Published online Mar 26, 2019. doi: 10.12998/wjcc.v7.i6.792
Peer-review started: December 13, 2018
First decision: January 12, 2019
Revised: February 16, 2019
Accepted: February 26, 2019
Article in press: February 26, 2019
Published online: March 26, 2019
Processing time: 103 Days and 6.8 Hours
Only a few cases of carcinoma ex pleomorphic adenoma arising in the submandibular gland have ever been reported, all with a poor prognosis. The standard treatment for salivary gland carcinoma ex pleomorphic adenoma is surgical resection combined with postoperative radiotherapy, but the necessity of chemotherapy as well as the most appropriate treatment regimen for patients with distant metastasis after radiotherapy remains controversial.
This report presents the case of a 73-year-old woman who suffered submandibular gland carcinoma ex pleomorphic adenoma. She accepted surgery to remove the mass; she was found to have lung metastasis after radiotherapy. Her condition was controlled by chemotherapy with liposomal doxorubicin plus cisplatin.
The positive clinical outcome in the patient reveals that this chemotherapy regimen may be an alternative treatment for such a condition.
Core tip: The standard treatment for salivary gland carcinoma ex pleomorphic adenoma is surgical resection combined with postoperative radiotherapy, but the necessity of chemotherapy as well as the most appropriate treatment regimen for patients with distant metastasis after radiotherapy remains controversial. The positive clinical outcome in this case reveals that this chemotherapy regimen may be an alternative treatment for such a condition.
- Citation: Chen ZY, Zhang Y, Tu Y, Zhao W, Li M. Effective chemotherapy for submandibular gland carcinoma ex pleomorphic adenoma with lung metastasis after radiotherapy: A case report. World J Clin Cases 2019; 7(6): 792-797
- URL: https://www.wjgnet.com/2307-8960/full/v7/i6/792.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i6.792
The most common pathological types of salivary gland malignant tumor are adenoid cystic carcinoma, mucoepidermoid carcinoma, adenocarcinoma, and malignant pleomorphic adenoma. Pleomorphic adenoma has three different forms of malignant changes, which are carcinoma ex pleomorphic adenoma (Ca ex PA), true malignant mixed tumor (carcinosarcoma), and metastasizing pleomorphic adenoma. Ca ex PA is a rare neoplasm that generally affects the major salivary glands especially the parotid gland and submandibular gland. Ca ex PA is rare because of its prevalence rate of 5.6 per 100000 malignant tumors and an annual incidence of 0.17 tumors per million people[1]. The pathological features of salivary Ca ex PA is that malignant components and benign pleomorphic adenoma should be noted together under a light microscope.
The most familiar clinical manifestation of Ca ex PA is a hard mass in the parotid gland[2]. It can be asymptomatic as PA since most tumors are not extensively invasive Ca ex PA[3,4]. Pain is usually caused by local expansion of the tumor to adjacent soft and hard tissues[3]. Local recurrence is more common than metastasis; the most common site of metastasis is the neck, followed by the lungs[5]. When the malignant component is completely contained within the fibrous sac that limits other benign pleomorphic adenomas, there is no difference between the prognosis of Ca ex PA and benign mixed tumors[1]. In contrast, if the malignant area extends beyond the tumor capsule, the prognosis is poor. The increased frequency of local recurrence and distant metastases are responsible for the poor prognosis of this rare malignancy, which has a high mortality rate, as more than half of patients (55.6%) die within 5 years after the diagnosis[5]. The standard treatment for pleomorphic adenoma is surgical resection combined with postoperative radiotherapy, but the necessity of chemotherapy as well as the most appropriate chemotherapy regimen for patients with distant metastasis after radiotherapy remains controversial. The use of chemotherapy drugs such as cisplatin, epirubicin, cyclophosphamide, and fluorouracil has been previously reported for the treatment of this condition in the literature. Importantly, in this case report we demonstrate that chemotherapy using liposomal doxorubicin plus cisplatin may be a novel treatment regimen for such patients.
A 73-year-old woman presented to our institution with a 17-mo history of a mass originating in the right submandibular gland and a one-year history of excision of the right submandibular gland tumor.
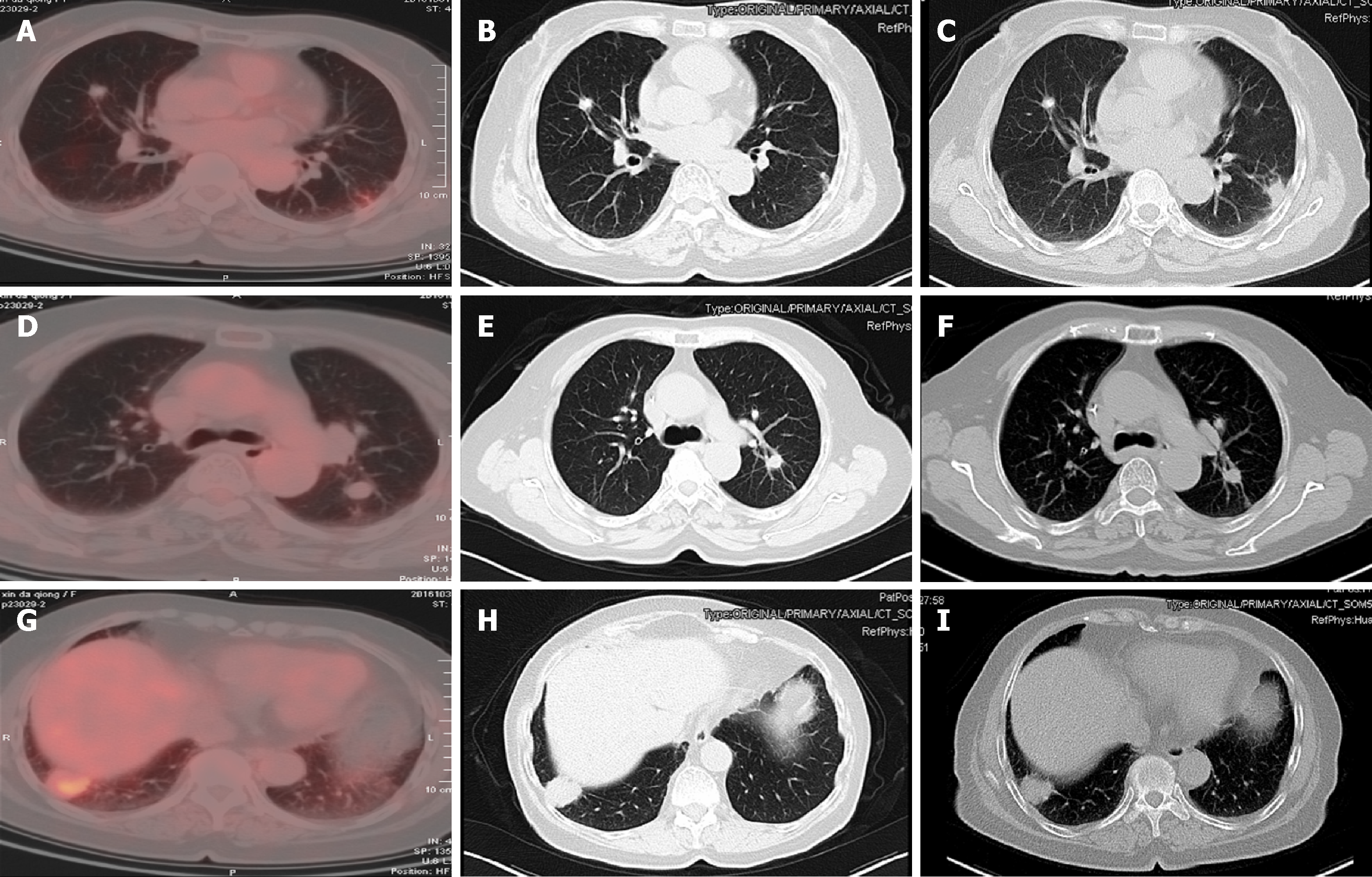
She found a 3 cm × 3 cm hard, fixed mass in her right submandibular region with local pain 17 mo prior. There was no restriction in mouth opening or facial nerve dysfunction. The nature of the mass was unclear until the surgery. Radiotherapy was recommended as an adjuvant therapy, so the patient accepted intensity modulated radiation therapy at a dose of CTV1 (right mandibular gland) 2.0 Gy/F; CTV2 1.8-2.0 Gy/F; and CTV1n 1.64 Gy/F. Regular follow-up was performed after radiotherapy. Local recurrence and metastasis were not observed until positron emission tomography-computed tomography (PET-CT) suggested bilateral lung metastasis a half month later.
She accepted surgery to remove the right submandibular gland and mass 5 months later; after the surgery, she received radiotherapy (66 Gy/33 fractions/45 d).
She denied smoking and had no personal or family history of the related disease.
Physical examination revealed old surgical scars about 6 cm in length on the right submandibular angle with no superficial lymphadenopathy or other signs. There was no local pain or facial nerve dysfunction.
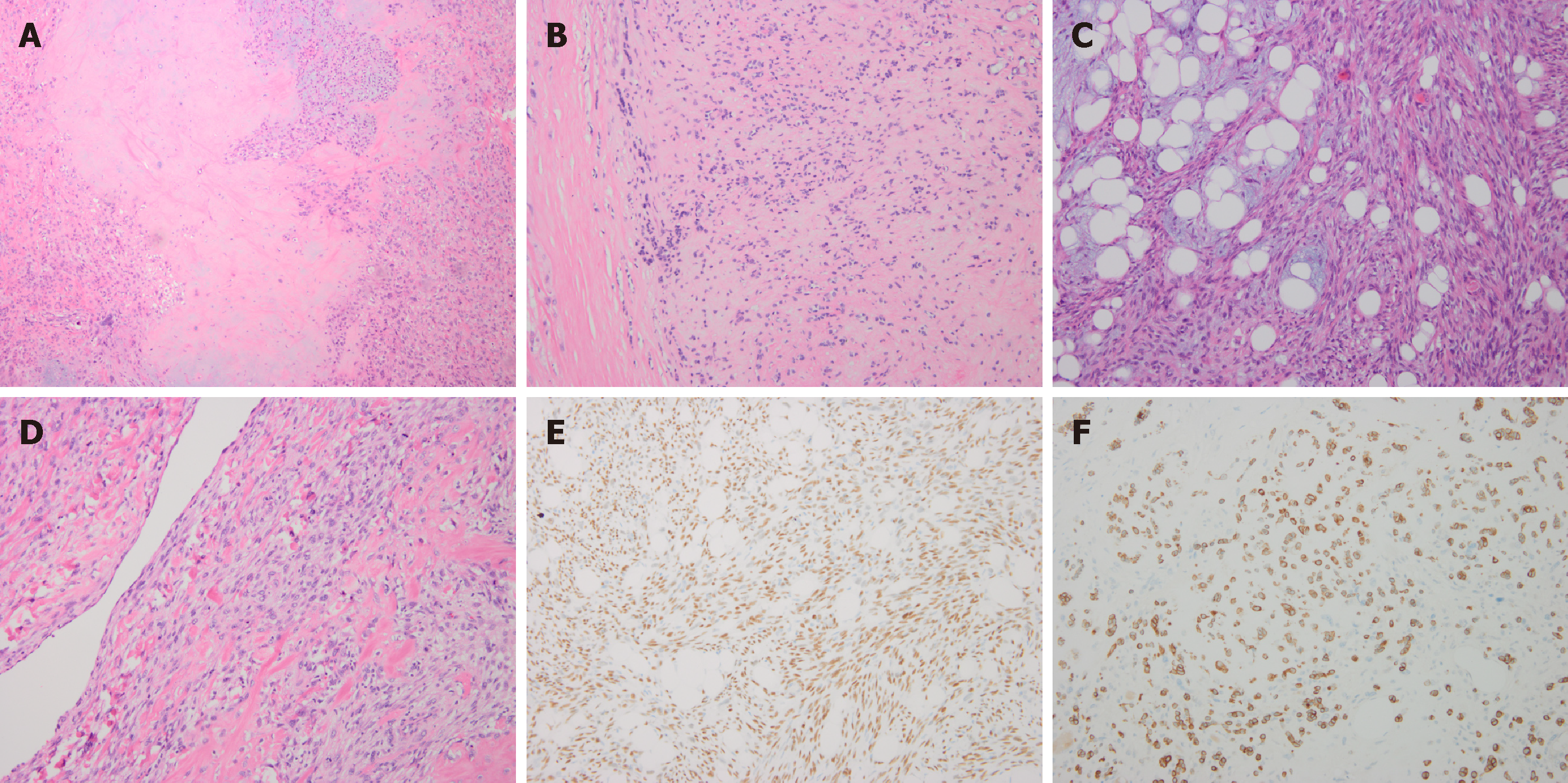
Pathological analysis revealed the tumor type to be Ca ex PA. The main components of this malignant tumor were poorly differentiated carcinomas, including myoepithelial carcinoma and spindle cell carcinoma, while the sarcoma was classified as osteosarcoma. Immunohistochemical (IHC) analysis showed that the tumor cells were positive for PCK, S-100 protein, CK14, P63, calponin, CK5/6, and desmin; the proliferative activity of Ki-67 was 20% (Figure 1).
After the second and fourth cycles of chemotherapy, head and neck enhanced magnetic resonance imaging (MRI), chest enhanced CT (Figure 2), and abdominal ultrasound were reviewed, which showed that the lung metastasis was controlled.
The patient was diagnosed with submandibular gland carcinoma ex pleomorphic adenoma with lung metastasis. The main components of this malignant tumor were poorly differentiated carcinomas, including myoepithelial carcinoma and spindle cell carcinoma, while the sarcoma was classified as osteosarcoma.
A total of four cycles of chemotherapy with cisplatin and liposomal doxorubicin were completed with one every 3 wk. No serious adverse events were observed during the chemotherapy.
Imaging showed that the lung metastasis was controlled. After the fourth cycle of chemotherapy, the patient and her family refused further treatment, and the patient had no evidence of disease progression 9 months after chemotherapy.
Clinical presentation, imaging, and cytology are the basis for the diagnosis and management of salivary gland tumors. Diagnostic modalities include CT, PET-CT, MRI, and biopsy, which were, respectively, used in 78.1%, 39.4%, 42.3%, and 43.7% of malignant salivary tumor patients according to a study[6]. The primary role of CT and MRI is to determine the size, location, and adjacent structures of tumors. MRI is more helpful in assessing facial nerve involvement. F-18-FDG PET is significantly more accurate than CT/MRI for the detection of head and neck cancer. Moreover, the sensitivity and specificity of PET are 87% and 67%, respectively, while those of CT/MRI are 67% and 44%[7].
Fine needle aspiration cytology (FNAC) and core biopsy are the main diagnostic tools for salivary gland lesions, which can help distinguish benign tumors from malignant ones, so FNAC combined with ultrasound is recommended[8]. FNAC is inexpensive, quick, and highly accurate, with an accuracy of 97.6%[9]. It also aids in preoperative diagnosis and surgical planning. However, the sensitivity and accuracy of FNAC in the detection of malignant characteristics in Ca ex PA are 60% and 46%, respectively[10]. Another retrospective study confirmed that clinical judgment of benign and malignant tumors in the submandibular gland had a low accuracy of 67%[11], so intraoperative frozen section analysis results were particularly important in determining the extent of surgical resection. Unfortunately, there was no accurate diagnosis made before surgery on our patient, so it can only be diagnosed by the intraoperative frozen section analysis.
Although hematoxylin-eosin staining is still the gold standard for the diagnosis of postoperative pathology, immunohistochemistry (IHC) can improve the diagnostic accuracy. Myoepithelial cells that are part of epithelial-myoepithelial carcinoma are strongly reactive for smooth muscle actin, P63, vimentin, and S-100 protein[12]. The high proliferative activity of Ki-67 is the strongest negative prognostic predictor in salivary gland cancer. Patients with a Ki-67 value of 15% or less had better survival rates than patients with a Ki-67 value more than 15% with early T1-T2 or N0 stage cancer[13]. The IHC characteristics of this patient are in accordance with the above reports, and her Ki-67 proliferation rate was 20%, which indicated a poor prognosis and survival rate. The patient and her family were fully informed of the disease and will be followed closely.
Treatment modalities for submandibular malignant tumors include exclusive surgery for early-stage tumors[14], surgery combined with radiotherapy for patients with high-grade or -stage tumors, clear histological risk factors (i.e., positive microscopic margins and perineural spread), and radiotherapy alone for patients who cannot tolerate surgery or patients with unresectable submandibular malignant tumors. Staffieri et al[15] found that surgery combined with radiotherapy can decrease the rate of recurrence compared with surgery alone. Several studies have suggested that adjuvant radiotherapy can improve local tumor control and patient survival rates, and serves as a salvage method for those who have inadequate surgical margins[16-18]. Thus, surgery combined with radiotherapy is essential for the treatment of submandibular gland Ca ex PA. Unfortunately, after one year of surgery and postoperative radiotherapy, our patient experienced bilateral lung metastasis.
Whether chemotherapy is indicated and which chemotherapy regimen is appropriate for patients with distant metastasis after radiotherapy remain controversial. It has been reported that chemotherapy and radiotherapy should be considered in patients who have suffered malignant pleomorphic adenoma with positive surgical margins, positive lymph nodes, highly advanced diseases, or surgically unresectable diseases[12]. Reports on the use of chemotherapy drugs such as cisplatin, epirubicin, cyclophosphamide, and fluorouracil had been previously published on this rare condition. Takahiro Wakasaki reported two cases of rare invasive myoepithelial Ca ex PA of the major salivary gland with distant metastasis (lung metastasis); the disease was controlled by chemotherapy including cyclophosphamide, cisplatin, and adriamycin[19]. A case of parotid gland car-cinosarcoma undergoing total parotidectomy with facial nerve preservation, radiotherapy, and chemotherapy (cisplatin, ifosfamide, and methotrexate) presented no evidence of recurrence at the 26-mo follow-up[15].
In our report, lung metastasis was controlled after chemotherapy with liposomal doxorubicin plus cisplatin. Such an outcome suggested that this chemotherapy regimen was effective in the treatment of lung metastasis of Ca ex PA. We chose liposomal doxorubicin instead of doxorubicin because as a carrier of doxorubicin, the liposome can prolong its pharmacological efficacy, reduce drug toxicity (especially cardiac toxicity and myelosuppression), improve efficacy, and mitigate drug resistance compared with doxorubicin alone. Our experience revealed that the above chemotherapy regimen may be an alternative regimen for such rare and complex conditions.
There is no uniform standard regimen for palliative chemotherapy of patients with distant metastasis after radiotherapy. Currently, there are also no large-sample clinical studies to guide treatment. This is a great challenge to clinicians and requires further exploration. We hope that this rare case report can provide a basis for novel chemotherapeutic recommendations. We will continue to follow this patient and accumulate data to try and resolve this clinical conundrum in the near future.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozyigit G, Vikey AK S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28 Pt 1:279-328. [PubMed] |
| 2. | Nouraei SA, Hope KL, Kelly CG, McLean NR, Soames JV. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg. 2005;116:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Zbären P, Zbären S, Caversaccio MD, Stauffer E. Carcinoma ex pleomorphic adenoma: diagnostic difficulty and outcome. Otolaryngol Head Neck Surg. 2008;138:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001;23:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Ye P, Gao Y, Mao C, Guo CB, Yu GY, Peng X. Carcinoma Ex Pleomorphic Adenoma: Is It a High-Grade Malignancy? J Oral Maxillofac Surg. 2016;74:2093-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Israel Y, Rachmiel A, Ziv G, Nagler R. Diagnostic and therapeutic modalities for 287 malignant and benign salivary tumors: A cohort study. J Craniomaxillofac Surg. 2017;45:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Nowak B, Di Martino E, Jänicke S, Cremerius U, Adam G, Zimny M, Reinartz P, Büll U. Diagnostic evaluation of malignant head and neck cancer by F-18-FDG PET compared to CT/MRI. Nuklearmedizin. 1999;38:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Sood S, McGurk M, Vaz F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 9. | Shetty A, Geethamani V. Role of fine-needle aspiration cytology in the diagnosis of major salivary gland tumors: A study with histological and clinical correlation. J Oral Maxillofac Pathol. 2016;20:224-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Stodulski D, Rzepko R, Kowalska B, Stankiewicz C. [Carcinoma ex pleomorphic adenoma of major salivary glands--a clinicopathologic review]. Otolaryngol Pol. 2007;61:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lee WH, Tseng TM, Hsu HT, Lee FP, Hung SH, Chen PY. Salivary gland tumors: A 20-year review of clinical diagnostic accuracy at a single center. Oncol Lett. 2014;7:583-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Politi M, Robiony M, Avellini C, Orsaria M. Epithelial-myoepithelial carcinoma of the parotid gland: Clinicopathological aspect, diagnosis and surgical consideration. Ann Maxillofac Surg. 2014;4:99-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Vacchi-Suzzi M, Bocciolini C, Bertarelli C, Dall'Olio D. Ki-67 proliferation rate as a prognostic marker in major salivary gland carcinomas. Ann Otol Rhinol Laryngol. 2010;119:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Lombardi D, McGurk M, Vander Poorten V, Guzzo M, Accorona R, Rampinelli V, Nicolai P. Surgical treatment of salivary malignant tumors. Oral Oncol. 2017;65:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Staffieri C, Marioni G, Ferraro SM, Marino F, Staffieri A. Carcinosarcoma de novo of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e35-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Dai D. Postoperative irradiation in malignant tumors of submandibular gland. Cancer Invest. 1999;17:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Noh JM, Ahn YC, Nam H, Park W, Baek CH, Son YI, Jeong HS. Treatment Results of Major Salivary Gland Cancer by Surgery with or without Postoperative Radiation Therapy. Clin Exp Otorhinolaryngol. 2010;3:96-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mahmood U, Koshy M, Goloubeva O, Suntharalingam M. Adjuvant radiation therapy for high-grade and/or locally advanced major salivary gland tumors. Arch Otolaryngol Head Neck Surg. 2011;137:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Wakasaki T, Kubota M, Nakashima Y, Tomonobe E, Mihara T, Fukushima J. Invasive myoepithelial carcinoma ex pleomorphic adenoma of the major salivary gland: two case reports. BMC Cancer. 2016;16:827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |