Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5730
Peer-review started: March 11, 2021
First decision: March 25, 2021
Revised: April 6, 2021
Accepted: May 6, 2021
Article in press: May 6, 2021
Published online: July 16, 2021
Processing time: 118 Days and 0.8 Hours
Cerebral proliferative angiopathy (CPA) is a rare vascular disease characterized by the presence of diffuse vascular proliferation, progressive vascular hyperflow and vasodilation of multiple vessels in the normal brain parenchyma. Unlike cerebral arteriovenous malformations, CPA has a mixed appearance between that of lesions with cell proliferation and endothelial proliferation. To date, the pathogenesis of CPA is unclear, in which changes induced by cortical ischemia in the elastic layer of the blood supply artery and smooth muscle cells may be involved.
In this article, we retrospectively analyzed a case of hemorrhagic transformation of ischemic CPA diagnosed by digital subtraction angiography and reviewed the related literature for further exploration of its pathogenesis, diagnosis and treatment.
The information in the present case report may facilitate further clinical research on this cerebrovascular disease.
Core Tip: This article reports a very rare case of ischemic cerebrovascular disease, namely ischemic cerebral proliferative angiopathy. After consulting major databases, we found that cases of ischemic and hemorrhagic cerebral proliferative angiopathy have been reported, but the biggest feature of this case is the phenomenon of hemorrhagic transformation after ischemia. So far, there are few reports of such diseases globally, and their pathogenesis is currently unclear. The diagnosis and treatment methods are limited. Therefore, this article reviews relevant literature to better understand this rare disease and provides clues for further clinical research.
- Citation: Xia Y, Yu XF, Ma ZJ, Sun ZW. Hemorrhagic transformation of ischemic cerebral proliferative angiopathy: A case report. World J Clin Cases 2021; 9(20): 5730-5736
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5730.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5730
Cerebral proliferative angiopathy (CPA) is defined as an atypically chronic ischemic cerebrovascular disease that appears to be significantly different from normal arteriovenous malformation (AVM) in epidemiology, clinical manifestations, angiographic and histopathological features, natural history and management[1]. In CPA, a large and sparse lesion caused by progressive angiogenesis can be seen in the normal brain parenchyma. Ischemia induced by hypoperfusion around the lesion is considered to lead to that excessive proliferation[2]. CPA is less common than AVM, and hemo
Our patient was a 76-year-old male with dizziness, headache and left hemiparesis and numbness.
The patient was diagnosed with hemorrhagic cerebral infarction and was treated with mannitol and glycerol fructose to reduce intracranial pressure.
The patient had a history of hypertension, and no oral medications were applied.
The patient smoked for decades and presently quit smoking. In addition, he occasionally drank alcohol. No family hereditary diseases and tumors were reported.
At the time of admission, the main clinical positive signs of this patient were as follows: positive horizontal nystagmus; both eyes were blurred to the left; the left limb muscle strength was grade IV; the left limbs’ shallow sensations were decreasing; and the left pathological sign was positive.
Cerebrospinal fluid routine, biochemistry and serum paraneoplastic neurological syndrome test results were negative. Additionally, there were no significant abnormalities in blood examinations.
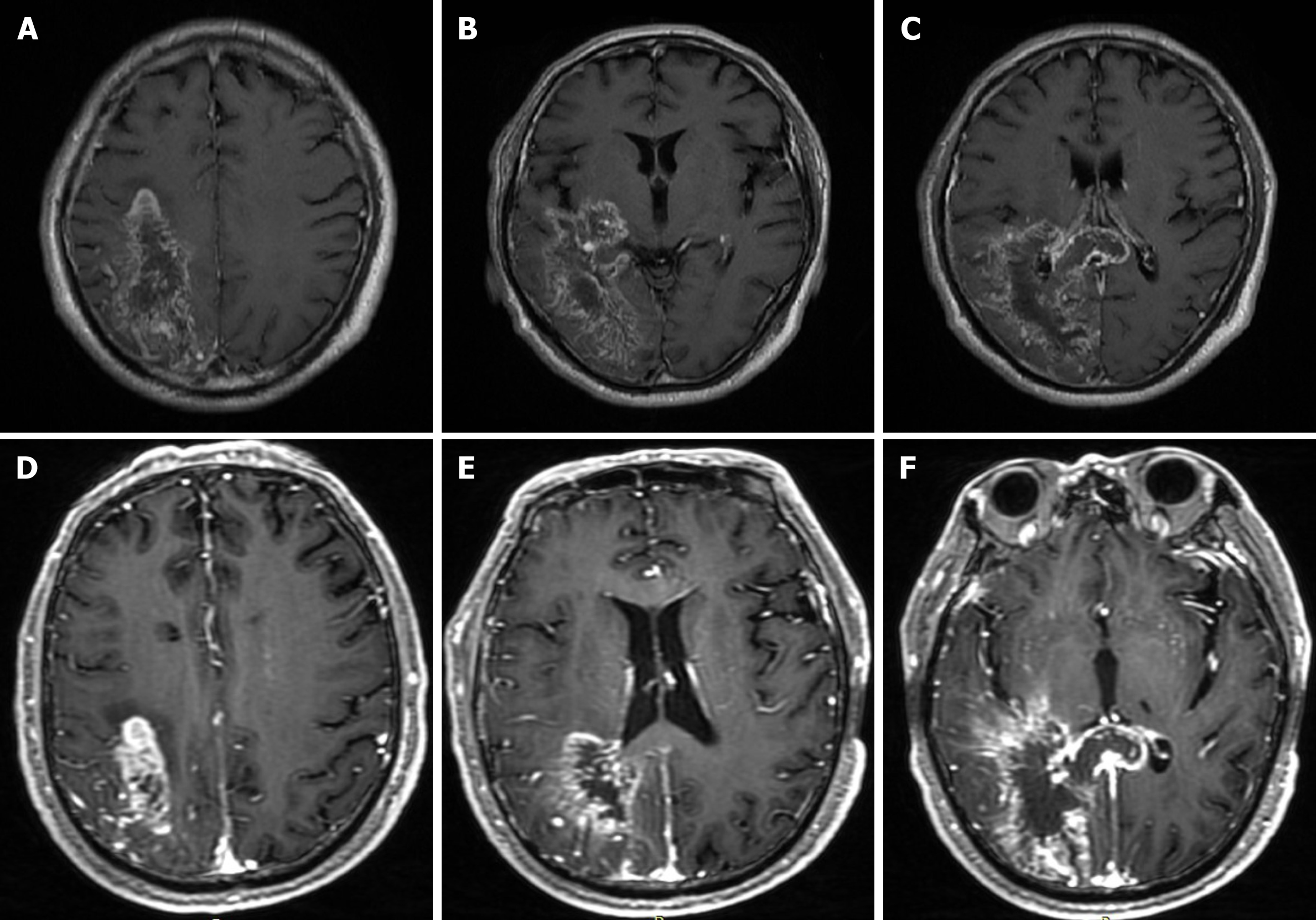
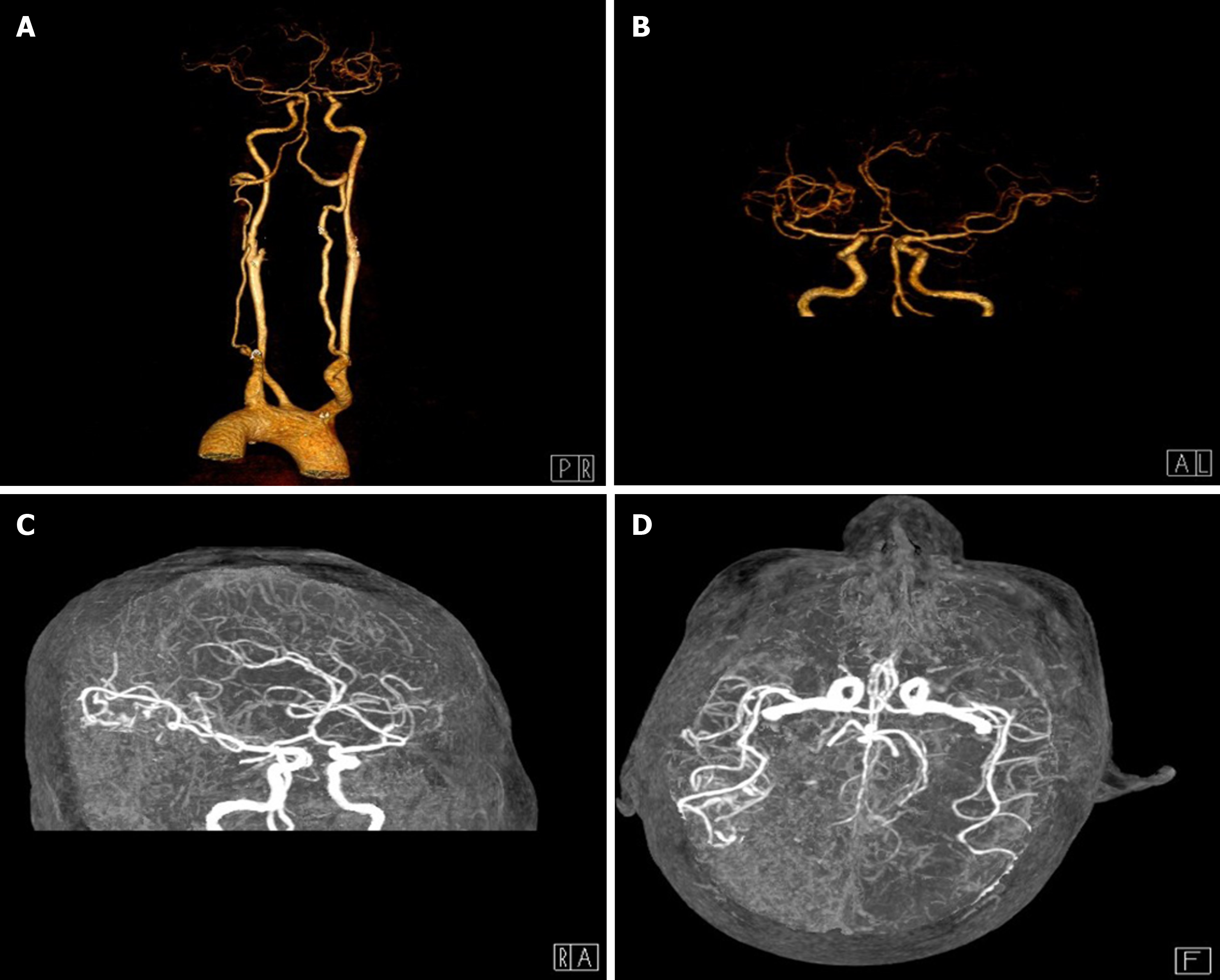
A magnetic resonance imaging (MRI) scan was performed, which revealed a massive cerebral infarction in the right temporal lobe, parietal lobe, occipital lobe and the corpus callosum accompanied by minor bleeding and gliosis within. In addition, brain enhanced MRI showed irregular ring enhancement in the right temporo-occipital junction area and corpus callosum compression, and the posterior horn of the right lateral ventricle was compressed (Figure 1). Brain computed tomography angiography revealed intracranial artery stenosis and hyperplasia (Figure 2).
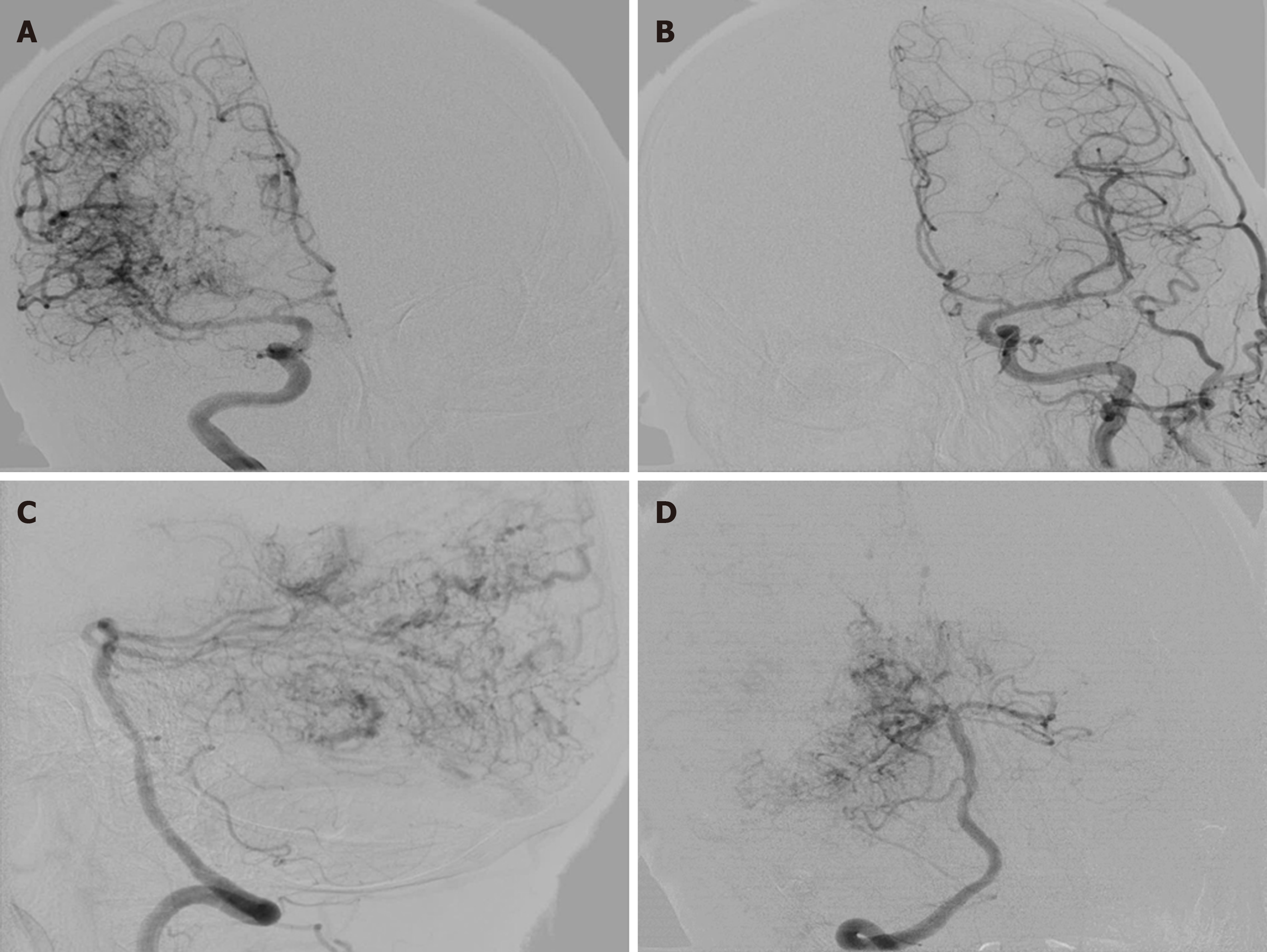
Chest and abdominal pelvic computed tomography and brain magnetic resonance venography. Digital subtraction angiography showed a large number of proliferative blood vessels in the blood supply area of the right middle cerebral artery. A small number of proliferative blood vessels were also seen in the A4 segment of the right anterior cerebral artery. A large number of proliferative blood vessels were visible in the P3 or P4 segment of the posterior artery (Figure 3).
Hemorrhagic CPA after ischemia
In this case, symptomatic treatments such as lowering intracranial pressure and fluid supplementation were the main treatments. Considering that the risk of surgical treatment was too high, the family refused.
At his 1-year telephone follow-up, we learned that the aforementioned clinical symptoms had been progressively worsening with a disturbance of consciousness, and the patient had eventually passed away.
Recently, Lasjaunias et al[1] retrospectively analyzed 1434 AVM databases, where they identified CPA in 49 cases with an age range of 10-65 years, with the highest proportion in young women. In addition, Puerta et al[3] reported an 8-year-old child suffering from CPA. However, our patient was a 76-year-old male. Other investigations[4-6] showed that CPA accounted for approximately 2%-4% of all AVMs, indicating that the right cerebral hemisphere was the most common location, especially the temporal lobe. CPA usually manifested as epilepsy, headache and progressive neurological deficit (predominantly ischemia). Our patient had lesions in the right temporal, parietal and occipital lobes. The most important finding was that in the case described here, the patient had postischemic hemorrhagic transformation. To date, this transformation phenomenon has not been largely reported in the literature, and thus we collected and summarized the information available on all cases of hemorrhagic CPA to establish its clinical features[7-13] (Table 1).
| Ref. | Age/sex | Clinical presentation | Location | Management | Follow-up |
| Maekawa et al[7], 2012 | 62/F | Gait instability and dysarthria | Tectum and cerebellar vermis | Symptomatic | 9 yr; died |
| Kumar et al[8], 2015 | 66/M | Headache | Left cerebellar hemisphere | Asymptomatic | 12 mo; no deficits |
| Bilaj et al[9], 2016 | 24/M | Headache and seizures | Left temporal lobe | Frontotemporal craniotomy | NA |
| Kimiwada et al[10], 2019 | 13/M | Headaches and reversible focal neurological deficits | Left frontal and parietal lobes | Indirect revascularization procedure | 2 yr; stable symptoms |
| Maekawa et al[11], 2018 | 12/F | Intractable headaches | Left cerebellar hemisphere | Symptomatic | 30 yr; recurrence of cerebral hemorrhage |
| Giragani et al[12], 2018 | 12/M | Headache | Cerebellar hemisphere andcerebellar vermis | Asymptomatic | 6 mo; no deficits |
| Beniwal et al[13], 2020 | 12/M | Trivial trauma and altered sensorium with hemorrhage in imaging | Right cerebellarhemisphere | Symptomatic | 18 mo; no deficits |
| Current case | 76/M | Dizziness, headache and left hemiparesis and numbness | Right cerebellarhemisphere | Symptomatic | 12 mo; died |
CPA was diagnosed by its typical vascular imaging features. The major manifestation of CPA on MRI was diffuse lesions with a densely enhanced vascular network structure mixed with normal brain parenchyma. In some CPA patients, the drainage veins were reduced, with varying degrees of vein thickening on MRI. On digital subtraction angiography, the blood vessels were in a muddy shape with a scattered distribution, manifested in a small shunt volume, lack of dominant arterial blood supply period, delayed arterial phase and early venous phase. The patient had a large number of proliferative blood vessels in the blood supply area of the right middle cerebral artery. Proliferated blood vessels were also visible in the A4 segment of the right anterior cerebral artery. No obvious abnormalities were detected in the left middle cerebral artery and the anterior cerebral artery. The vertebral artery angiography showed extensive hyperplasia in the P3 or P4 segment of the right posterior cerebral artery. The draining veins were clearly visible, and no abnormality was found in the left posterior cerebral.
CPA may be cured surgically by embolization and using stereotactic radiotherapy, which could increase the blood flow to the hypoperfused brain tissue and prevent future ischemia[14,15]. Considering that the aforementioned treatment methods are risky with poor long-term prognosis, conservative treatment is still the mainstay[16]. In our case, the most characteristic feature of CPA that distinguished it from cerebral AVMs was the presence of recognizable neurons and normal brain tissue intertwined with abnormal blood vessels[17]. The vascular lesions of CPA were unstable, and their continuous growth was detected by neuroimaging at multiple time points. The lesions gradually expanded, accompanied by aggravated clinical symptoms, which indicated that CPA was progressive. After the patient was discharged from the hospital on September 3, 2019, his dysfunction of the limbs worsened again. However, due to the unstable vital signs of the patient, his digital subtraction angiography images were not reviewed. Larger clinical trials are required for a further exploration of the patho
CPA is a rare ischemic vascular disease, and hemorrhagic transformation after ischemia has been seldom reported. In recent years, increased research attention has been paid to this disease. The pathogenesis, clinical manifestations, diagnosis and treatment of CPA have been initially established. However, in the future, more high-quality clinical research is to be conducted to further explore its pathogenesis, improve therapeutic schedules for minimizing its disability and reduce mortality.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pérez-Cabezas V S-Editor: Gao CC L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Lasjaunias PL, Landrieu P, Rodesch G, Alvarez H, Ozanne A, Holmin S, Zhao WY, Geibprasert S, Ducreux D, Krings T. Cerebral proliferative angiopathy: clinical and angiographic description of an entity different from cerebral AVMs. Stroke. 2008;39:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Sakata H, Fujimura M, Sato K, Niizuma K, Endo H, Tominaga T. Development of Abnormal Hemispheric Vascular Networks Mimicking Cerebral Proliferative Angiopathy in a Child Originally Diagnosed with Deep-Seated Arteriovenous Fistula. J Stroke Cerebrovasc Dis. 2016;25:e200-e204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Puerta P, Guillén A, Muchart J, González V, Ferrer E. Cerebral Proliferative Angiopathy in a Child. Pediatr Neurosurg. 2017;52:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lopci E, Olivari L, Bello L, Navarria P, Chiti A. Cerebral Proliferative Angiopathy (CPA): Imaging Findings and Response to Therapy. Clin Nucl Med. 2016;41:e527-e529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kono K, Terada T. Encephaloduroarteriosynangiosis for cerebral proliferative angiopathy with cerebral ischemia. J Neurosurg. 2014;121:1411-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1512] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 7. | Maekawa H, Tanaka M, Hadeishi H. Fatal hemorrhage in cerebral proliferative angiopathy. Interv Neuroradiol. 2012;18:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Sharma M, Srivastava T, Sinha VD. Infratentorial hemorrhagic cerebral proliferative angiopathy: A rare presentation of a rare disease. Asian J Neurosurg. 2015;10:240-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Bilaj F, Rroji A, Enesi E, Ruka M, Petrela M. Cerebral proliferative angiopathy with tumor-like hemorrhage: A case report and literature review. Neuroradiol J. 2016;29:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Kimiwada T, Hayashi T, Takahashi M, Shirane R, Tominaga T. Progressive Cerebral Ischemia and Intracerebral Hemorrhage after Indirect Revascularization for a Patient with Cerebral Proliferative Angiopathy. J Stroke Cerebrovasc Dis. 2019;28:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Maekawa H, Terada A, Ishiguro T, Komiyama M, Lenck S, Renieri L, Krings T. Recurrent periventricular hemorrhage in cerebral proliferative angiopathy: Case report. Interv Neuroradiol. 2018;24:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Giragani S, Pavunesan SK, Balasubramaniam A. Targeted endovascular treatment of haemorrhagic posterior fossa proliferative angiopathy. Interv Neuroradiol. 2018;24:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Beniwal M, Kandregula S, Aravind, Rao KVLN, Vikas V, Srinivas D. Pediatric cerebral proliferative angiopathy presenting infratentorial hemorrhage. Childs Nerv Syst. 2020;36:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Chaddad-Neto F, Joaquim AF, dos Santos MJ, Linhares PW, de Oliveira E. Microsurgical approach of arteriovenous malformations in the central lobule. Arq Neuropsiquiatr. 2008;66:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Hashimoto N, Nozaki K, Takagi Y, Kikuta K, Mikuni N. Surgery of cerebral arteriovenous malformations. Neurosurgery. 2007;61:375-87; discussion 387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Yamaki VN, Solla DJF, Telles JPM, Liem GLJ, da Silva SA, Caldas JGMP, Teixeira MJ, Paschoal EHA, Figueiredo EG. The current clinical picture of cerebral proliferative angiopathy: systematic review. Acta Neurochir (Wien). 2020;162:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Lehman LL, Bruccoleri R, Danehy A, Swanson J, Mrakotsky C, Smith E, Orbach DB, Burstein R. Adverse effects of erenumab on cerebral proliferative angiopathy: A case report. Cephalalgia. 2021;41:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |