical analysis. The measurement indexes of age, body mass, gestational age, MUC-1 protein expression and IL-11 protein expression in the two groups are expressed as mean ± SD. In addition, comparisons between the two groups were performed by t-tests, the sex of the two groups was compared by the χ2 test, and correlation analysis was performed by Pearson linear correlation analysis.
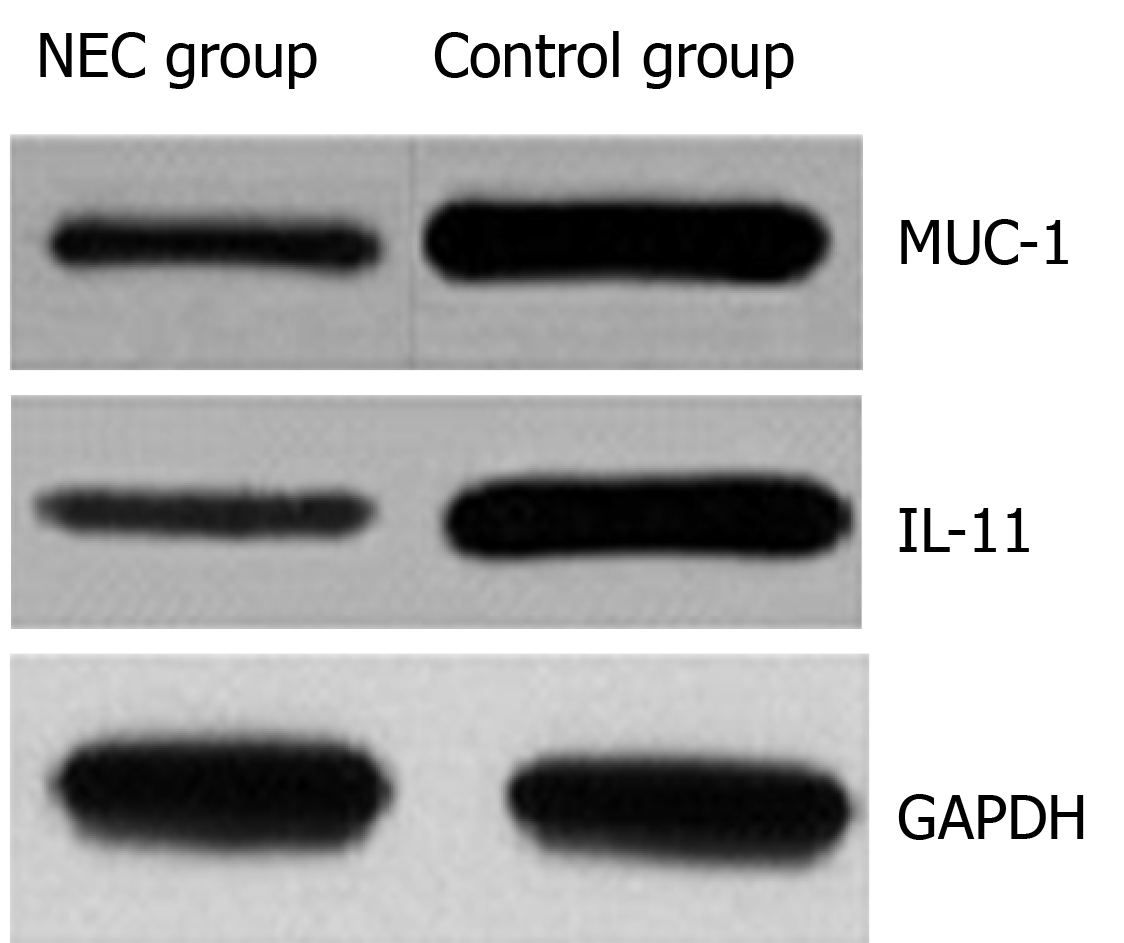
The protein expression of MUC-1 and IL-11 in the NEC group was significantly lower than that in the control group, and the difference was statistically significant (P < 0.05) (Table 1, Figure 1).
| Groups | n | MUC-1 protein | IL-11 protein |
| NEC group | 48 | 0.461 ± 0.108 | 0.391 ± 0.085 |
| Control group | 22 | 0.958 ± 0.177 | 0.920 ± 0.204 |
| t | -14.494 | -15.381 | |
| P value | 0.000 | 0.000 |
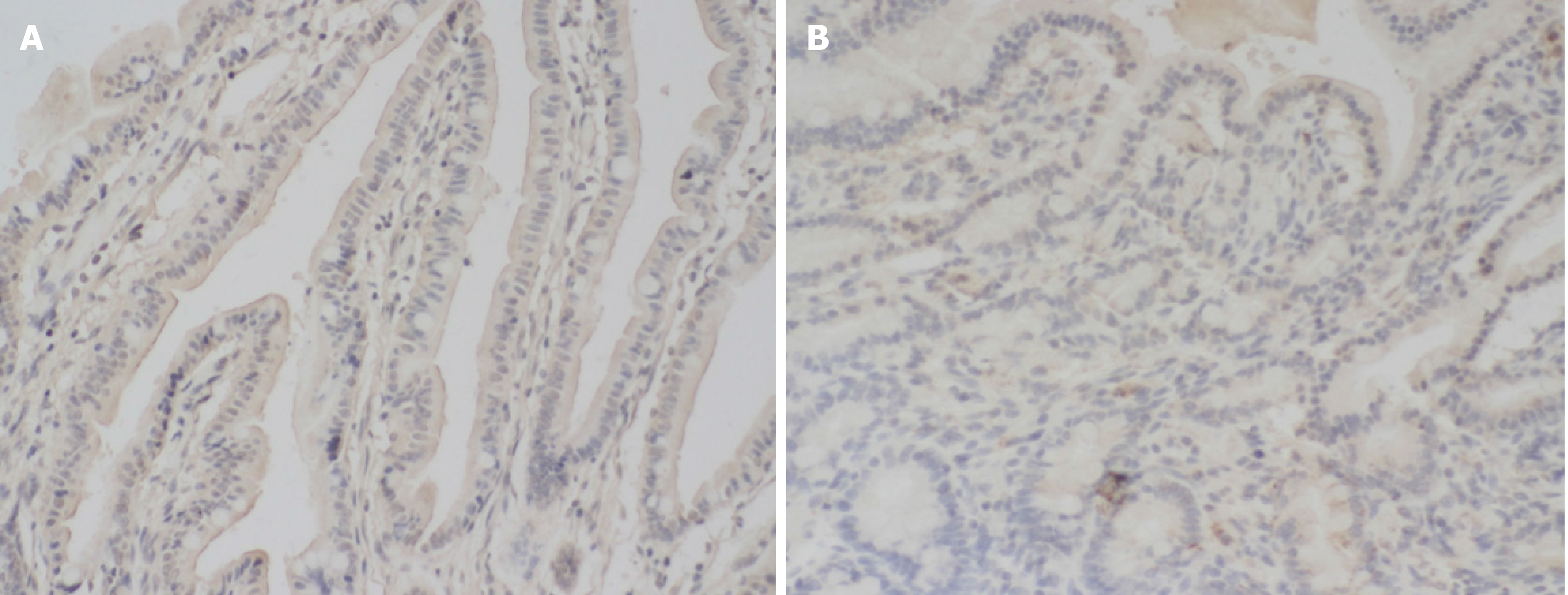
The positive expression rate of MUC-1 in the NEC group (33.33%) was lower than that in the control group (77.27%), and the difference was statistically significant (P > 0.05). (Table 2, Figure 2).
| Group | n | MUC-1protein | |
| Positive | Negative | ||
| NEC group | 48 | 16 (33.33) | 32 (66.67) |
| Control group | 22 | 17 (77.27) | 5 (22.73) |
| χ2 | 11.688 | ||
| P value | 0.001 | ||
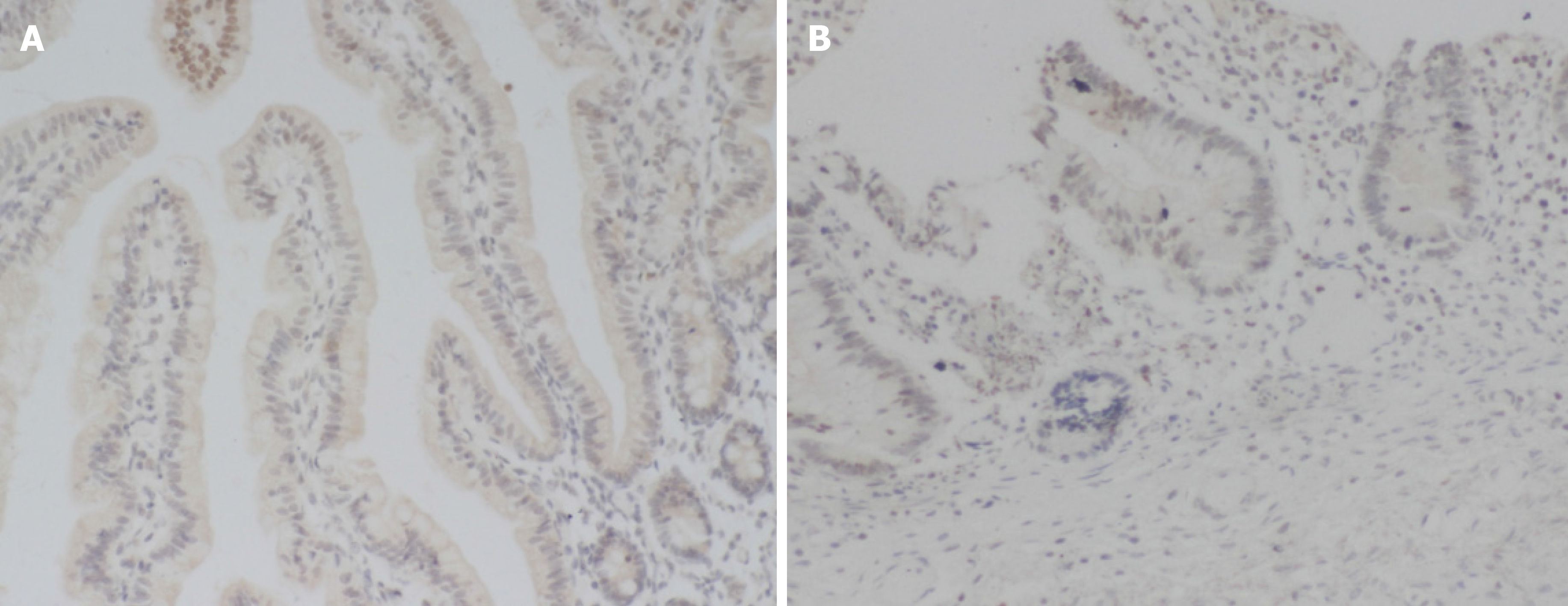
The positive expression rate of IL-11 in the NEC group (27.08%) was lower than that in the control group (81.82%), and the difference was statistically significant (P > 0.05). (Table 3, Figure 3)
| Groups | n | IL-11protein | |
| Positive | Negative | ||
| NEC group | 48 | 13 (27.08) | 35 (72.92) |
| Control group | 22 | 18 (81.82) | 4 (18.18) |
| χ2 | 18.317 | ||
| P value | 0.000 | ||
The levels of serum tumor necrosis factor-α (TNF-α) and IL-1β in the NEC group were significantly higher than those in the control group (P < 0 05) (Table 4).
| Group | n | TNF-α (ng/L) | IL-1β (ng/mL) |
| NEC group | 48 | 386.6 ± 76.4 | 18.63 ± 3.02 |
| Control group | 22 | 260.7 ± 48.8 | 14.19 ± 2.50 |
| t | 7.080 | 6.010 | |
| P value | 0.000 | 0.000 |
There was a significant negative correlation between the protein expression of MUC-1 and IL-11 and the serum levels of TNF-α and IL-1β in the NEC group (P < 0.05) (Table 5).
| Index | Correlation | TNF-α | IL-1β |
| MUC-1 protein | r | -0.619 | -0.486 |
| P value | 0.000 | 0.000 | |
| IL-11 protein | r | -0.577 | -0.528 |
| P value | 0.000 | 0.000 |
In the clinic, neonatal NEC is a common clinical disease. The incidence of NEC is increasing, and this disease has recently had serious impacts on the late development and life expectancy of newborns. Factors such as premature delivery, hypoxia, and immune factors impact the intestinal mucosal blood supply and weaken intestinal peristalsis, resulting in the accumulation of food in the intestinal cavity, which affects intestinal function[4,5]. Studies have shown that preterm delivery is an important factor that leads to the occurrence of NEC due to the immature intestinal epithelium, high permeability, underdeveloped immune mechanisms, susceptibility to bacterial translocation, and activated inflammatory cascade reactions, all of which lead to disease. In addition, the development of the intestinal lumen and gastrointestinal peristalsis in premature infants are not perfect, the digestion and hydrolysis of various pathogens and toxins are inadequate, and intestinal peristalsis is slow, which allows food to accumulate in the intestine. This accumulation can lead to gastrointestinal damage, bacterial overgrowth and intestinal flatulence, resulting in the occurrence of NEC[6,7]. On the other hand, studies have shown that hypoxia-ischemia and reperfusion injury to the intestinal wall are also important factors that lead to the occurrence of NEC. To protect the blood vessels of important organs, such as the heart and brain, the gastrointestinal blood supply decreases sharply, and intestinal wall damage is caused by hypoxia. In addition, hypoxia can also cause free radical release and increase local tissue nitric oxide synthesis, leading to organ damage[8,9]. At present, clinical research on the role of cytokines in the pathogenesis of NEC has mainly focused on proinflammatory factors. However, research on protective factors has been relatively rare[10].
MUC1 and IL-11 in the intestinal mucosa of infants after NEC surgery were analyzed. IL-11 is a platelet-promoting factor produced by bone marrow-derived cells in primates and can participate in a variety of physiological and pathological processes in the human body[11]. Studies have shown that IL-11 can reduce intestinal injury due to various causes. Exogenous IL-11 can promote crypt cell proliferation, reduce apoptosis, and protect against intestinal injury[12]. The function of human cytokines is to bind to specific receptors on the target cell membrane and transmit signals to the cell to induce signal transduction. The receptor is an important functional protein that mediates the effects of cytokines. IL-11 receptors can form complexes by homologous dimerization and activate intracellular signaling pathways. IL-11 is expressed on the surface of intestinal epithelial cells. The expression of IL-11 on intestinal epithelial cells protects cells and inhibits apoptosis, and enhanced expression of the IL-11 receptor protects cells and inhibits apoptosis, which can delay disease progression[13]. As a consequence, the expression level of IL-11 in NEC-associated intestinal injury is downregulated. This downregulation is not conducive to ligand engagement, can activate the corresponding signaling pathway and plays a protective role in the intestinal tract[14].
As a highly glycosylated transmembrane mucin with a high molecular weight, the structure of MUC1 is highly complex. MUC1 can be expressed in a variety of tissues and organs under normal physiological conditions. MUC1 has a polar distribution and is not easily recognized by the immune system. MUC1 plays roles in lubrication, protecting cells, maintaining stickiness, identifying cells and maintaining the functional integrity of epithelial cells. In addition, MUC1 can participate in intercellular signal transduction and immune regulation[15,16]. Studies have shown that damage to and repair of the intestinal mucosa can lead to abnormal glycosylation, weaken intestinal mucosal epithelial barrier function, and lead to the release of large amounts of MUC1[17]. Some scholars have pointed out that the MUC1 protein is an important part of maintaining the stability of the intestinal environment and affects cell proliferation, apoptosis and self-phagocytosis through a variety of signaling pathways. The destruction of MUC1 Leads to the formation of an infectious environment, inflammatory diseases or inflammation-related tumors[18]. In addition, MUC1 can regulate the inflammatory response, membrane-bound mucin can regulate inflammation through MUC1, and its extracellular part can bind with bacteria to release its receptor from the cell membrane and activate the nuclear factor kappa B pathway to regulate the inflammatory response. Membrane-bound mucin plays a very important role in regulating the intestinal mucosal barrier, as the last mucin barrier is distributed on the surface of the cell membrane[19,20].
The results showed that the protein expression of MUC-1 and IL-11 in the NEC group was significantly lower than that in the control group. This result indicated that there was a decrease in the protein expression of MUC-1 and IL-11 in children with NEC. The levels of serum TNF-α and IL-1β in the NEC group were higher than those in the control group. This result indicated that the levels of TNF-α and IL-1β were significantly increased in children with NEC, and the degree of the inflammatory reaction in vivo was exacerbated. There was a significant negative correlation between the protein expression of MUC-1 and IL-11 and the levels of serum TNF-α and IL-1β in the NEC group. This study confirmed that there was abnormal protein expression of MUC-1 and IL-11 in children with NEC and that there was a negative correlation with the degree of inflammation. This study provides a new basis for reasonable clinical prediction of the pathogenesis and disease progression in children with NEC. However, the follow-up time in this study was short, and there were few cases in the group. As a consequence, it is necessary to expand the sample size and perform long-term follow-up for in-depth analysis.
Overall, the protein expression of MUC1 and IL-11 in the intestinal mucosa of children with NEC was significantly downregulated after surgery. This downregulation may be involved in the occurrence of the disease and has a certain correlation with inflammatory response factors in children with NEC.
In the clinic, neonatal necrotizing enterocolitis (NEC) is a frequently occurring pediatric intestinal inflammatory disease. The mortality of premature infants with NEC is as high as 50%, and the incidence shows an increasing trend, which has serious impacts on the quality of life of children.
This study provides help for the clinical diagnosis and treatment of neonatal NEC.
This study aimed to investigate the expression and significance of mucin 1 (MUC1) and interleukin-11 (IL-11) in the intestinal mucosa of children with neonatal NEC after surgery.
Total 48 postoperative intestinal mucosal specimens from children with NEC (NEC group) and 22 intestinal mucosal specimens from congenital intestinal atresia (control group) were collected in our hospital. Immunohistochemical staining and Western-blot technique were used to detect the expression of MUC-1 protein and IL-11 protein in the two groups. The serum levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the two groups were detected by enzyme-linked immunosorbent assay, and the relationship between MUC-1 protein, IL-11 protein and serum TNF-α and IL-1β was analyzed by linear correlation method.
The protein expression of MUC-1 and IL-11 in the NEC group was significantly lower than that in the control group, and the difference was statistically significant. The levels of serum TNF-α and IL-1β in the NEC group were significantly higher than those in the control group. The protein expression of MUC-1 and IL-11 in the NEC group negatively correlated with serum TNF-α and IL-1β levels. There was a significant negative correlation between the protein expression of MUC-1 and IL-11 and the levels of serum TNF-α and IL-1β in the NEC group.
The protein expression of MUC1 and IL-11 in the intestinal mucosa of children with NEC is significantly downregulated after surgery. This down regulation may be involved in the pathogenesis of the disease and has a certain correlation with inflammatory response factors in children with NEC.
MUC1 and IL-11 proteins may be related to the pathogenesis of the disease and have a certain correlation with inflammatory response factors in children with NEC, which has profound clinical significance.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kurokawa R S-Editor: Wang JL L-Editor: A P-Editor: Xing YX
| 1. | Maheshwari A. Role of platelets in neonatal necrotizing enterocolitis. Pediatr Res. 2021;89:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Evidence-Based Medicine Group. [Clinical guidelines for the diagnosis and treatment of neonatal necrotizing enterocolitis (2020)]. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Mattern J, Marin T. Neonatal Microbiome and Its Relationship to Necrotizing Enterocolitis: A Review of the Science. J Perinat Neonatal Nurs. 2020;34:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | El-Abd Ahmed A, Hassan MH, Abo-Halawa N, Abdel-Razik GM, Moubarak FA, Sakhr HM. Lactate and intestinal fatty acid binding protein as essential biomarkers in neonates with necrotizing enterocolitis: ultrasonographic and surgical considerations. Pediatr Neonatol. 2020;61:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Oldenburg KS, O'Shea TM, Fry RC. Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Semin Fetal Neonatal Med. 2020;25:101115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Surlis C, McNamara K, O'Hara E, Waters S, Beltman M, Cassidy J, Kenny D. Birth delivery method affects expression of immune genes in lung and jejunum tissue of neonatal beef calves. BMC Vet Res. 2017;13:391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Tadesse S, Corner G, Dhima E, Houston M, Guha C, Augenlicht L, Velcich A. MUC2 mucin deficiency alters inflammatory and metabolic pathways in the mouse intestinal mucosa. Oncotarget. 2017;8:71456-71470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Al-Ezzy AIA. Immunopathological and Modulatory Effects of Cag A+ Genotype on Gastric Mucosa, Inflammatory Response, Pepsinogens, and Gastrin-17 Secretion in Iraqi Patients infected with H. pylori. Open Access Maced J Med Sci. 2018;6:794-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Nitkin CR, Rajasingh J, Pisano C, Besner GE, Thébaud B, Sampath V. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr Res. 2020;87:265-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Wagener K, Pothmann H, Prunner I, Peter S, Erber R, Aurich C, Drillich M, Gabler C. Endometrial mRNA expression of selected pro-inflammatory factors and mucins in repeat breeder cows with and without subclinical endometritis. Theriogenology. 2017;90:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Mahmutovic Persson I, Menzel M, Ramu S, Cerps S, Akbarshahi H, Uller L. IL-1β mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir Res. 2018;19:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Shelby RD, Raab R, Besner GE, McElroy SJ. Hope on the horizon: promising novel therapies for necrotizing enterocolitis. Pediatr Res. 2020;88:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ypsilantis P, Lambropoulou M, Evagellou A, Papadopoulos N, Simopoulos C. Immune and Inflammatory Responses of the Intestinal Mucosa following Extended Liver Radiofrequency Ablation. Gastroenterol Res Pract. 2017;2017:3450635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Djuric Z, Turgeon DK, Sen A, Ren J, Herman K, Ramaswamy D, Zhao L, Ruffin MT 4th, Normolle DP, Smith WL, Brenner DE. The Anti-inflammatory Effect of Personalized Omega-3 Fatty Acid Dosing for Reducing Prostaglandin E2 in the Colonic Mucosa Is Attenuated in Obesity. Cancer Prev Res (Phila). 2017;10:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Cloots RHE, Sankaranarayanan S, Poynter ME, Terwindt E, van Dijk P, Lamers WH, Eleonore Köhler S. Arginase 1 deletion in myeloid cells affects the inflammatory response in allergic asthma, but not lung mechanics, in female mice. BMC Pulm Med. 2017;17:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Pisano C, Galley J, Elbahrawy M, Wang Y, Farrell A, Brigstock D, Besner GE. Human Breast Milk-Derived Extracellular Vesicles in the Protection Against Experimental Necrotizing Enterocolitis. J Pediatr Surg. 2020;55:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 17. | Vitale S, Strisciuglio C, Pisapia L, Miele E, Barba P, Vitale A, Cenni S, Bassi V, Maglio M, Del Pozzo G, Troncone R, Staiano A, Gianfrani C. Cytokine production profile in intestinal mucosa of paediatric inflammatory bowel disease. PLoS One. 2017;12:e0182313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Ungaro F, Massimino L, Furfaro F, Rimoldi V, Peyrin-Biroulet L, D'Alessio S, Danese S. Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory bowel disease. Gut Microbes. 2019;10:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Sznurkowska K, Żawrocki A, Sznurkowski J, Iżycka-Świeszewska E, Landowski P, Szlagatys-Sidorkiewicz A, Plata-Nazar K, Kamińska B. Indoleamine 2,3-dioxygenase and regulatory t cells in intestinal mucosa in children with inflammatory bowel disease. J Biol Regul Homeost Agents. 2017;31:125-131. [PubMed] |
| 20. | Patel AL, Meier PP, Canvasser J. Strategies to increase the use of mother's own milk for infants at risk of necrotizing enterocolitis. Pediatr Res. 2020;88:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |