Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6278
Peer-review started: April 11, 2021
First decision: April 23, 2021
Revised: May 6, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: August 6, 2021
Processing time: 107 Days and 18.3 Hours
Bile duct cyst (BDC) is a rare congenital bile duct malformation. The incidence of bile duct malignancy in BDC patients is markedly higher than that in the general population. However, few studies have been conducted on the risk factors for preoperative carcinogenesis in BDC patients.
To analyze the risk factors associated with preoperative carcinogenesis in BDC patients.
The medical records of BDC patients treated at our hospital between January 2012 and December 2018 were retrospectively reviewed. We constructed a database and compared the characteristics of BDC patients with dysplasia and carcinoma against those with benign cysts. The risk factors for preoperative carcinogenesis were identified using univariate and multivariate analyses.
The cohort comprised 109 BDC patients. Ten patients had preoperative dysplasia or adenocarcinoma. Univariate and multivariate analyses showed that gallbladder wall thickness > 0.3 cm [odds ratio (OR), 6.551; 95% confidence interval (CI), 1.351 to 31.763; P = 0.020] and Todani type IV (OR, 7.675; 95%CI, 1.584 to 37.192; P = 0.011) were independent factors associated with preoperative carcinogenesis.
BDC is a premalignant condition. Our findings show that gallbladder wall thickness > 0.3 cm and Todani type IV are independent risk factors for preoperative carcinogenesis of BDC. They are therefore useful for deciding on the appropriate treatment strategy, especially in asymptomatic patients.
Core Tip: Bile duct cyst (BDC) is a rare congenital bile duct malformation that is more common in Asian countries. The incidence of bile duct malignancy in BDC patients is 20- to 30-fold higher than that in the general population. However, few studies have been conducted on the risk factors for preoperative carcinogenesis of BDC. The present study retrospectively analyzed 109 BDC patients and found that gallbladder wall thickness > 0.3 cm and Todani type IV were independently associated with preope
- Citation: Wu X, Li BL, Zheng CJ, He XD. Risk factors for preoperative carcinogenesis of bile duct cysts in adults. World J Clin Cases 2021; 9(22): 6278-6286
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6278.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6278
Bile duct cyst (BDC), or biliary dilatation, is a rare congenital bile duct malformation that can occur in the intrahepatic biliary system, extrahepatic biliary tree, or both. Todani et al[1-3] systematically described and classified BDC in 1977 and then updated the classification in 1997 and 2003. BDC is more common in women, with a female-to-male ratio of 4:1[4]. The incidence rate of BDC is higher in Asian countries than in Western countries[5]. Most patients with BDC are diagnosed in the first decade of life, and only around 20% go undiagnosed into adulthood[6].
The currently recommended treatment modality for BDC is complete cyst excision plus Roux-en-Y hepaticojejunostomy[7,8]. Liver resection and transplantation are treatment choices of BDC type V[6]. For patients with symptoms like abdominal pain, jaundice, and fever, surgery is more acceptable. However, the necessity and timing of surgery are a difficult choice in asymptomatic patients due to the high incidence of postoperative complications. Most studies recommend complete cyst removal even in asymptomatic patients because the incidence of bile duct malignancy in BDC patients is 20- to 30-fold higher than that in the general population[6,9-11]. However, few studies have been conducted on the risk factors for preoperative carcinogenesis of BDC patients[12,13]. Precise estimates of the risk of preoperative carcinogenesis in BDC are lacking[14], and the patient features that are indications for surgery are still unknown. Thus, the present study aimed to analyze the potential risk factors associated with preoperative carcinogenesis in patients with BDC to provide a deeper understanding of BDC and determine the optimal treatment options for asymptomatic BDC patients.
All the medical records of BDC patients treated at our hospital between January 2012 and December 2018 were retrospectively reviewed. Patients who were diagnosed with BDC by both preoperative imaging and postoperative pathology, and at least 18 years old were selected, while those who did not undergo operation or had incomplete medical records were excluded. Clinical data were compiled from both inpatient and outpatient medical records, and a retrospective database was constructed. The demographic characteristics, symptoms, laboratory tests, operation details, pathology information, and prognoses were analyzed. This study was approved by the Peking Union Medical College Hospital Institutional Review Board (S-K1483). The requirement of informed consent for publication of data was waived owing to the retrospective nature of the study.
Statistical analyses were performed using the Statistical Package for Social Sciences software (version 25.0, IBM Corp, Armonk, NY, United States). Continuous variables are presented as the mean ± SD and were analyzed using Student’s t test. Categorical variables are shown as an absolute number or frequency and were analyzed using the χ2 test or Fisher’s exact test as appropriate. Logistic multivariate regression analysis was performed to identify potential independent risk factors for preoperative carcinogenesis of BDC patients. A P value < 0.05 was considered statistically significant.
A total of 129 adult patients with BDC were treated at our institution during the study period, and we excluded 20 patients who did not undergo operation due to severe underlying disease. Thus, the cohort comprised 109 patients with BDC, and they were further divided into two groups based on pathology results: Group A comprised patients with benign pathology (n = 99), while group B comprised patients with dysplasia or carcinoma (n = 10). The preoperative dysplasia/carcinoma rate was 9.2% (10/109). The demographic characteristics, symptoms, and laboratory tests by group are shown in Table 1. There was no significant difference between the two groups. The mean age at admission was 40.4 ± 15.0 years (range, 18-80 years), and the male-to-female ratio was 1:4.7. Abdominal pain (82.6%) was the most common preoperative symptom, followed by fever (26.6%) and jaundice (12.8%). Abnormal pancreatic biliary duct was confirmed in 55 (50.5%) patients via endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography. Stratified analysis by age group was also conducted to further analyze the correlation between age and dysplasia/carcinoma rates (Table 2).
| Overall cohort (n = 109) | Group A (n = 99) | Group B (n = 10) | P value | |
| Male/female (n) | 19/90 | 19/80 | 0/10 | 0.277 |
| Age at symptom onset (yr) | 34.6 ± 15.5 | 34.7 ± 15.4 | 34.0 ± 16.5 | 0.892 |
| Age at admission (yr) | 40.4 ± 15.0 | 40.6 ± 15.0 | 38.1 ± 16.0 | 0.611 |
| BMI (kg/m2) | 22.0 ± 3.3 | 22.1 ± 3.4 | 21.6 ± 2.2 | 0.634 |
| Smoking (n) | 7 | 7 | 0 | 1.000 |
| Abdominal pain (n) | 90 | 82 | 8 | 1.000 |
| Fever (n) | 29 | 26 | 3 | 1.000 |
| Jaundice (n) | 14 | 11 | 3 | 0.228 |
| CA19-9 > 34 U/mL (n) | 9 | 8 | 1 | 0.594 |
| CEA > 5 ng/mL (n) | 1 | 0 | 1 | 0.092 |
| APBD (n) | 55 | 51 | 4 | 0.717 |
| Age (yr) | Total, n | Dysplasia/carcinoma, n (%) | P value |
| 18-30 | 35 | 4 (11.4) | 0.866 |
| 31-40 | 21 | 2 (9.5) | |
| 41-50 | 24 | 2 (8.3) | |
| 51-60 | 13 | 0 (0) | |
| ≥ 61 | 16 | 2 (12.5) |
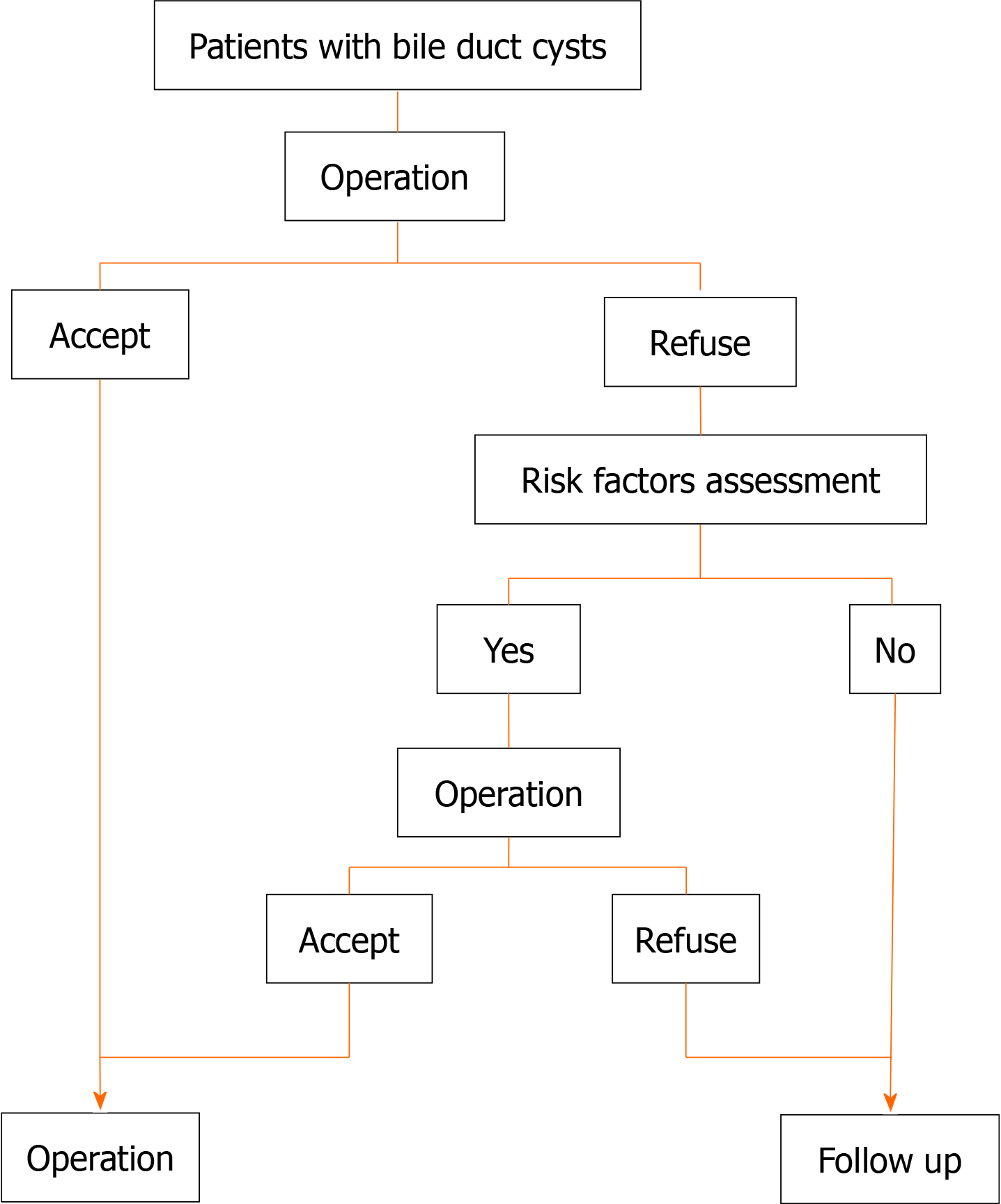
All patients were diagnosed with BDC by both preoperative imaging and postoperative pathology. The operation details, pathology information, and Todani classification are shown in Table 3. The number of patients with gallbladder wall thickness > 0.3 cm, cyst transverse diameter > 3 cm, and Todani type IV was significantly higher in group B than in group A. Multivariate logistic regression analysis showed that gallbladder wall thickness > 0.3 cm and Todani type IV were independently associated with preoperative carcinogenesis, with odds ratios of 6.551 and 7.675, respectively (Table 4). A flow chart of treatment recommendations for patients with BDC is shown in Figure 1.
| Overall cohort (n = 109) | Group A (n = 99) | Group B (n = 10) | P value | |
| Operative time (min) | 219.2 ± 64.8 | 215.8 ± 61.6 | 249.5 ± 86.6 | 0.119 |
| Bleeding amount (mL) | 212.3 ± 210.0 | 204.8 ± 202.0 | 283.0 ± 277.5 | 0.265 |
| Max diameter of GB (cm) | 7.8 ± 1.7 | 7.9 ± 1.7 | 7.0 ± 1.7 | 0.147 |
| GB wall thickness > 0.3 cm (n) | 20 | 15 | 5 | 0.022 |
| TD of cyst > 3 cm (n) | 46 | 38 | 8 | 0.028 |
| Cyst wall thickness > 0.3 cm (n) | 8 | 8 | 0 | 1.000 |
| Bile duct stone (n) | 22 | 21 | 1 | 0.668 |
| Todani classification (n)1 | 0.031 | |||
| I | 74 | 71 | 3 | |
| III | 1 | 1 | 0 | |
| IVa | 31 | 24 | 7 | |
| V | 3 | 3 | 0 |
| P value | OR | 95%CI | |
| Gallbladder wall thickness > 0.3 cm | 0.020 | 6.551 | 1.351-31.763 |
| Todani type IV | 0.011 | 7.675 | 1.584-37.192 |
| Transverse diameter of cyst > 3 cm | 0.051 | 5.479 | 0.990-30.333 |
As of December 2020, 97 (89.0%) patients were followed for a mean duration of 59.7 ± 24.6 mo (range, 6-102 mo). No postoperative carcinoma was observed. In group B, nine out of ten patients were followed. The detailed clinical features of patients in group B are presented in Table 5. In total, 4, 3, and 3 patients had dysplasia or carcinoma in the cyst, the gallbladder, and in both the cyst and the gallbladder, respectively. Five patients had dysplasia and the other five had adenocarcinoma. The longest follow-up time was 81 mo, and the patient remains alive and disease free to date.
| No. | Sex | Age (yr) | Todani type | Operation | Lesion location | Pathology | TNM stage1 | Follow-up time (mo) | Outcome |
| 1 | F | 39 | IVa | CH + CE + HJ | Cyst | Mucinous adenocarcinoma | T4N0M0 | 40 | Recurred 10 mo after surgery, alive with tumor |
| 2 | F | 46 | IVa | CH + CE + HJ | Gallbladder | Mucinous adenocarcinoma | T3N0M0 | 6 | Survived disease free for 6 mo after surgery, then lost to follow-up |
| 3 | F | 44 | IVa | CH + CE + HJ | Cyst | Moderate dysplasia | -- | 52 | Disease-free survival |
| 4 | F | 26 | IVa | CH + CE + HJ | Cyst + gallbladder | Mild dysplasia | -- | 53 | Disease-free survival |
| 5 | F | 61 | I | CH + CE + HJ | Gallbladder | Adenocarcinoma | T2N0M0 | 40 | Disease-free survival |
| 6 | F | 27 | IVa | CH + CE + HJ | Cyst + gallbladder | Moderate dysplasia | -- | 66 | Disease-free survival |
| 7 | F | 33 | IVa | Pancreaticoduodenectomy | Cyst | Adenocarcinoma | TisN0M0 | 81 | Disease-free survival |
| 8 | F | 18 | I | CH + CE + HJ | Cyst + gallbladder | Mild dysplasia | -- | 40 | Disease-free survival |
| 9 | F | 65 | I | CH + CE + HJ | Gallbladder | Adenocarcinoma | T1N0M0 | 65 | Disease-free survival |
| 10 | F | 22 | IVa | CH + CE + HJ | Cyst | Mild dysplasia | -- | -- | Lost to follow-up |
The incidence of BDC diagnoses in adult patients has increased worldwide due to the widespread use of health screening and improvements in noninvasive bile duct imaging[15,16]. Approximately 10%-30% of adult patients with BDC develop carcinoma[9,17,18]. The first case of neoplastic change within BDC was reported by Irwin et al[19] in 1944. The tumor may arise in the cyst wall, gallbladder, undilated parts of the biliary tree, and even in remnant tissue after operation. The pathogenesis of preoperative and postoperative carcinogenesis of BDC is entirely different[20]. Preoperative carcinoma is mainly caused by abnormal confluent pancreatic juice that can erode the bile duct epithelium[21,22]. This problem is resolved after operation because the pancreatic and biliary drainage is separated. Meanwhile, postoperative carcinogenesis is primarily caused by recurrent cholangitis, which can be avoided via complete cyst dissection and proper bile duct flow. In the present study, we only focused on preoperative carcinogenesis.
As observed in esophageal and colon cancer, the progression of carcinoma in BDC involves simple hyperplasia and dysplasia that ultimately leads to the formation of invasive carcinoma[23-26]. Carcinogenesis may be related to dysplasia of the bile duct epithelium, and thus we analyzed the risk factors for both dysplasia and carcinoma in this study to further clarify the risk factors for carcinogenesis and determine the optimal treatment modality given that the need to treat in asymptomatic patients is based on the risk of developing a malignancy[27,28]. A meta-analysis reported that the preoperative malignancy rate of BDC is 7.3%[14]. The preoperative rates of both dysplasia and carcinoma and only carcinoma in this study (9.2% and 4.6%, respec
Chronic inflammation of the bile duct leads to K-ras mutations, cellular atypia, overexpression of the p53 encoding protein, and loss of heterozygosity of p53 at the molecular level[30-33]. These mutations could result in malignant transformation and cause an association between chronic inflammation and bile duct carcinoma. Concurrently, metaplastic changes of the bile duct epithelium are considered premalignant lesions that progress to bile duct carcinoma[32], and this is frequently observed in chronic bile duct inflammation. Bile duct inflammation causes the gallbladder wall to thicken to > 0.3 cm, and this could explain why such thickness is a risk factor for carcinogenesis. Some previous studies also reported the thickness of the gallbladder wall as a predictor of premalignant mucosal transformation[34,35]. The incidence of cholangiocarcinoma varies between different types of BDC, with type I and type IV having the highest risk of malignant transformation[6,9,14]. Todani IV cysts were strongly associated with chronic inflammation of the bile duct and abnormal pancreaticobiliary duct junction[26,36]. Prolonged reflux of pancreatic secretions could lead to malignant degeneration of the bile duct epithelium[28], and these factors cause the high dysplasia and carcinoma rate. Ten Hove et al[14] reported Todani type I and IV as risk factors for preoperative carcinogenesis, and He et al[12] reported a higher carcinogenesis rate in type I than in type IV. The difference between the findings of the current study and those in the literature might be caused by the limited number of patients with dysplasia and carcinoma in the current study.
Age has been consistently reported as an independent risk factor for carcinogenesis[9,12,13], and the incidence of carcinogenesis particularly increased with high age at presentation[37,38]. However, we found no relationship between incidence and age in our study (Table 2) and this may be due to the following: First, the reported age-dependent increase in incidence was only for tumors, while the present study calculated the incidence of both dysplasia and carcinoma. Second, because of the popularity of health examination, the number of young patients diagnosed and treated is increasing, while the number of patients diagnosed at an older age is decreasing. Third, the sample size, particularly the number of patients with dysplasia or carcinoma was limited, and the possibility of selection bias could not be ruled out.
In the present study, all the patients with dysplasia and carcinoma were women. The reason may be that BDC is more common in women than in men. Further, at the molecular level, increased estrogen receptor expression in the biliary epithelium was found in patients with neoplastic changes[39]. Of the ten patients with dysplasia and carcinoma, nine were followed and had a mean survival time of 49.2 ± 21.5 mo, and seven were still alive and disease free at the last follow-up. The patient prognosis in the current study was markedly better than that in the literature[12], and this may be because not only patients with carcinoma, but also those with dysplasia were also included in this study. Another possible reason was that the exclusion of 20 patients with severe underlying disease would lead to an improvement in overall outcomes.
This study has some limitations. First, the registration information and patient number could not be designated in advance due to its retrospective nature. Second, the study is confined to a single institution, and the number of patients is limited. Given that the number of BDC cases diagnosed and treated is increasing, prospective, observational, controlled, and multi-center clinical trials are needed to identify reliable risk factors for malignancy in BDC.
BDC is a rare congenital bile duct malformation. Prophylactic operation is recommended but not well accepted by all asymptomatic patients. Precise estimates of the risk of preoperative carcinogenesis in BDC are lacking. Our findings show that gallbladder wall thickness > 0.3 cm and Todani type IV are independent risk factors for carcinogenesis of BDC in adults and are thus valuable in choosing the appropriate treatment strategy in these patients.
The currently recommended treatment modality for bile duct cyst (BDC) is operation due to the high incidence of bile duct malignancy. However, few studies have been conducted on the risk factors for preoperative carcinogenesis of BDC patients.
To find out the patient features that are indications for surgery in BDC and provide better treatment recommendation.
To analyze the risk factors associated with preoperative carcinogenesis in BDC patients.
This retrospective study included patients with BDC treated at our hospital between January 2012 and December 2018. A database containing demographic characteristics, symptoms, laboratory tests, operation details, pathology information, and prognoses was constructed. The characteristics of BDC with dysplasia/carcinoma were compared with benign cysts. Univariate and multivariate analyses were used to analyze the risk factors for preoperative carcinogenesis.
A total of 109 patients with BDC were included. Ten patients had preoperative dysplasia or adenocarcinoma. Univariate analysis showed that gallbladder wall thickness > 0.3 cm, cyst transverse diameter > 3 cm, and Todani type IV were associated with preoperative carcinogenesis. Multivariate logistic regression analysis showed that gallbladder wall thickness > 0.3 cm and Todani type IV were independently associated with preoperative carcinogenesis. The follow-up information of ten patients with dysplasia/carcinoma was presented.
BDC is a premalignant condition. Gallbladder wall thickness > 0.3 cm and Todani type IV are independent risk factors for preoperative carcinogenesis.
The two risk factors are useful for deciding on the appropriate treatment strategy, especially in asymptomatic patients.
We wish to thank our colleagues in the Department of Medical Records for their cooperation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bain V, Gumbs A S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 835] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 2. | Todani T. Congenital choledochal dilatation: classification, clinical features, and long-term results. J Hepatobiliary Pancreat Surg. 1997;4:276-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Todani T, Watanabe Y, Toki A, Morotomi Y. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg. 2003;10:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 4. | Atkinson HD, Fischer CP, de Jong CH, Madhavan KK, Parks RW, Garden OJ. Choledochal cysts in adults and their complications. HPB (Oxford). 2003;5:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Akaraviputh T, Boonnuch W, Watanapa P, Lert-Akayamanee N, Lohsiriwat D. Surgical management of adult choledochal cysts. J Med Assoc Thai. 2005;88:939-943. [PubMed] |
| 6. | Mabrut JY, Bozio G, Hubert C, Gigot JF. Management of congenital bile duct cysts. Dig Surg. 2010;27:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Jan YY, Chen HM, Chen MF. Malignancy in choledochal cysts. Hepatogastroenterology. 2000;47:337-340. [PubMed] |
| 8. | Xia HT, Dong JH, Yang T, Liang B, Zeng JP. Selection of the surgical approach for reoperation of adult choledochal cysts. J Gastrointest Surg. 2015;19:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Söreide K, Körner H, Havnen J, Söreide JA. Bile duct cysts in adults. Br J Surg. 2004;91:1538-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Kamisawa T, Okamoto A, Tsuruta K, Tu Y, Egawa N. Carcinoma arising in congenital choledochal cysts. Hepatogastroenterology. 2008;55:329-332. [PubMed] |
| 11. | Morine Y, Shimada M, Takamatsu H, Araida T, Endo I, Kubota M, Toki A, Noda T, Matsumura T, Miyakawa S, Ishibashi H, Kamisawa T, Shimada H. Clinical features of pancreaticobiliary maljunction: update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci. 2013;20:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | He XD, Wang L, Liu W, Liu Q, Qu Q, Li BL, Hong T. The risk of carcinogenesis in congenital choledochal cyst patients: an analysis of 214 cases. Ann Hepatol. 2014;13:819-826. [PubMed] |
| 13. | Sastry AV, Abbadessa B, Wayne MG, Steele JG, Cooperman AM. What is the incidence of biliary carcinoma in choledochal cysts, when do they develop, and how should it affect management? World J Surg. 2015;39:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Ten Hove A, de Meijer VE, Hulscher JBF, de Kleine RHJ. Meta-analysis of risk of developing malignancy in congenital choledochal malformation. Br J Surg. 2018;105:482-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Dhupar R, Gulack B, Geller DA, Marsh JW, Gamblin TC. The changing presentation of choledochal cyst disease: an incidental diagnosis. HPB Surg. 2009;2009:103739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Cho MJ, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Lee SK, Kim MH, Lee SS, Park DH, Lee SG. Surgical experience of 204 cases of adult choledochal cyst disease over 14 years. World J Surg. 2011;35:1094-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 17. | Liu CL, Fan ST, Lo CM, Lam CM, Poon RT, Wong J. Choledochal cysts in adults. Arch Surg. 2002;137:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, Shimada H, Takamatsu H, Miyake H, Todani T; Committee for Registration of the Japanese Study Group on Pancreaticobiliary Maljunction. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Irwin ST, Morison JE. Congenital cyst of the common bile duct containing stones and undergoing cancerous change. Br J Surg. 1944;32:319-321. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Xia HT, Yang T, Liu Y, Liang B, Wang J, Dong JH. Proper bile duct flow, rather than radical excision, is the most critical factor determining treatment outcomes of bile duct cysts. BMC Gastroenterol. 2018;18:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Kim JW, Moon SH, Park DH, Lee SS, Seo DW, Kim MH, Lee SK. Course of choledochal cysts according to the type of treatment. Scand J Gastroenterol. 2010;45:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, Koizumi S, Kurata M, Honda G, Itoi T. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol. 2015;50:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Nagai M, Watanabe M, Iwase T, Yamao K, Isaji S. Clinical and genetic analysis of noncancerous and cancerous biliary epithelium in patients with pancreaticobiliary maljunction. World J Surg. 2002;26:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Matsumoto Y, Fujii H, Itakura J, Matsuda M, Yang Y, Nobukawa B, Suda K. Pancreaticobiliary maljunction: pathophysiological and clinical aspects and the impact on biliary carcinogenesis. Langenbecks Arch Surg. 2003;388:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Yang B, House MG, Guo M, Herman JG, Clark DP. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol. 2005;18:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Søreide K, Søreide JA. Bile duct cyst as precursor to biliary tract cancer. Ann Surg Oncol. 2007;14:1200-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Jordan PH Jr, Goss JA Jr, Rosenberg WR, Woods KL. Some considerations for management of choledochal cysts. Am J Surg. 2004;187:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kim Y, Hyun JJ, Lee JM, Lee HS, Kim CD. Anomalous union of the pancreaticobiliary duct without choledochal cyst: is cholecystectomy alone sufficient? Langenbecks Arch Surg. 2014;399:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Madadi-Sanjani O, Wirth TC, Kuebler JF, Petersen C, Ure BM. Choledochal Cyst and Malignancy: A Plea for Lifelong Follow-Up. Eur J Pediatr Surg. 2019;29:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Wee A, Teh M, Raju GC. Clinical importance of p53 protein in gall bladder carcinoma and its precursor lesions. J Clin Pathol. 1994;47:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Wistuba II, Sugio K, Hung J, Kishimoto Y, Virmani AK, Roa I, Albores-Saavedra J, Gazdar AF. Allele-specific mutations involved in the pathogenesis of endemic gallbladder carcinoma in Chile. Cancer Res. 1995;55:2511-2515. [PubMed] |
| 32. | Tazuma S, Kajiyama G. Carcinogenesis of malignant lesions of the gall bladder. The impact of chronic inflammation and gallstones. Langenbecks Arch Surg. 2001;386:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Cerwenka H. Bile duct cyst in adults: interventional treatment, resection, or transplantation? World J Gastroenterol. 2013;19:5207-5211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Seretis C, Lagoudianakis E, Gemenetzis G, Seretis F, Pappas A, Gourgiotis S. Metaplastic changes in chronic cholecystitis: implications for early diagnosis and surgical intervention to prevent the gallbladder metaplasia-dysplasia-carcinoma sequence. J Clin Med Res. 2014;6:26-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Bangash M, Alvi AR, Shahzad N, Shariff AH, Gill RC. Factors Associated with Premalignant Epithelial Changes in Chronic Calculous Cholecystitis: A Case-Control Study. World J Surg. 2018;42:1701-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Ohashi T, Wakai T, Kubota M, Matsuda Y, Arai Y, Ohyama T, Nakaya K, Okuyama N, Sakata J, Shirai Y, Ajioka Y. Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cysts. J Gastroenterol Hepatol. 2013;28:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Benjamin IS. Biliary cystic disease: the risk of cancer. J Hepatobiliary Pancreat Surg. 2003;10:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Nicholl M, Pitt HA, Wolf P, Cooney J, Kalayoglu M, Shilyansky J, Rikkers LF. Choledochal cysts in western adults: complexities compared to children. J Gastrointest Surg. 2004;8:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Fumino S, Iwai N, Deguchi E, Kimura O, Ono S, Iwabuchi T. Estrogen receptor expression in anomalous arrangement of the pancreaticobiliary duct. J Pediatr Surg. 2005;40:1716-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |