Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.3951
Peer-review started: November 22, 2020
First decision: December 24, 2020
Revised: January 2, 2021
Accepted: March 5, 2021
Article in press: March 5, 2021
Published online: June 6, 2021
Processing time: 160 Days and 19 Hours
Neuromyelitis optica spectrum disorder (NMOSD) is a demyelinating autoimmune disease that affects the central nervous system. It typically manifests as optic neuritis or extensive longitudinal myelitis, with or without the presence of anti-aquaporin protein 4 autoantibodies (immunoglobulin G).
We report the case of a 45-year-old woman with a history of Sjogren's syndrome who was diagnosed with NMOSD accompanied by spinal cord injury and left calf intermuscular vein thrombosis. The patient received hormone shock and gamma globulin therapy in the acute phase and standard rehabilitation treatment during convalescence. Upon discharge, the patient was able to control urination and defecation, stand independently, and walk short distances with the aid of a walker.
This case suggests that pharmacotherapy and standard rehabilitation treatment can improve the prognosis of NMSOD patients.
Core Tip: Neuromyelitis optica spectrum disorder (NMOSD) is a demyelinating autoimmune disorder of the central nervous system. It typically manifests as optic neuritis or extensive longitudinal myelitis, with or without the presence of anti-aquaporin protein 4 autoantibodies (immunoglobulin G). We report the case of a 45-year-old woman with a history of Sjogren's syndrome who was diagnosed with NMOSD accompanied by spinal cord injury and left calf intermuscular vein thrombosis. The patient received hormone shock and gamma globulin therapy in the acute phase and standard rehabilitation treatment during convalescence.
- Citation: Wang XJ, Xia P, Yang T, Cheng K, Chen AL, Li XP. Rehabilitation and pharmacotherapy of neuromyelitis optica spectrum disorder: A case report. World J Clin Cases 2021; 9(16): 3951-3959
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/3951.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.3951
Neuromyelitis optica spectrum disorder (NMOSD) is a demyelinating autoimmune disorder of the central nervous system (CNS) with high recurrence and disability rates[1]. It typically manifests as optic neuritis or extensive longitudinal myelitis, with or without the presence of anti-aquaporin protein 4 (AQP4) autoantibodies [immunoglobulin G (IgG)]. AQP4, an astrocyte-specific aqueous channel protein, is involved in the formation of the blood brain barrier, and regulates the movement of water molecules across the cerebrospinal fluid (CSF), blood and brain[2]. The diagnosis of NMOSD needs to be distinguished from other diseases of the nervous system, such as multiple sclerosis, which is often difficult to distinguish due to their similar clinical symptoms. Hormone shock therapy is an important treatment in the early stage, but many patients are not sensitive to hormone therapy, and even have serious electrolyte disorder. NMSOD patients exhibit varying degrees of disability after early clinical pharmacotherapy and need continued rehabilitation. However, in the late stage of rehabilitation, some patients will have repeated attacks and aggravation of symptoms, which pose a great challenge in the diagnosis and treatment of NMOSD. We report a patient with NMOSD accompanied by spinal cord injury, Sjogren's syndrome and deep venous thrombosis in the lower extremity who received rehabilitation and pharmacotherapy.
A 45-year-old female patient presented at our hospital on March 11, 2020 due to numbness in both lower extremities without obvious inducement, which gradually spread to the waist.
The patient experienced weakness in her lower extremities and walked with difficulty which she described as “stepping on cotton”. In addition, the patient also complained of transient acupuncture-like pain in the chest and back, although she could control defecation and urination. A head computed tomography scan showed subdural effusion on both frontal sides. The patient was then admitted to the Neurology Department of our hospital on March 16, 2020. On the second day after admission, the weakness in her lower limbs worsened and the sensation of a “banding” pain in the chest and back became more tangible. There was also difficulty in urination but not in passing stool. Once the patient’s condition stabilized, she was transferred to the Department of Rehabilitation Medicine on March 27, 2020. At the time of admission, her lower limbs were still weak but not swollen, with obvious chest and back banding, urinary catheter retention and self-dissolving stool.
The patient was diagnosed with Sjogren's syndrome more than 2 years previously, and had been taking 0.2 g hydroxychloroquine sulfate tablets once daily for 1 year, which had been discontinued for about 6 mo before the onset of NMOSD. The patient denied a history of hypertension, diabetes and heart disease.
There was no similar medical history in her family. She also denied previous exposure to toxins, smoking, drinking, or any food and drug allergies.
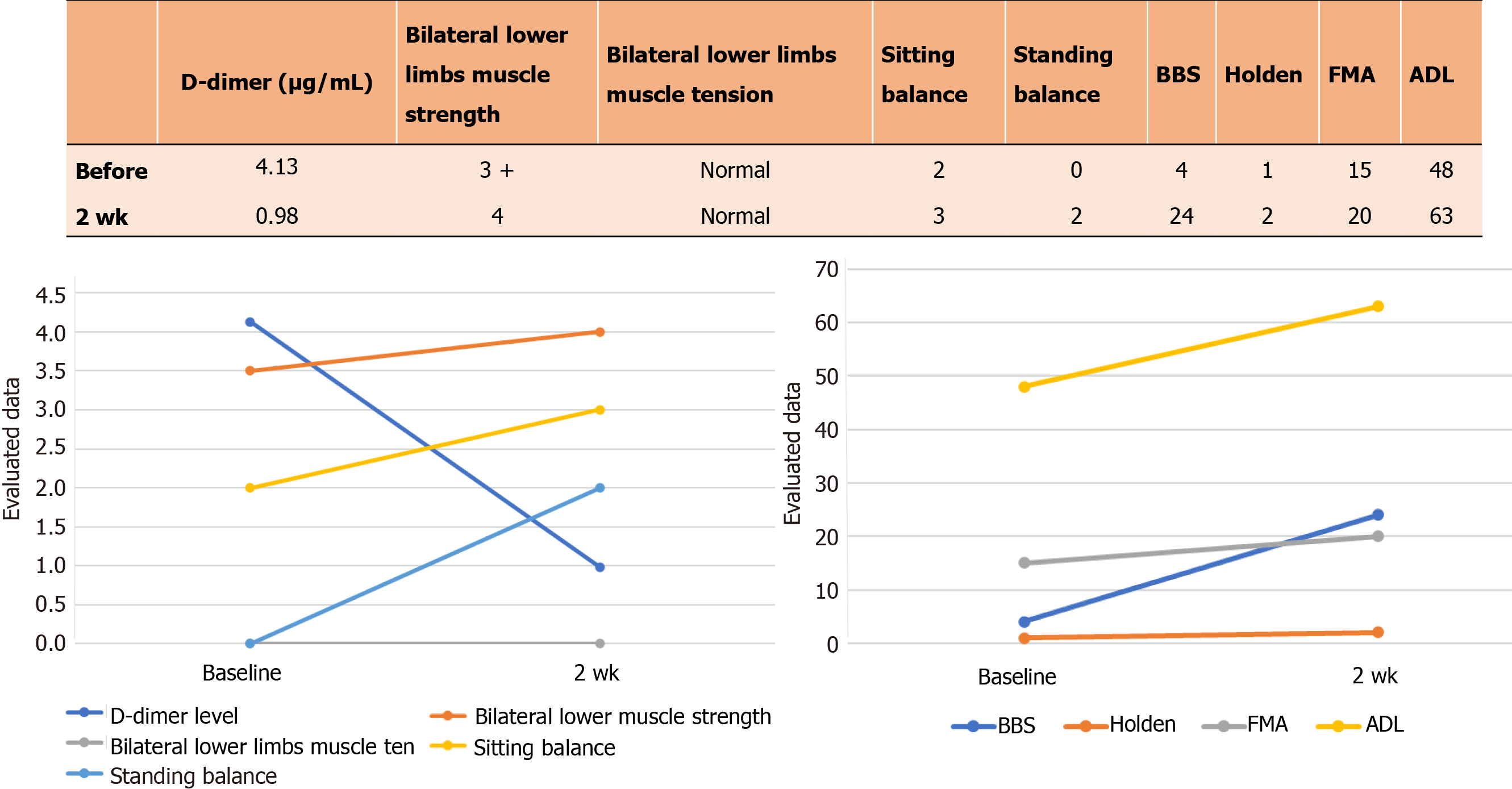
Physical examination showed hypoesthesia from the T5 plane, with retention of sacrococcygeal sensation. The patient did not have any sensation in the T6-T11 plane but experienced a pronounced corset-like pain in her chest and abdomen. In addition, although the muscular strength of the lower limbs had decreased, the muscle tension was normal. The patient's bilateral eye movements, vision and visual field tests were normal, which ruled out optic neuritis. Her Berg Balance Scale (BBS) score was 4/56, bilateral Fugl-Meyer assessment (FMA) score for lower extremities was 15/34, and the modified Barthel index (MBI) score for activities of daily living (ADL) was only 48/100.
The routine laboratory tests for blood, urine, and liver, kidney and thyroid function did not show any obvious abnormalities. The D-dimer level was 4.13 μg/mL (Figure 1) (the normal range is 0.5 μg/mL) in the coagulation test. The patient’s serum was positive for antibodies against rheumatoid factor, Sjogren's syndrome antigen A (SSA), Sjogren's syndrome antigen B (SSB), Ro-52 (an antinuclear antibody of 52 kDa peptide fragment), AQP4 (IgG) and myelin oligodendrocyte glycoprotein (MOG; IgG), and negative for anti-myelin basic protein (MBP) IgG (Table 1). The CSF was colorless and transparent, with an abnormally high leukocyte count and protein levels, which indicated an inflammatory response. However, the CSF was negative for the anti-AQP4, MBP and MOG antibodies (Table 2).
| Immunology | Patient | Normal |
| Rheumatoid factor | 158.00 IU/mL | 0 - 20 IU/mL |
| SS-A antibody | > 400.00 RU/mL | 0 - 20 RU/mL |
| Ro-52 antibody | > 400.00 RU/mL | 0 - 20 RU/mL |
| SS-B antibody | > 400.00 RU/mL | 0 - 20 RU/mL |
| AQP4 antibody IgG | 1:320 | Negative |
| MOG antibody IgG | 1:32 | Negative |
| MBP antibody IgG | Negative | Negative |
| CSF | Patient | Normal |
| Leukocyte count | 0.050 × 109/L | 0-0.008 × 109/L |
| Lactate dehydrogenase | 61.00 U/L | 120-250 U/L |
| Protein | 1154.80 mg/L | 200-400 mg/L |
| APQ4 antibody IgG | Negative | Negative |
| MOG antibody IgG | Negative | Negative |
| MBP antibody IgG | Negative | Negative |
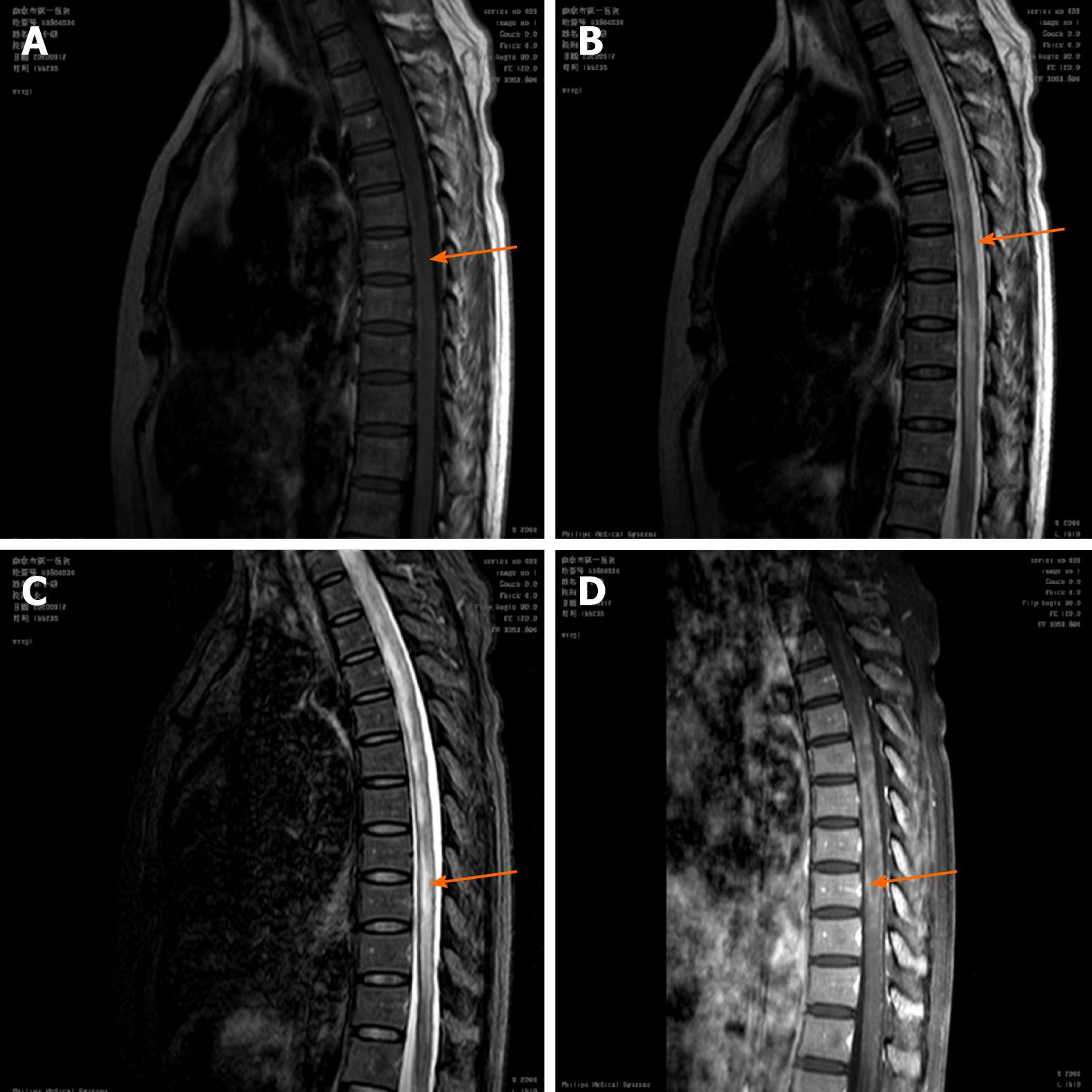
Thoracic and lumbar magnetic resonance imaging (MRI) showed an abnormal signal involving more than 3 spinal segments in the medulla (T4-T9) (Figure 2). Somatosensory evoked potential on electromyogram suggested central impairment, probably originating from the spinal cord. The patient’s electrocardiograph was normal, while deep vein ultrasound of the lower limbs indicated thrombosis of the left calf intermuscular vein.
The patient was given intravenous shock therapy with 1 g methylprednisolone for three days during the acute stage. However, the treatment was switched to daily intravenous administration of 20 g gamma globulin for five days due to her poor response and severe electrolyte imbalance. During the rehabilitation period, the patient took 40 mg of prednisone daily and one tablet of mecobalamin and vitamin B1 thrice daily. At the same time, Rabeprazole enteric-coated capsules were given to protect gastric mucosa, and 2 g potassium chloride sustained-release tablets per day were administered to treat hypokalemia. In addition, the patient took 0.25 g mycophenolate mofetil capsule (CellCept) daily to relieve inflammatory symptoms. Given the thrombosis of the left calf intermuscular vein and the high risk of bleeding due to hormone therapy, the patient received daily subcutaneous injections of 4100 IU low molecular weight heparin calcium as an anticoagulant.
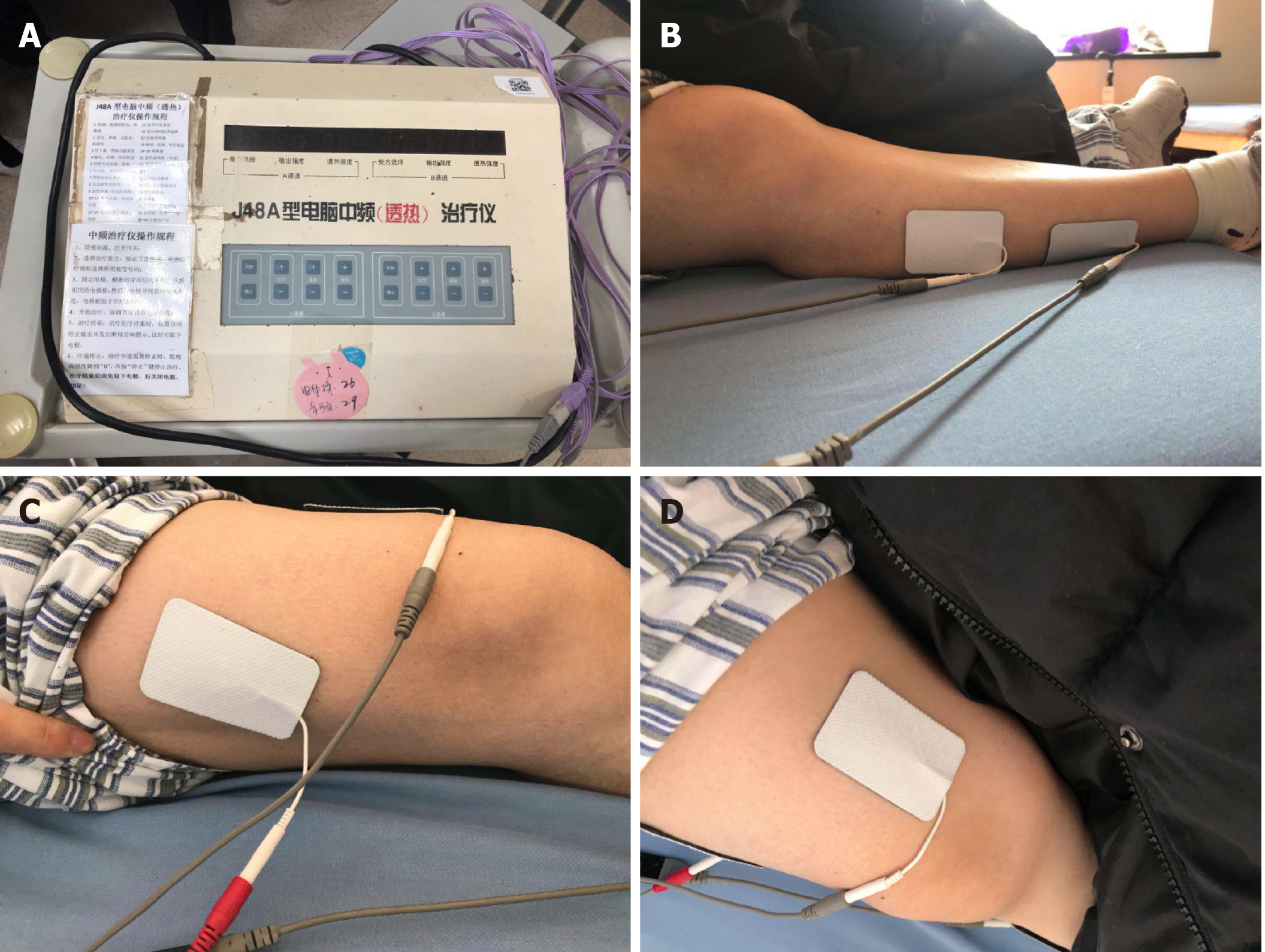
The patient underwent training for 20 min to 30 min twice daily during the rehabilitation phase to improve mobility of the trunk and key lower limb muscles. Stretching exercises for the hamstring, adductor and Achilles tendon, passive movement training of the hip, knee and ankle joints, as well as body support and transfer training in the sitting position were performed to improve sitting posture and balance. As the limb function gradually increased, standing and gait training was started with the aid of walkers (Figures 3 and 4). In addition, neuromuscular electrical stimulation (Type J48A; Jinhao Trading Co., Ltd., Beijing, China) was used to improve the muscle strength of the lower extremities, mainly that of the right quadriceps femoris and tibialis anterior muscle (Figure 5). The frequency, pulse duration and intensity of the electrical stimulus were 25 Hz, 0.2 ms and 30 mA, respectively, and was performed twice daily for 20 min. The patient only felt a pleasant tingling sensation. The left lower extremity could not be treated with neuromuscular electrical stimulation due to intermuscular venous thrombosis. The urinary catheter was removed on the third day of rehabilitation, and the patient was able to control urination as ultrasound detected less than 50 mL residual urine in the bladder. The patient showed no obvious symptoms of anxiety and depression and actively cooperated with the rehabilitation treatment. Nevertheless, we established a good rapport with the patient during the rehabilitation stage, and explained her condition and the importance of rehabilitative treatment. We also prepared the patient to cope with this condition in the future.
NMOSD.
The patient received hormone shock and gamma globulin therapy in the acute phase and standard rehabilitation treatment during convalescence.
The patient was discharged after 2 wk of rehabilitation. The D-dimer level had decreased to 0.98 μg/mL (Figure 1) and blood potassium levels were normal upon discharge. The sensory plane was the same as that at admission, although motor function and ADL improved significantly (Figure 1). The BBS, FMA and MBI scores also markedly increased to 24/56, 20/34 and 63/100, respectively, after rehabilitation. The patient was able to stand independently and walk with an aid. The attending physicians recommended warfarin anticoagulant therapy after discharge, which was refused by the patient who insisted on taking rivaroxaban 20 mg tablets daily. Other recommendations at discharge were anticoagulant therapy for 3 mo, daily dose of prednisone starting at 40 mg and decreased by 5 mg every 2 wk, 0.25 g mycophenolate mofetil twice daily (morning and evening), and other drugs at the same dosage as during hospitalization. One month after discharge, the patient had been taking 30 mg prednisone daily, and reported improvement in the chest and back banding sensations, as well as the ability to walk about 300 m independently and perform daily activities.
We report a patient with NMOSD accompanied by spinal cord injury, Sjogren's syndrome and deep venous thrombosis in the lower extremities who received two weeks of rehabilitation and pharmacotherapy. The patient showed considerable improvement in her ability to sit, stand and walk independently and perform daily activities, along with achieving better control of defecation and urination. The patient was overall satisfied with the treatment and outcomes. This typical NMOSD patient was not sensitive to hormone therapy, and had deep vein thrombosis. After effective drug treatment and standardized rehabilitation treatment, the patient recovered well, which may provide a basis for the treatment of other NMOSD patients.
Classic neuromyelitis optica (NMO) is an acute or subacute demyelinating lesion that affects the optic nerve and spinal cord either sequentially or simultaneously. It usually involves the cervicothoracic segments of the spinal cord longitudinally, with only sporadic intracranial involvement. Wingerchuk et al[3] first defined NMOSD in 2007 as a group of localized demyelinating diseases that did not meet the NMO diagnostic criteria, such as idiopathic long segmental myelitis (longitudinal involvement of three or more spinal cord segments), recurrent optic neuritis, and optic neuritis or long segmental myelitis with autoimmune disease or the presence of autoantibodies. According to the 2015 international diagnostic criteria of NMOSD for adult patients[4], its core clinical characteristics include optic neuritis, acute myelitis, area postrema syndrome, acute brainstem syndrome, symptomatic narcolepsy or acute diencephalic clinical syndrome and symptomatic cerebral syndrome. Our patient was a 45-year-old woman with normal menstruation and a history of Sjogren's syndrome, although the typical symptoms like dry mouth and dry eyes were absent. She exhibited spinal cord injury without optic nerve injury, complicated by left calf intermuscular vein thrombosis.
NMOSD affects women more than men, and the age of onset is 30 to 40 years. Therefore, many women with NMOSD exhibit the first symptoms during the childbearing years. Although its exact pathological basis is unclear, studies have routinely implicated vascular disorders and autoimmune demyelinating disease. Pereira et al[5] found that around 27.3% of patients with NMOSD had accompanying autoimmune diseases such as systemic lupus erythematosus and Sjogren's syndrome. NMOSD can be clinically classified into AQP4-IgG positive, negative and unidentified serological types[6,7]. AQP4 is mainly expressed in the astrocytes of the CNS and is involved in neuro-immune induction. The AQP4-IgG autoantibody is a characteristic biomarker of NMOSD[8] and can differentiate it from multiple sclerosis. Our patient was serum positive for the anti-AQP4 and anti-MOG antibodies, as well as for the anti-SSA, anti-SSB and anti-Ro52 antibodies specific for Sjogren's syndrome.
High-dose hormones, gamma globulin shock and plasmapheresis are generally used to treated NMSOD during the acute phase[9]. However, since it is prone to relapse, drug maintenance is essential during the recovery period, usually with small doses of hormones and/or non-specific immunosuppressants such as azathi-oprine[10]. Studies showed that maintenance therapy should be continued for at least 5 years[11]. The monoclonal antibody rituximab[12] has been proved to be more effective in treating NMOSD and reducing the risk of recurrence[13]. Mycophenolate mofetil or CellCept, a non-competitive, selective and reversible hypoxanthine nucleoside phosphate dehydrogenase I/II inhibitor, is an effective and safe option for maintenance therapy[11]. Our patient received methylprednisolone shock therapy and gamma globulin in the acute phase, which significantly strengthened the lower limb muscles. Low-dose prednisone plus mycophenolate mofetil was given to maintain the therapeutic effects, and low molecular-weight heparin calcium was injected subcutaneously for anticoagulation. The serum D-dimer levels decreased gradually and no obvious swelling was observed in the lower limbs, which indicated an optimal anticoagulation effect. We recommended oral warfarin after discharge, which was refused by the patient due to the inconvenience of regularly monitoring the INR value. Therefore, she was prescribed oral rivaroxaban (Xarelto).
NMSOD-mediated spinal cord injury results in varying degrees of disability that requires rehabilitation[14,15]. Patients with spinal cord injury frequently exhibit motor and sensory dysfunction in the limbs, as well as lack of control over defecation and urination. Thus, patients need further rehabilitation such as strength training[16] of paralyzed limbs, joint movement, balance and gait training[17], neuromuscular electrical stimulation[18] to promote muscle contraction, and management training of intestine and bladder. Our patient underwent strength training of the trunk and key lower limb muscles, muscle stretching, and postural balance training, followed by supported standing/walking and gait training. Neuromuscular electrical stimulation was used to improve the muscle strength of the lower extremities, primarily that of the right quadriceps femoris and tibialis anterior muscles. Two weeks of rehabilitation significantly increased the BBS, FMA and ADL scores, which manifested as improvements in sitting balance, walking stability, gait and execution of daily activities. One month after discharge, the patient experienced less pain in her chest and back, and could walk 300 m without aid and perform daily activities independently.
This study has several limitations that ought to be discussed. First, the rehabilitation duration was short due to time constraints, and neuromuscular electrical stimulation and some equipment training could not be performed. Although we provided post-discharge rehabilitation guidance for the patient, there was no way to determine whether the patient could complete daily rehabilitation exercises on time or whether the methods of rehabilitation exercises were correct. Furthermore, due to the considerable distance between the patient’s home and the hospital, only online follow-up was possible. Finally, it is impossible to comment on the possibility of any future recurrence due to the short follow-up duration.
MRI also allows a quantitative assessment of spine recovery. At the first MRI examination, the patient asked for a routine examination due to the additional cost of quantitative MRI. Compared with the international literature, our country and hospitals have more strict management of inpatient MRI examination. When the patient's condition changes, the MRI can be checked again. There are two main reasons why the patient did not have MRI examination again: (1) The clinical symptoms of the patient gradually improved without sudden aggravation; and (2) Considering the high cost of re-examination, the patient refused MRI re-examination in such a short time. In addition, the patient did not show any symptoms of optic nerve injury. In the first MRI examination, there was no abnormal signal in the occipital lobe and brainstem, so no further optic nerve enhancement was performed.
NMOSD is a recurrent and disabling disease that can be managed by early diagnosis and treatment. Standardized rehabilitation training can reduce the neuromotor dysfunction and improve ADL ability. However, it is crucial to avoid excessive fatigue during rehabilitation in order to minimize the risk of symptom aggravation and recurrence.
We thank the Neurology Department of Nanjing First Hospital for clinical support.
Manuscript source: Unsolicited manuscript
Specialty type: Rehabilitation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roldan-Valadez E S-Editor: Liu M L-Editor: Webster JR P-Editor: Wang LL
| 1. | Huda S, Whittam D, Bhojak M, Chamberlain J, Noonan C, Jacob A. Neuromyelitis optica spectrum disorders. Clin Med (Lond). 2019;19:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 2. | Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 433] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1489] [Cited by in RCA: 1597] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 4. | Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG; International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2353] [Cited by in RCA: 3281] [Article Influence: 328.1] [Reference Citation Analysis (0)] |
| 5. | Pereira WLCJ, Reiche EMV, Kallaur AP, Oliveira SR, Simão ANC, Lozovoy MAB, Schiavão LJV, Rodrigues PRDVP, Alfieri DF, Flauzino T, Kaimen-Maciel DR. Frequency of autoimmune disorders and autoantibodies in patients with neuromyelitis optica. Acta Neuropsychiatr. 2017;29:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Wingerchuk DM, Weinshenker BG. The emerging relationship between neuromyelitis optica and systemic rheumatologic autoimmune disease. Mult Scler. 2012;18:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2214] [Cited by in RCA: 2307] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 8. | Prasad S, Chen J. What You Need to Know About AQP4, MOG, and NMOSD. Semin Neurol. 2019;39:718-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Romeo AR, Segal BM. Treatment of neuromyelitis optica spectrum disorders. Curr Opin Rheumatol. 2019;31:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Costanzi C, Matiello M, Lucchinetti CF, Weinshenker BG, Pittock SJ, Mandrekar J, Thapa P, McKeon A. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Montcuquet A, Collongues N, Papeix C, Zephir H, Audoin B, Laplaud D, Bourre B, Brochet B, Camdessanche JP, Labauge P, Moreau T, Brassat D, Stankoff B, de Seze J, Vukusic S, Marignier R; NOMADMUS study group and the Observatoire Français de la Sclérose en Plaques (OFSEP). Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler. 2017;23:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Jeong IH, Park B, Kim SH, Hyun JW, Joo J, Kim HJ. Comparative analysis of treatment outcomes in patients with neuromyelitis optica spectrum disorder using multifaceted endpoints. Mult Scler. 2016;22:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Yang Y, Wang CJ, Wang BJ, Zeng ZL, Guo SG. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and lower dosages of rituximab among patients with neuromyelitis optica spectrum disorder. J Neurol Sci. 2018;385:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Maribo T, Jensen CM, Madsen LS, Handberg C. Experiences with and perspectives on goal setting in spinal cord injury rehabilitation: a systematic review of qualitative studies. Spinal Cord. 2020;58:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Mackiewicz-Milewska M, Cisowska-Adamiak M, Rość D, Głowacka-Mrotek I, Świątkiewicz I. Effects of Four-Week Rehabilitation Program on Hemostasis Disorders in Patients with Spinal Cord Injury. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Eitivipart AC, de Oliveira CQ, Arora M, Middleton J, Davis GM. Overview of Systematic Reviews of Aerobic Fitness and Muscle Strength Training after Spinal Cord Injury. J Neurotrauma. 2019;36:2943-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Rigot S, Worobey L, Boninger ML. Gait Training in Acute Spinal Cord Injury Rehabilitation-Utilization and Outcomes Among Nonambulatory Individuals: Findings From the SCIRehab Project. Arch Phys Med Rehabil. 2018;99:1591-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Arpin DJ, Ugiliweneza B, Forrest G, Harkema SJ, Rejc E. Optimizing Neuromuscular Electrical Stimulation Pulse Width and Amplitude to Promote Central Activation in Individuals With Severe Spinal Cord Injury. Front Physiol. 2019;10:1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |