Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6091
Peer-review started: March 22, 2021
First decision: April 29, 2021
Revised: May 9, 2021
Accepted: May 26, 2021
Article in press: May 26, 2021
Published online: July 26, 2021
Processing time: 121 Days and 2.8 Hours
Congenital factor VII deficiency (FVIID) is a rare autosomal recessive genetic disorder. The clinical manifestations of this deficiency vary greatly. Predicting the risk of bleeding during and after childbirth of pregnant women with congenital FVIID is difficult. Recombinant factor VIIa is the most common replacement therapy for FVIID. However, no unified diagnosis and treatment plan for pregnant women with congenital FVIID has been established.
We report the clinical history of a pregnant woman who was considered to have congenital FVIID. Recombinant factor VIIa was prophylactically administered to the pregnant woman at the time of cervical fully opening. She successfully delivered a live infant without any complications, such as postpartum hemorr
Prophylaxis of recombinant factor VIIa during delivery can effectively reduce the incidence of postpartum hemorrhage among pregnant women with congenital FVIID associated with a high risk of bleeding.
Core Tip: The clinical manifestations of congenital factor VII deficiency vary greatly and range from a mild asymptomatic case to fatal bleeding. During pregnancy, predicting the risk of bleeding during and after childbirth of pregnant women with congenital factor VII deficiency is difficult, causing serious challenges to obstetricians and gynecologists. We hereby report a case of a pregnant woman with congenital factor VII deficiency and discuss the perinatal period and delivery management of this disease.
- Citation: Yang Y, Zeng YC, Rumende P, Wang CG, Chen Y. Diagnosis and treatment discussion of congenital factor VII deficiency in pregnancy: A case report. World J Clin Cases 2021; 9(21): 6091-6101
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6091.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6091
Factor VII (FVII) is a vitamin-K-dependent glycoprotein synthesized and secreted by the liver. FVII is a determining factor in activating the exogenous coagulation pathway[1]. Congenital factor VII deficiency (FVIID) is a rare autosomal recessive hemorrhagic disease caused by F7 gene mutation and leads to a decrease in FVII number or functional defect[2]. The F7 gene is located on chromosome 13 (13q34) and consists of 9 exons and 8 introns, covering a 12.8 kb genome area. As of February 2014, the human gene mutation database (http://www.hgmd.cf.ac.uk/ac/all.php) published 283 F7 gene mutations, including 180 missense and nonsense mutations, 39 cutting site mutations, and 33 small mutations inserted or deleted; single nucleotide polymor
The International Society of Thrombosis and Hemostasis classifies FVIID as follows: severe: FVII < 10%, with the risk of spontaneous bleeding; moderate: FVII 10%-20%, with the risk of mild spontaneous or trigger bleeding; mild: FVII 20%-50%, most of them are asymptomatic[5]. The Seven Treatment Evaluation Registry defined FVII activity among severe patients as ≤ 5%[6]. Patients with FVIID are usually asympto
The clinical manifestations of FVIID are quite different compared with the general cases. Mild patients only have slight bleeding or post-traumatic bleeding, such as gingival bleeding, epistaxis, ecchymosis of skin and mucous membrane, menorrhagia, and persistent post-traumatic bleeding. By contrast, severe manifestations occur in 4.4% to 8.0% of patients with FVIID[7], which may provoke life-threatening bleeding, such as gastrointestinal bleeding, intracranial bleeding, and joint bleeding. Based on analytical data of the cooperative registry of the International Registry of Factor VII deficiency and Seven Treatment Evaluation Registry, menorrhagia is the most common bleeding symptom, and 46% of female patients with this deficiency have menorrhagia[8]. The severe clinical manifestation of pregnant women with FVIID during delivery is bleeding, which may occur during placental detachment, genital tract laceration, vulvar incision, or cesarean section[9].
At present, only a few cases of congenital FVIID in pregnancy have been reported. Kulkarni et al[10] reported the full-term delivery history of four pregnant women with congenital FVIID; these women had increased mean FVII activity from 33 IU/dL to 73 IU/dL at full term and received prophylactics of recombinant FVIIa (rFVIIa) at delivery. Three of these women underwent cesarean section, and one had a vaginal delivery. One of the pregnant women suffered excessive blood loss (1400 mL) during cesarean section and did not require a blood transfusion. The average blood loss of three pregnant women who had cesarean section was 800 mL. Eskandari et al[11] admitted a 22-year-old primipara with congenital FVIID who had 1% FVII activity and received rFVIIa at 50 µg/kg at cervix full opening and 35 µg/kg 4 h later. No bleeding complication was recorded. The activity of FVII was more than 900% at 30 min after each administration. Therefore, the authors believed they used excessive rFVIIa.
Jiménez-Yuste et al[12] reported a 30-year-old woman with moderate FVII deficiency (FVII activity was 5%) and HIV infection who delivered by cesarean section after continuous infusion of rFVIIa to maintain the plasma FVII level to approximately 100%. No bleeding complications occurred. Ariffin et al[13] reported a couple with congenital heterozygous FVIID whose first two newborns died of massive intracranial hemorrhage secondary to severe congenital FVIID. No samples were obtained from the two children who died, but the level of FVII activity was consistent with that of conjugal complex heterozygotes with genetic damage. Three years later, when the mother became pregnant again, a villus specimen was taken at 10 wk of gestation to rule out severe coagulation FVIID. Exon polymerase chain reaction amplification and villus sequence analysis revealed that the fetus inherited only the father’s genotype as a heterozygous FVIID. A male infant was delivered by elective cesarean section at term and performed well clinically at the time of delivery and 1-year follow-up.
Loddo et al[14] described a 35-year-old woman with coagulation FVIID who received an intravenous dose of 20 μg/kg rFVIIa at cervix full opening at 40 wk of gestation, followed by the same dose 4 h later. The woman had an initial FVII activity of 18% (normal range 60%-150%). The pregnant woman had a smooth vaginal delivery, and neither the pregnant woman nor the newborn had any bleeding-related complications.
A low correlation was found between the level of FVII activity and the risk of bleeding. FVII genotype, coagulation test, and bleeding history cannot predict the risk of bleeding in patients with FVIID[14]. Therefore, predicting the risk of bleeding and determining the corresponding treatment in clinical practice is difficult. In this report, we present the course of pregnancy and delivery of a patient with congenital FVIID who was admitted to our hospital. We present a discussion of the diagnosis, monitoring, and delivery management process. We then provide literature reviews of pregnancies with congenital FVIID.
A 23-year-old woman Gravida 1 Para 0 appeared at our hospital to confirm her pregnancy after amenorrhea for 33 d, and the deficiency of coagulation FVII was considered (the congenital possibility was excellent).
On April 1, 2019, a 23-year-old woman Gravida 1 Para 0 appeared at our hospital to confirm her pregnancy after amenorrhea for 33 d. The coagulation test showed prothrombin time (PT) of 62.10 s (normal range 9.00-15.00), International Standard Ratio (INR) of 5.09 (normal range 0.80-1.40), and prothrombin activity (PTA) of 12% (normal range 70%-130%). The laboratory reports revealed no remarkable result of activated partial thromboplastin time (APTT), fibrinogen, plasma thrombin time, and complete blood count, and the liver function was normal. On April 25, 2019, she visited the Department of Hematology of our hospital and checked four items of the coagulation test: + D2 quantification + AT + FDP: PT 50.7 s, INR 4.11, PTA 15%, and the others were normal. PT correction (mixing equal volume of patient’s plasma and normal plasma) can be conducted immediately and after incubation for 2 h. For the complete set of coagulation factors, the activity of factor VII was 2.00% (normal range: 50.00%-129.00%), and the rest of the coagulation factors were normal. The lupus anticoagulant and immune-related indices were negative. The deficiency of coagulation FVII was considered (the congenital possibility was excellent).
The patient was generally healthy. During pregnancy, she had no history of abnormal bleeding, such as gum bleeding, nose bleeding, joint bleeding, gastroin
Liver and biliary system diseases, blood system, and rheumatic immune system diseases were denied. No anticoagulant drug intake history, rodenticide administration history, operation history, and trauma history were recorded.
The patient had normal menstrual volume. Nothing out of the ordinary was found on the woman’s personal history and her family history (no history of abnormal bleeding in her parents and sister).
The patient was conscious and had no bleeding spots and ecchymosis. No remarkable result of physical examination and no special obstetric conditions were found.
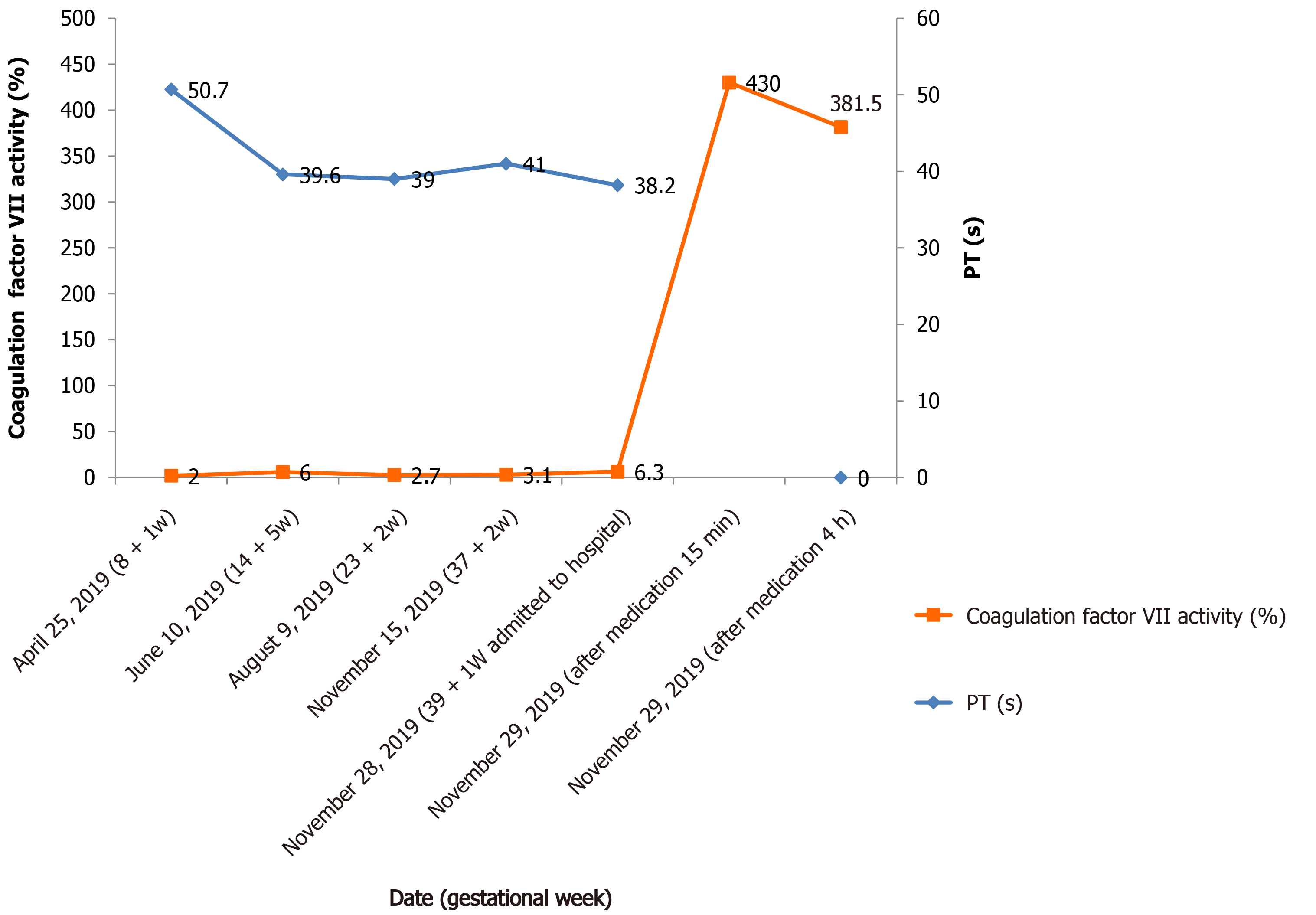
The fluctuation of coagulation indices was monitored during pregnancy: Activity of FVII was 2.00%-6.00%, PT 39-62.10 s, INR 3.24-5.09, and PTA 12%-20%. On November 28, 2019, 39 + 1 wk gestational age, the woman was admitted to our hospital due to regular lower abdominal pain. Laboratory examination results were as follows: PT 38.2 s, INR 3.2, PTA 21%, FVII activity 6.30%, D-dimer 710 ng/mL (normal range 0-450), and fibrinogen 6.09 g/L (normal range 2-5). FDP, AT, and thrombelastogram were normal.
The fluctuation of FVII activity and corresponding PT value of the pregnant woman during pregnancy and childbirth were as follows (Figure 1).
The genetic testing for the inherited disease of the patient’s blood system showed that she carried the homozygous mutation of the F7 gene, which is a missense mutation (it is expected to change the 241st amino acid of the encoded protein from Thr to Asn). The mutation detected in patients with FVIID: ESP6500, thousand human genome, and dbSNP database were not included. One sample with clinical manifestation of lack of FVII in the sample laboratory’s reference population database also detected the homozygous mutation. Bioinformatics software predicted that it might be pathogenic. According to the American College of Medical Genetics and Genomics guidelines, the variation was classified as suspicious pathological variation[15]. The genetic result of the pregnant woman’s blood system genetic disease is presented in Table 1.
| Gene | Chromosome location | Reference sequence | Position | cDNA level | Protein level | State | Variation type |
| F7 | 13q34 | NM_000131.4 | Exon 8 | c.722C>A | p.(Thr241Asn) | Homozygosity | Suspicious pathology |
The pregnant woman was diagnosed with congenital FVIID.
Considering the extremely low FVII activity for this case, the clearance rate of the coagulation factor in pregnant women is higher than that in non-pregnant women. Given that the half-life of the drug is short, no adverse complications, such as thrombosis, were detected. The drug used was 90 μg/kg[6]. The patient was assessed to have normal fetal weight, good pelvic condition, and no indication of a cesarean section before delivery. Before medication, the thromboelastogram was normal.
On November 29, 2019, when the cervix was fully dilated, she was intravenously given 5 mg (90 μg/kg) of rFVIIa (NovoSeven®, Novo Nordisk A/S, Bagsvaerd, Denmark) for injection. The activity of FVII was 430.00% in 15 min after medication and 381.50% in 4 h. The activity exceeded the normal range of FVII activity (50%-129%) in this case, and the use of rFVIIa was considered to be excessive. She had a normal spontaneous vaginal delivery of a 2780 g male without episiotomy, and the Apgar score was 10. No laceration of the soft birth canal was detected, and the cumulative amount of postpartum hemorrhage was about 120 mL. rFVIIa was not used again due to less bleeding during labor.
She gave birth to a live baby boy by vaginal delivery and had 120 mL bleeding during delivery. No evidence of delayed postpartum hemorrhage, thrombosis, and neonatal hemorrhage was detected. The newborn was transferred to the neonatal department for further observation after birth, and no abnormality was found in the blood examination routine. The four items of the coagulation test were PT 15.3 s, APTT 55.5 s, and PTA 64%. No abnormality was recorded in the rest, and no coagulation factor was detected. Doctors suggested that parent and newborn should get tested for the genetic disease of the blood system, but the family members refused. After 3 mo of follow-up, the mother and baby were in very good condition.
Congenital FVIID is a rare hereditary bleeding disease, and cases of pregnancy with congenital FVIID are even rarer. Given that congenital FVIID is a rare disease, it limits the feasibility of prospective clinical trials and case control studies. Therefore, no information summary is given about the diagnosis and treatment for this disease and how to prevent bleeding during delivery (vaginal delivery and cesarean section). The significant factor that causes bleeding risk in patients with congenital FVIID is still yet to be determined, which results in a huge clinical challenge to find the treatment for acute bleeding and how to take preventive measures. The procedure of ensuring smooth pregnancy and safe passage during childbirth entails careful planning and managing as well as multidisciplinary diagnosis and management for the entire duration of pregnancy and childbirth. Taking this reason into consideration, we have concocted the following diagnosis process and delivery medication plan for pregnancy with congenital FVIID.
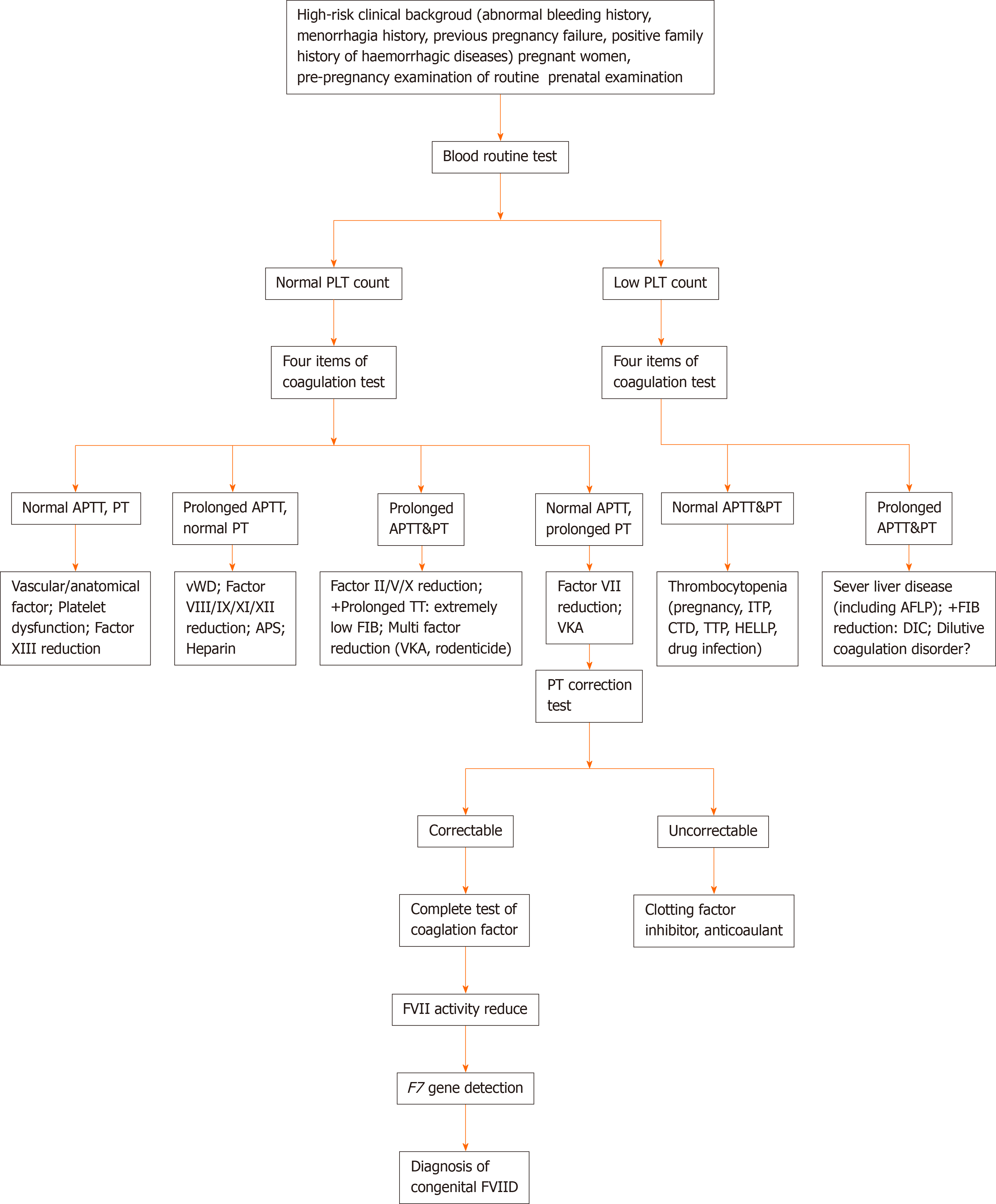
In pregnant women with a high-risk clinical background (history of abnormal bleeding, menorrhagia, pregnancy failure, and a family history of bleeding disease)[9], the blood routine and four items of coagulation test need to be completed for the pre-pregnancy examination along with routine prenatal examination. When the patient’s blood routine test is normal, the following items are excluded: History of vitamin K deficiency, liver disease, biliary system disease, rheumatic and immune system disease (lupus disease, antiphospholipid antibody syndrome, and so on)[16], malabsorption, diffuse intravascular coagulation, anticoagulant treatment, malignant tumor, long-term antibiotics, severe infection, sepsis, etc. When the laboratory examination shows prolonged PT with normal APTT or both PT and APTT are normal[17] (with an exception for the causes of abnormal bleeding and pregnancy failure caused by related diseases), then FVIID should be suspected. The prolonged PT can be corrected by normal plasma[18], while the decrease in the FVII activity and the detection of the F7 gene[2] can further confirm congenital FVIID in patients. We summarize the diagnosis process of pregnancy with congenital FVIID in Figure 2.
After further analysis of the reported cases, the most common conclusion is that the FVII activity level, FVII genotype, coagulation test, and bleeding history could not predict the bleeding risk of patients with FVIID[14]. In preliminary studies, some patients with FVII activity lower than 8% showed mild symptoms or no symptoms; 12 patients with no previous bleeding history and 44% FVII activity had delayed intracranial hemorrhage[5]. In this case, the activity of FVII fluctuated between 2.00%-6.30% and PT fluctuated between 38.2-62.10 s when the drug was not used. The mutation of the F7 gene was homozygous. No abnormal bleeding history was recorded during the pregnancy and in the past. This case also showed the limitation of using the above indicators solely to predict the bleeding risk in patients. In addition to the indicators mentioned above, the risk of bleeding of pregnant women should be evaluated by combining the method of delivery, bleeding tendency, pregnancy, bleeding history of family members, and the age of the patient.
Vitamin K supplementation has proven to be ineffective in managing congenital FVIID cases[1]. The treatment plan for pregnant women with congenital FVIID largely depends on the infusion of fresh frozen plasma, prothrombin complex concentrate, activated prothrombin complex concentrate, plasma-derived FVII concentrate, rFVIIa, etc. rFVIIa can be combined with tissue factor to stop bleeding at specific sites, with a slight risk of thrombosis (< 0.4%) and virus transmission[14]. Currently, rFVIIa is the preferred alternative treatment[8].
However, at present, the use of rFVIIa prophylactic as a treatment during the delivery of congenital FVIID in pregnant women is still considered a controversy. Kulkarni et al[10] retrospectively analyzed 62 women and 94 newborns with FVIID and found that preventive measures are 2.9 times more likely to be received by patients undergoing cesarean section than patients undergoing vaginal delivery. Studies have reported only 10% cases of postpartum hemorrhage during labor with preventive measures and 13% cases of postpartum hemorrhage during labor without preventive measures. The median of FVII activity in the serum of pregnant women in the two groups before pregnancy was 5.5%. The authors thought that conducting a preventative measure was unnecessary and suggested that rFVIIa should only be used under an unavoidable circumstance[4]. Kolucki et al[19] believed that maintaining FVII activity above 15%-25% can achieve a sufficient hemostasis effect for most surgical operations. Hasoon and Rivers[4] hold the view that vaginal delivery does not have to be prevented with rFVIIa unless evidence of postpartum hemorrhage is present, while cesarean section needs to have a prevention method by using rFVIIa.
Mumford et al[20] suggested that the use of rFVIIa (15-30 μg/kg, every 4 h) should be considered for prevention of at least 3 d in late pregnancy for pregnant women with FVII < 20% (with a history of abnormal bleeding or cesarean section). Loddo et al[14] deemed that pregnant women with FVII activity level lower than 10%-20% are at high risk of postpartum hemorrhage, especially for patients with a history of abnormal bleeding and are more likely to need rFVIIa prevention and treatment. The recommended dosage for treatment of congenital FVIID pregnant women during delivery is 15-30 μg/kg every 4-6 h, and the increasing dosage depends on the bleeding situation. The bleeding is suggested to be prevented 30-60 min before the elective cesarean section and in vaginal delivery when the cervix is opening fully. Murray et al[17] recommended that the activity of FVII in cesarean section patients should reach 50%.
The decision of alternative treatment in delivery should be individualized according to each delivery method, bleeding tendency, FVII activity in late pregnancy, FVII genotype, bleeding history of individuals and family members, coagulation-related indicators, pregnancy status, and age of the patient[16]. rFVIIa can be used for bleeding treatment or surgical intervention but not as a mandatory preventive measure.
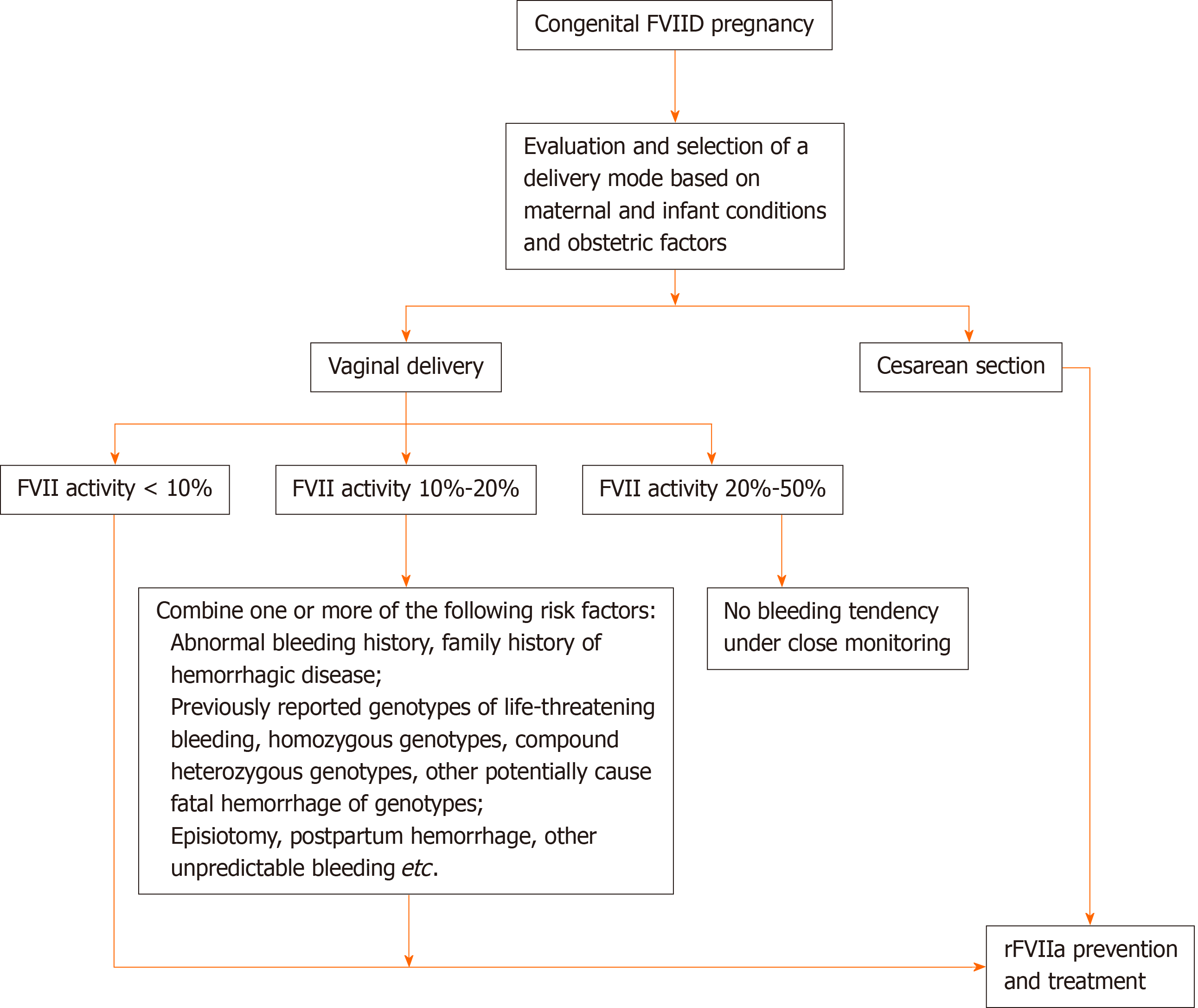
According to the classification of patients with FVIID by the International Society of Thrombosis and Hemostasis, with the previously reported cases and rFVIIa drug instructions, we suggest that congenital patients with FVIID delivered by a cesarean section should receive rFVIIa prevention and treatment. Pregnant women who deliver vaginally are classified as follows: Patients with FVII activity less than 10% should use rFVIIa for prevention; patients with FVII activity between 10%-20% with one or more of the following risk factors (abnormal bleeding history, family history of hemorrhagic disease, previously reported genotypes of life-threatening bleeding, homozygous genotypes, compound heterozygous genotypes, other potentially cause fatal hemorrhage of genotypes, episiotomy) also need to undertake preventive replacement therapy, or without the above situations, when there is evidence of postpartum hemorrhage (estimated blood loss exceeding 500 mL), alternative preventive treatment should be carried out immediately[4]; and rFVIIa prophylaxis is not necessary for patients with FVII activity of 20% to 50% under the condition of being closely monitored and no bleeding tendency. The recommended dose of rFVIIa in the treatment of bleeding attack and prevention of bleeding in surgical and invasive operation is 15-30 μg/kg. rFVIIa is administered intravenously every 4 to 6 h until hemostasis is achieved. The dosage and frequency of rFVIIa should be individualized. We suggest that rFVIIa should be administered 30-60 min before elective cesarean section[14], and the activity of FVII should reach 50%[4]. In a vaginal delivery, the patient should be treated with rFVIIa in the full open cervix, and the activity of FVII should meet 20%[14].
Tranexamic acid, aminocaproic acid[19], and other antifibrinolytic drugs can be used for skin or mucous membrane bleeding (oral bleeding, nose bleeding), small surgery, or low risk of bleeding patient[8,17,20]. Antifibrinolytic drugs are also combined with rFVIIa to increase the hemostasis effect[16]. When necessary, the use of contractile agents[8] (oxytocin, rectal misoprostol, ergotamine, carboprost trometamol)[16] can help prevent and control perinatal bleeding effectively.
Pregnant women with congenital FVIID should carry out multidisciplinary management before delivery. Obstetrics, hematology, anesthesiology, clinical laboratory, and pediatrics should discuss and formulate appropriate delivery methods and management countermeasures for pregnant women and newborns. On the premise of ensuring the supply of rFVIIa, vaginal delivery should be the primary choice for patients with congenital FVIID. Although the risk of fetal intracranial hemorrhage is lower in cesarean delivery than in vaginal delivery, the risk of maternal bleeding in the vaginal delivery is the lowest. In cases of emergency delivery or obstetric cesarean delivery indications, cesarean delivery should be performed. Congenital FVIID should not be used as the indication for a cesarean section. The surgical treatment for pregnant women with congenital FVIID should refer to the indications of obstetric operation. If necessary, epidural anesthesia could be used in vaginal delivery, intraspinal anesthesia for elective cesarean section, and general anesthesia for emergency cesarean section. We recommend 72 h postpartum observation to monitor the risk of delayed postpartum hemorrhage because the activity of FVII in the maternal body will drop sharply after delivery[4]. Newborns with congenital FVIID should avoid a traumatic operation, such as fetal scalp sampling, fetal scalp electrode, vacuum suction, or forceps delivery[16].
The selection of delivery mode and the corresponding rFVIIa medication scheme of pregnant women with congenital FVIID during delivery are presented in Figure 3.
Using PT, FVII activity, or FVII specific test[18] is suggested to monitor the therapeutic effects of rFVIIa. However, due to the difference in test reagents and laboratories, FVII activity and PT results measured in different laboratories could have different results. The FVII specific test kit developed in recent years appears to be more suitable for rFVIIa treatment monitoring of patients with FVII[18,21]. In addition to the indices mentioned previously, INR (the results measured in different laboratories are comparable), FDP, D-dimer, and thrombelastogram can be monitored selectively at the same time, which can better reflect the coagulation and thrombotic state of patients.
Previous work reported spontaneous deep vein thrombosis and pulmonary embolism of pregnant women with FVIID[1]. Some mutations in the FVIID gene, such as Ala294Val and Arg364Gln, have been claimed to be related to venous thrombosis. The risk of thrombosis in patients with congenital FVIID should also be calculated during the clinical practice, especially in patients with mild defects (FVII activity is 30%-50%)[22]. In November 2017, the European Society of Anesthesiology issued a guideline for the prevention of postoperative venous thromboembolism, which suggested risk factors of thrombosis in patients with FVIID. Thrombus prevention drugs (grade 2C) can be considered if antithrombotic treatment is necessary after risk assessment. Low-molecular-weight heparin is suggested for patients without hemophilia to keep the activity of factor VIII/IX at the level of 0.6-1.0 IU/mL (grade 2C)[23].
In this case, no remarkable history of the patient was recorded. The laboratory examination result showed that the PT was prolonged, while the APTT was normal. The plasma PT value of the pregnant woman could be rectified by normal plasma, and the FVII activity decreased. The gene result showed a homozygous mutation of the F7 gene, making the diagnosis of congenital FVIID clear. The patient had no abnormal bleeding history during pregnancy and in the past, and the FVII activity fluctuated from 2.00% to 6.30% when the drug was not used. According to the severity classification guidelines for FVIID by the International Society of Thrombosis and Hemostasis, the patient belonged to severe FVIID (FVII activity < 10%), with a risk of spontaneous massive bleeding[5]; Seven Treatment Evaluation Registry defined the patient as a severe patient (FVII activity ≤ 5%)[6]. We established the delivery response process for the patient at 37 wk of gestation. Under strict management and preparation, combined with obstetric factors, the patient should undergo vaginal delivery and use rFVIIa during delivery.
Gene diagnosis is an effective tool for prenatal diagnosis of congenital FVIID. Genetic counseling and prenatal diagnosis are crucial for families with F7 gene mutation[16]. Primary prevention treatment dating as early as possible is the only option to prevent death and disability of severe patients with congenital FVIID (Farah et al[24]). In principle, gene therapy, with its therapeutic potential will be the most appropriate treatment intervention for congenital FVIID cases[25] but perfecting this treatment may take longer to achieve[26].
This article systematically introduced the overview of congenital FVIID, and the experience of the patient in perinatal management, diagnosis, and treatment process. The bleeding performance of each pregnant woman with congenital FVIID varies greatly. We should integrate many factors to find the best treatment plan accordingly. At the moment, rFVIIa is the main drug used to be an alternative preventive therapy. However, the clinical use of rFVIIa is rather limited due to the low number of cases of pregnant women with congenital FVIID. The management of perinatal safety of congenital FVIID in pregnant women still needs further research due to the lack of prospective studies and case control trials to confirm.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aziret M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | He J, Zhou W, Lv H, Tao L, Chen X, Wang L. Novel IVS7+1G>T mutation of life-threatening congenital factor VII deficiency in neonates: A retrospective study in China. Medicine (Baltimore). 2019;98:e17360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Lee YJ, Ju DH, Yi SW, Lee SS, Sohn WS. Successful management of maternal factor VII deficiency in a cesarean section. Obstet Gynecol Sci. 2014;57:314-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Hunault M, Bauer KA. Recombinant factor VIIa for the treatment of congenital factor VII deficiency. Semin Thromb Hemost. 2000;26:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Hasoon J, Rivers JM. A case of heterozygous factor VII deficiency in pregnancy. J Obstet Gynaecol. 2020;40:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Jain S, Donkin J, Frey MJ, Peltier S, Gunawardena S, Cooper DL. Phenotypical variability in congenital FVII deficiency follows the ISTH-SSC severity classification guidelines: a review with illustrative examples from the clinic. J Blood Med. 2018;9:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Cramer TJ, Anderson K, Navaz K, Brown JM, Mosnier LO, von Drygalski A. Heterozygous congenital Factor VII deficiency with the 9729del4 mutation, associated with severe spontaneous intracranial bleeding in an adolescent male. Blood Cells Mol Dis. 2016;57:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Alam MM, Moiz B, Rehman KA, Jethwani P, Fadoo Z. Congenital Factor VII Deficiency in Children at Tertiary Health Care Facility in Pakistan. Clin Appl Thromb Hemost. 2015;21:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Shapiro A. The use of prophylaxis in the treatment of rare bleeding disorders. Thromb Res. 2020;196:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Zaidi SM, Qureshi RN, Adil SN. Factor VII deficiency and pregnancy: a case report and review of literature. J Pak Med Assoc. 2010;60:136-138. [PubMed] |
| 10. | Kulkarni AA, Lee CA, Kadir RA. Pregnancy in women with congenital factor VII deficiency. Haemophilia. 2006;12:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Eskandari N, Feldman N, Greenspoon JS. Factor VII deficiency in pregnancy treated with recombinant factor VIIa. Obstet Gynecol. 2002;99:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Jiménez-Yuste V, Villar A, Morado M, Canales M, Hernández MC, Sanjurjo MJ, Quintana M, Hernández-Navarro F. Continuous infusion of recombinant activated factor VII during caesarean section delivery in a patient with congenital factor VII deficiency. Haemophilia. 2000;6:588-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Ariffin H, Millar DS, Cooper DN, Chow T, Lin HP. Prenatal exclusion of severe factor VII deficiency. J Pediatr Hematol Oncol. 2003;25:418-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Loddo A, Cornacchia S, Cane FL, Barcellona D, Marongiu F, Melis GB, Angioni S, Paoletti AM, Neri M. Prophylaxis of peripartum haemorrhage using recombinant factor VIIa (rfVIIa) in pregnant women with congenital factor VII deficiency: A case report and literature review. Eur J Obstet Gynecol Reprod Biol. 2019;235:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Millar DS, Kemball-Cook G, McVey JH, Tuddenham EG, Mumford AD, Attock GB, Reverter JC, Lanir N, Parapia LA, Reynaud J, Meili E, von Felton A, Martinowitz U, Prangnell DR, Krawczak M, Cooper DN. Molecular analysis of the genotype-phenotype relationship in factor VII deficiency. Hum Genet. 2000;107:327-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Matei A, Dolan S, Andrews J, Rivard GÉ. Management of Labour and Delivery in a Patient With Acquired Factor VII Deficiency With Inhibitor: A Case Report. J Obstet Gynaecol Can. 2016;38:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Murray NP, Garcia C, Ilabaca J, Lagos N. Management of Pregnancy in a Chilean Patient with Congenital Deficiency of Factor VII and Glanzmann's Thrombasthenia Variant. Case Rep Obstet Gynecol. 2014;2014:628386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Sevenet PO, Kaczor DA, Depasse F. Factor VII Deficiency: From Basics to Clinical Laboratory Diagnosis and Patient Management. Clin Appl Thromb Hemost. 2017;23:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Kolucki FR Jr, Morris GJ, Thomas LC, Scialla S. Factor VII deficiency in pregnancy and delivery: a case report. Haemophilia. 2011;17:e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Mumford AD, Ackroyd S, Alikhan R, Bowles L, Chowdary P, Grainger J, Mainwaring J, Mathias M, O'Connell N; BCSH Committee. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors' Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2014;167:304-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Morfini M, Batorova A, Mariani G, Auerswald G, Bernardi F, Di Minno G, Dolce A, Fede C, Giansily-Blaizot M, Ingerslev J, Martinowitz U, Napolitano M, Pinotti M, Schved JF; International FVII [IF7] and Seven Treatment Evaluation Registry [STER] Study Groups. Pharmacokinetic properties of recombinant FVIIa in inherited FVII deficiency account for a large volume of distribution at steady state and a prolonged pharmacodynamic effect. Thromb Haemost. 2014;112:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Klovaite J, Friis-Hansen L, Larsen FS, Toffner-Clausen N, Bjerrum OW. Vena porta thrombosis in patient with inherited factor VII deficiency. Blood Coagul Fibrinolysis. 2010;21:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Afshari A, Ageno W, Ahmed A, Duranteau J, Faraoni D, Kozek-Langenecker S, Llau J, Nizard J, Solca M, Stensballe J, Thienpont E, Tsiridis E, Venclauskas L, Samama CM; ESA VTE Guidelines Task Force. European Guidelines on perioperative venous thromboembolism prophylaxis: Executive summary. Eur J Anaesthesiol. 2018;35:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Farah RA, Hamod D, Melick N, Giansily-Blaizot M, Sallah S. Successful prophylaxis against intracranial hemorrhage using weekly administration of activated recombinant factor VII in a newborn with severe factor VII deficiency. J Thromb Haemost. 2007;5:433-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Binny C, McIntosh J, Della Peruta M, Kymalainen H, Tuddenham EG, Buckley SM, Waddington SN, McVey JH, Spence Y, Morton CL, Thrasher AJ, Gray JT, Castellino FJ, Tarantal AF, Davidoff AM, Nathwani AC. AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage. Blood. 2012;119:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Farah R, Al Danaf J, Braiteh N, Costa JM, Farhat H, Mariani G, Giansily-Blaizot M. Life-threatening bleeding in factor VII deficiency: the role of prenatal diagnosis and primary prophylaxis. Br J Haematol. 2015;168:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |