Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6810
Peer-review started: February 1, 2021
First decision: April 25, 2021
Revised: May 6, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: August 16, 2021
Processing time: 185 Days and 4.9 Hours
Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) is relatively common in several cancers, such as small cell lung cancer. However, nedaplatin-induced SIADH is rare. We describe a case of SIADH mediated by nedaplatin.
A 54-year-old female with nasopharyngeal carcinoma was treated with nedaplatin and developed severe hyponatremia due to SIADH. The side effects were successfully treated by fluid restriction and sodium supplementation.
This case report highlights the importance of cautiously treating life-threatening hyponatremia in patients treated with nedaplatin.
Core Tip: Nedaplatin has been shown to be an effective drug in a variety of cancers. Due to its wide application, it is important to pay attention to its side effects, especially rare side effects. Here, we report a case of a severe, rare syndrome of inappropriate secretion of antidiuretic hormone after initiating nedaplatin chemotherapy. Timely diagnosis, fluid restriction and sodium supplementation may be the most appropriate treatments.
- Citation: Tian L, He LY, Zhang HZ. Nedaplatin-induced syndrome of inappropriate secretion of antidiuretic hormone: A case report and review of the literature. World J Clin Cases 2021; 9(23): 6810-6815
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6810.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6810
Nedaplatin is a second-generation platinum medication that was developed in Japan in 1983. Its antitumor mechanism is similar to that of cisplatin—binding with DNA, inhibiting the growth of tumor cells and producing antitumor activity[1]. It has been widely used for the treatment of head and neck, lung, esophageal, bladder, and gynecological cancers and many other malignancies[2-4]. The dose-limiting toxicity of nedaplatin is myelosuppression, and other common adverse reactions include gastrointestinal symptoms, kidney dysfunction, ototoxicity and alopecia[3,5]. Rare complications, such as hypersensitivity reactions and Adams-Stokes syndrome, have also been reported in clinical practice[6]. We report the case of a rare, severe side effect of nedaplatin: syndrome of inappropriate secretion of antidiuretic hormone (SIADH).
A 54-year-old female was admitted to our hospital in November 2020 with a 1-year history of nasal congestion, tinnitus, and hearing loss.
One year before admission, the patient had nasal congestion, tinnitus, and hearing loss. She had no dizziness or syncope. She was admitted to a local hospital and treated with antibiotics. Due to persistence of her symptoms, the patient was referred to our hospital for further assessment.
Her past medical history included hypertension.
There was no family history of malignancy or any other family history.
Upon physical examination, multiple enlarged lymph nodes fused in the bilateral neck were observed. The largest was approximately 30 mm × 20 mm and located at level II of the right neck.
Routine blood examination, coagulation function, urinalysis results, stool analysis, liver chemistry tests, urea, creatinine, uric acid and electrocardiogram results were all within normal limits. Her serum electrolytes were as follows: Sodium 134 mmol/L, potassium 3.8 mmol/L, chloride 93 mmol/L, calcium 2.53 mmol/L, magnesium 0.73 mmol/L and phosphorus 1.50 mmol/L.
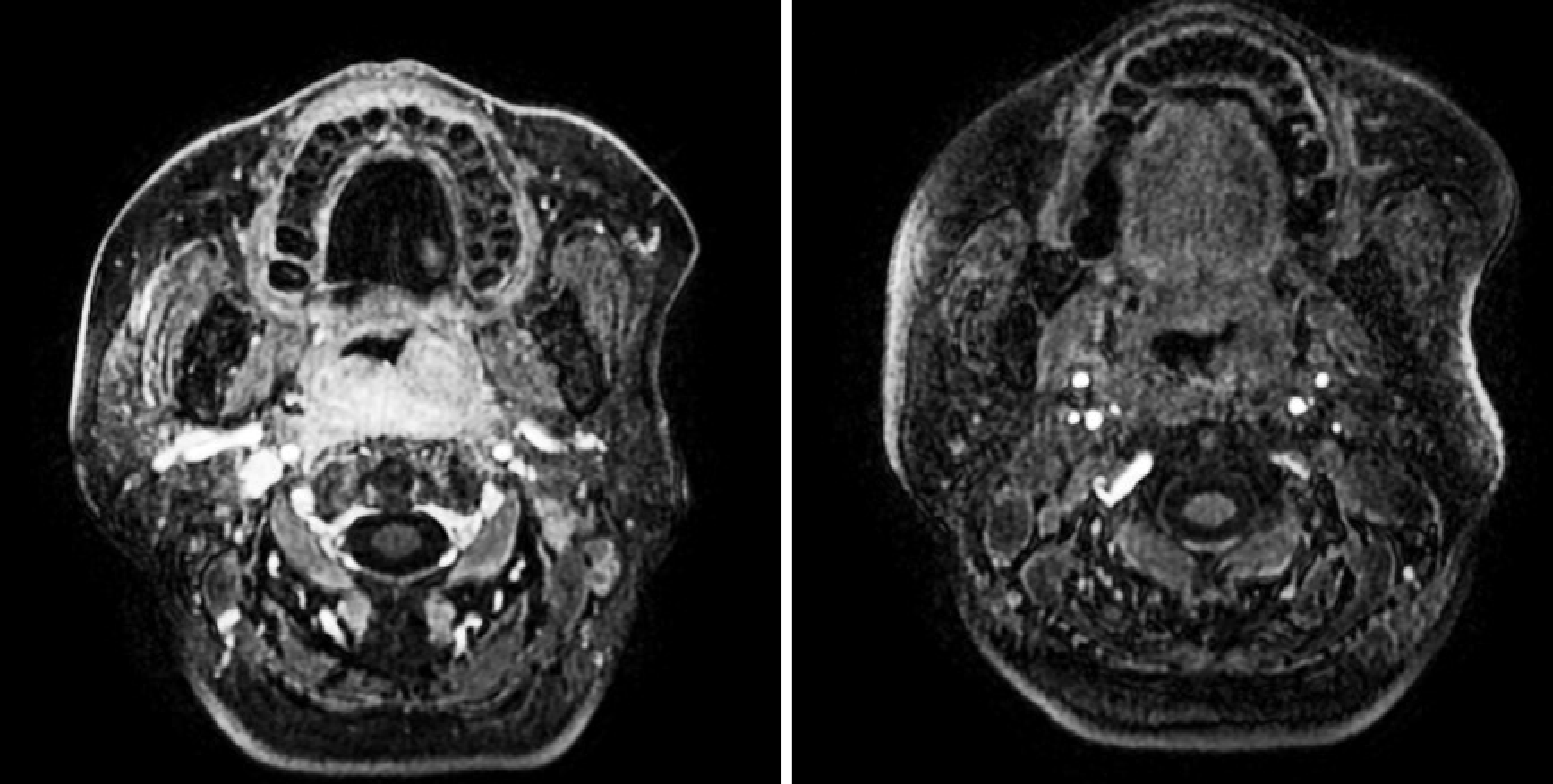
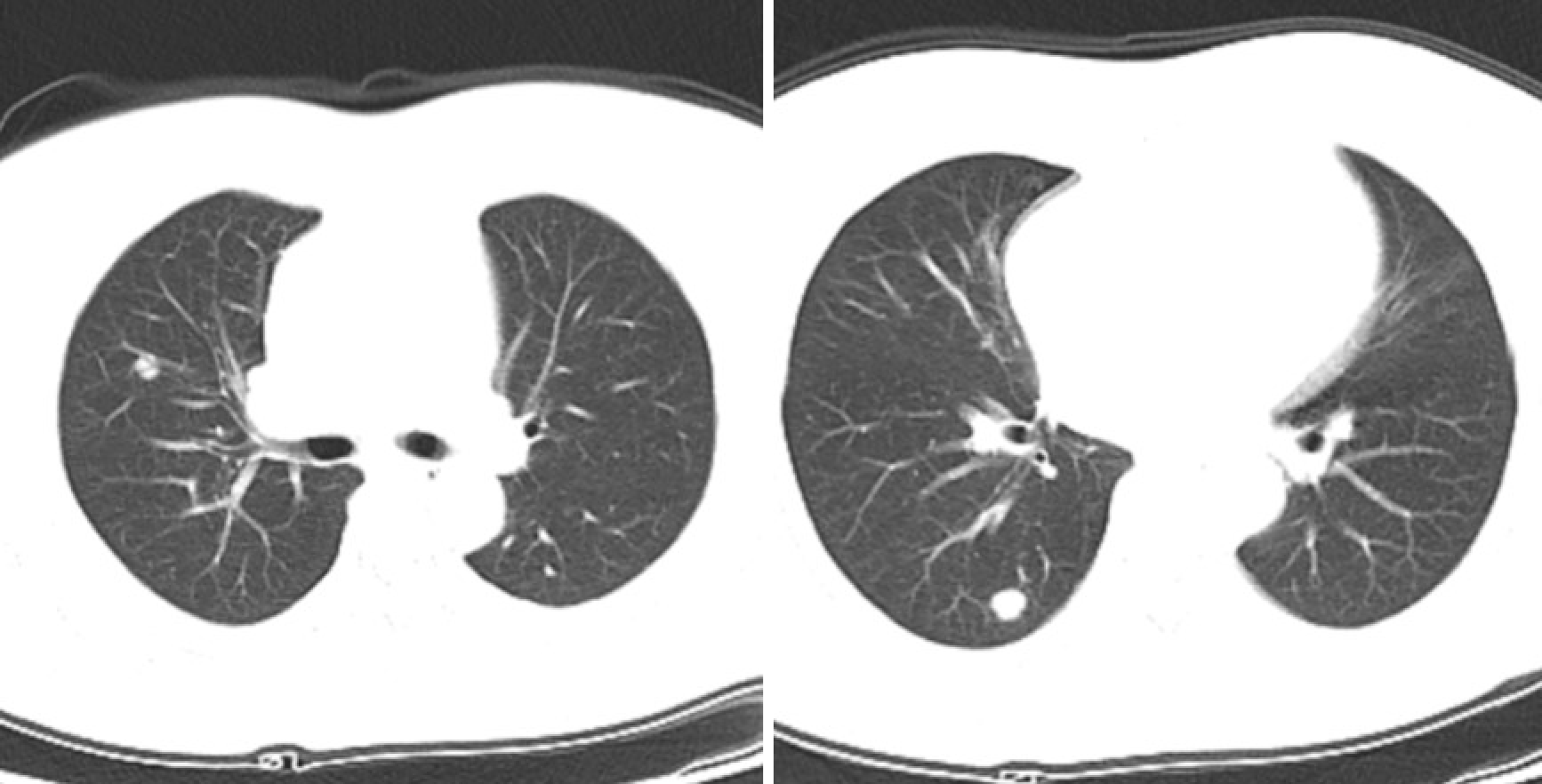
A magnetic resonance imaging scan of the neck revealed a lesion (51 mm × 28 mm × 40 mm) that occupied the posterior wall of the nasopharynx (Figure 1). A computed tomography (CT) scan of the chest showed multiple nodules, that were possibly metastases (Figure 2). There were no lesions on CT scans of the head and abdomen, and bone scans were normal.
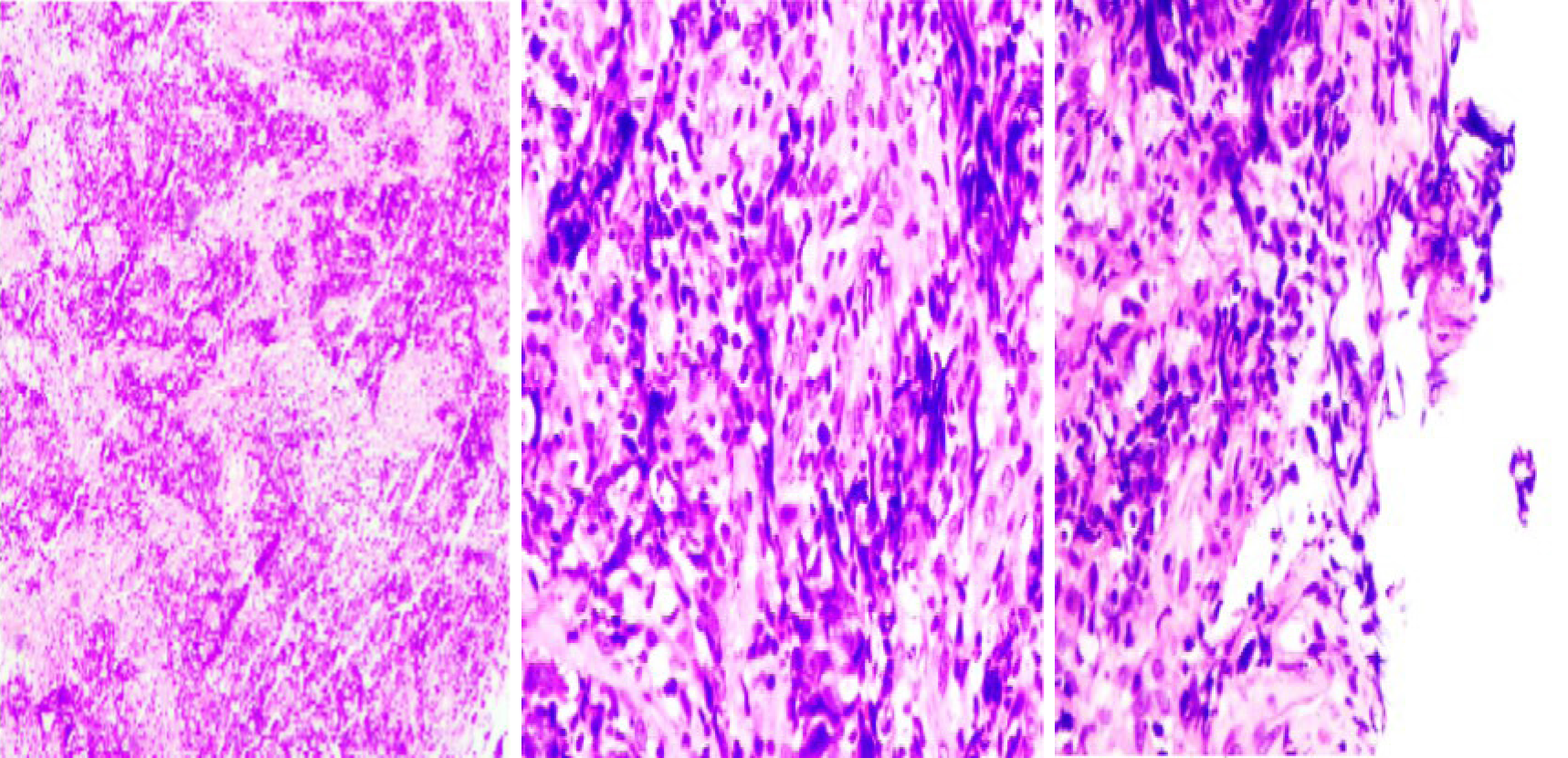
A nasopharyngeal biopsy confirmed a histopathological diagnosis of non-keratogenic carcinoma. Immunohistochemistry was positive for creatine kinase (CK), epidermal growth factor receptor, P40, P63, CK19 and CK5/6 and negative for Vim, S-100, leukocyte common antigen and CD34, which is consistent with nasopharyngeal carcinoma (NPC) (Figure 3). In situ hybridization showed Epstein-Barr encoding region (-).
She was diagnosed with NPC, with a classification of clinical stage IV.
According to the National Comprehensive Cancer Network guidelines, the patient received 1 cycle of chemotherapy with docetaxel and nedaplatin on November 25, 2020. The doses of docetaxel and nedaplatin were 75 mg/m2 (day 1) and 80 mg/m2 (days 1–3), respectively. She had sudden-onset convulsions, palpitations and sweating four days after nedaplatin initiation. On examination, she was hemodynamically stable and afebrile, with a heart rate of 86 bpm, blood pressure 152/113 mmHg, respiratory rate of 20 breaths/min, and oxygen saturation of 100% on room air. Her lungs were clear, and an abdominal exam was unremarkable. An electrocardiogram showed no acute pathologic processes. Her serum electrolytes were as follows: sodium 120 mmol/L, potassium 3.0 mmol/L, chloride 77 mmol/L, calcium 2.16 mmol/L, magnesium 0.57 mmol/L and phosphorus 1.05 mmol/L. Her blood urea nitrogen and creatinine were 4.32 mmol/L and 40.9 µmol/L, respectively. Serum osmolality was 257 mOsm/kg, with a urine osmolality of 570 mOsm/kg and urine sodium of 144 mmol/L. Her urinalysis was unremarkable. Thyroid-stimulating hormone, blood glucose, cholesterol, adrenocorticotropic hormone and cortisol stimulation tests were normal. These findings were consistent with SIADH.
She was treated with fluid restriction, and approximately 12 g of sodium was intravenously administered. Oral sodium replacement therapy was initiated at 3 g daily. Two days later (December 1, 2020), she experienced convulsions and sweating again, and her serum sodium had decreased to 108 mmol/L. Stricter fluid restriction (< 1200 mL/d) and more sodium were administered (20 g of sodium was intrave
Serum sodium levels gradually returned to within normal limits approximately 1 wk after initial presentation. Intravenous and oral sodium supplementation were discontinued approximately 2 wk later without sequelae.
In our patient, other possible causes of hyponatremia included inappetence and vomiting attributed to chemotherapy, but she had no clinical signs of dehydration or obvious digestive symptoms. The Naranjo Adverse Drug Reactions Probability Scale (NADRPS) is widely used to evaluate the relationship between drugs and adverse drug reactions (ADRs). NADRPS involves 10 ‘yes,’ ‘no,’ or ‘unknown or non-applicable’ questions. The ADR is assigned to a probability category on the basis of the total score of ‘definite’ ≥ 9; ‘likely’, 5-8; ‘possible’, 1-4; and ‘unlikely’, ≤ 0. For nedaplatin-induced SIADH, the results are shown in Table 1. The total score was 7, and the causality was assessed as ‘likely’.
| Axis | Numerical score | ||
| Yes | No | Unknown | |
| Previous reports on the reaction | 1 | ||
| The reaction occurred after the use of nedaplatin | 2 | ||
| Sodium increased after nedaplatin withdrawal | 1 | ||
| Nedaplatin rechallenge | 0 | ||
| Exclusion of alternative causes of SIADH | 2 | ||
| Placebo response | 0 | ||
| Drug concentration and monitoring | 0 | ||
| Dose relationship | 0 | ||
| Previous exposure and cross-reactivity | 0 | ||
| Presence of any objective evidence | 1 | ||
| Results | 7 = ‘likely’ | ||
SIADH was first described by Schwartz et al[7] in 1957. The diagnostic criteria for SIADH include serum Na < 135 mmol/L, serum osmolarity < 275 mOsm/kg, urine Na > 40 mEq/L, and urine concentration > 100 mOsm/kg. There was no evidence of hyper- or hypovolemia or diuretics usage. The functions of the thyroid, adrenal glands, heart, liver, and kidneys were normal[8]. The most frequent causes of SIADH include malignancies, lung diseases, central nervous system disorders and different kinds of drugs, especially anticancer medications, such as cyclophosphamide and platinum compounds[8,9].
Cisplatin-induced SIADH is more common than nedaplatin-induced SIADH[10-12]. To date, SIADH following the use of nedaplatin has only been reported by several Japanese authors[13,14]. In 2001, Iwabuchi et al[13] described a case of nedaplatin-induced SIADH in a patient with mandibular gingival cancer. Subsequently, it has been reported in patients with thoracic esophageal cancer[14]. In both cases, nedaplatin was combined with 5-fluorouracil, which differed from our patient.
Nedaplatin-induced SIADH is rare. Due to the small number of cases, the mechanism for nedaplatin-induced SIADH remains unclear, but the condition may be life-threatening. We report this case to highlight the importance of monitoring the plasma sodium concentration in patients treated with nedaplatin.
In conclusion, we report an extremely rare side effect of nedaplatin: SIADH. Treatment options include fluid restriction and sodium supplementation. Considering the lethality of severe hyponatremia, it should be taken seriously.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Raj R S-Editor: Wang JL L-Editor: Webster JR P-Editor: Liu JH
| 1. | Zhou J, Kang Y, Chen L, Wang H, Liu J, Zeng S, Yu L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front Pharmacol. 2020;11:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 2. | Chen NB, Li QW, Li S, Guo SP, Wu YJ, Cheng ZJ, Li JB, Wang DQ, Liu FJ, Ai XL, Hu N, Qiu B, Liu H. Docetaxel and nedaplatin twice a week with concurrent definitive radiotherapy in inoperable esophageal squamous cell carcinoma: A phase I trial (GASTO-1021). Radiother Oncol. 2021;155:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS, Qi B, Tang QN, Wang P, Li XY, Li JB, Liu Q, Gao YH, Xie FY, Liu LT, Li Y, Liu SL, Xie HJ, Liang YJ, Sun XS, Yan JJ, Wu YS, Luo DH, Huang PY, Xiang YQ, Sun R, Chen MY, Lv X, Wang L, Xia WX, Zhao C, Cao KJ, Qian CN, Guo X, Hong MH, Nie ZQ, Chen QY, Mai HQ. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Chikazawa K, Netsu S, Imai K, Ishiguro A, Kimura A, Wang L, Kuwata T, Konno R. Nedaplatin use in patients with hypersensitivity reaction episodes to carboplatin. Taiwan J Obstet Gynecol. 2020;59:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Zhong LZ, Xu HY, Zhao ZM, Zhang GM, Lin FW. Comparison of efficacy and toxicity between nedaplatin and cisplatin in treating malignant pleural effusion. Onco Targets Ther. 2018;11:5509-5512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Kawarada Y, Miyazaki M, Itoh A, Araki R, Iwamizu H, Kataoka T, Kumakura Y, Ota A, Nagai T, Yamada K. Incidence of and risk factors associated with nedaplatin-related hypersensitivity reactions. Int J Clin Oncol. 2017;22:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | SCHWARTZ WB, BENNETT W, CURELOP S, BARTTER FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957;23:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 656] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Fernández Miró M, Marin Arguedas S, Ferrer Ruscalleda L. [Syndrome of inappropriate antidiuretic hormone secretion associated with a SARS-CoV-2 pneumonia]. Med Clin (Barc). 2021;156:195-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Krishnamurthy A, Bhattacharya S, Lathia T, Kantroo V, Kalra S, Dutta D. Anticancer Medications and Sodium Dysmetabolism. Eur Endocrinol. 2020;16:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Abid H, Siddiqui N, Gnanajothy R. Severe Hyponatremia Due to Cisplatin-induced Syndrome of Inappropriate Secretion of Antidiuretic Hormone. Cureus. 2019;11:e5458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Tan AC, Marx GM. Cisplatin-induced syndrome of inappropriate antidiuretic hormone secretion (SIADH) with life-threatening hyponatraemia. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Ohtaka M, Hattori Y, Kumano Y, Maeda Y, Kondo T, Mochizuki T, Kawahara T, Teranishi J, Miyoshi Y, Yumura Y, Uemura H. [Severe Hyponatremia after Cisplatin-Based Chemotherapy : Two Case Reports]. Hinyokika Kiyo. 2016;62:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Iwabuchi H, Takamori K, Honma H, Asanami S, Tanaka Y. [A case of mandibular gingival cancer T4N0M0 which markedly responded to a combined therapy of nedaplatin with 5-fluorouracil]. Gan To Kagaku Ryoho. 2001;28:1273-1276. [PubMed] |
| 14. | Matsuda Y, Lee S, Kishida S, Mori K, Isohata N, Iwasaki H, Hashiba R, Gyobu K, Osugi H. [A case of SIADH developed during neoadjuvant chemotherapy using nedaplatin and 5-fluorouracil in a patient with esophageal cancer]. Gan To Kagaku Ryoho. 2010;37:1787-1790. [PubMed] |