Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2644
Peer-review started: October 25, 2021
First decision: December 17, 2021
Revised: December 28, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 16, 2022
Processing time: 136 Days and 8.4 Hours
Serrated polyposis syndrome (SPS) is a relatively rare disease that is characterized by multiple serrated lesions/polyps. Very little is known regarding the extra
A 67-year-old male patient initially presented with belching and abdominal distension for a year as well as diarrhea for over 2 mo. The patient underwent colonoscopy and was diagnosed with serrated polyposis syndrome. Half a year later, a gastroscopy was performed during the postoperative re-examination to screen for other lesions of the upper gastrointestinal tract. An elevated lesion was detected in the anterior wall of the gastric antrum. Curative en bloc resection of the lesion was achieved via endoscopic submucosal dissection. The pathological result was high-grade dysplasia with focal intramucosal carcinoma. Exome sequencing was performed for the patient and five gastric cancer-associated variants (methylenetetrahydrofolate reductase, metaxin 1, coiled-coil domain containing 6, glutamate ionotropic receptor delta type subunit 1, and aldehyde dehydrogenase 1) were identified.
This paper reports a case that presented with both SPS and early gastric cancer. Genetic mutations that were potentially responsible for this condition were sought by exome sequencing.
Core Tip: Serrated polyposis syndrome (SPS) is a relatively rare disease. Very little is known regarding the extracolonic cancers associated with SPS. The genetic basis of the process remains unknown. Here, we report a case that presented with SPS and synchronized early gastric cancer. Genetic mutations that were potentially responsible for this condition were sought by exome sequencing.
- Citation: Ning YZ, Liu GY, Rao XL, Ma YC, Rong L. Synchronized early gastric cancer occurred in a patient with serrated polyposis syndrome: A case report. World J Clin Cases 2022; 10(8): 2644-2649
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2644.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2644
Serrated polyposis syndrome (SPS), previously known as hyperplastic polyposis, is a relatively rare disease that is characterized by multiple serrated lesions/polyps (SL/Ps), mainly in the proximal colon[1]. An increasing body of evidence suggests that patients with SPS have an increased risk of colorectal cancer (CRC) but the genetic basis of the process remains unknown[2]. Also, very little is known regarding the extracolonic cancers associated with SPS. To understand the molecular basis of SPS, it is important to identify the corresponding disease-causing genes. Because whole-exome sequencing can almost cover the entirety of protein-coding regions in the genome, which contains approximately 85% of disease-relevant mutations, it can serve as a powerful tool for cost-effective disease mechanistic research[3].
This paper reports a patient with SPS and synchronized early gastric cancer (GC) treated with endoscopic submucosal dissection (ESD), along with some potential causative mutations found in exome sequencing.
A 67-year-old male patient initially presented with belching and abdominal distension for a year as well as diarrhea for over 2 mo.
The patient had no history of present symptoms.
The patient had a history of hypertension that was well controlled with medication.
No personal or family history of SPS or cancers was reported.
Physical examination was unremarkable.
Since the patient was Helicobacter pylori negative, the diagnosis of H. pylori infection-related GC was excluded.
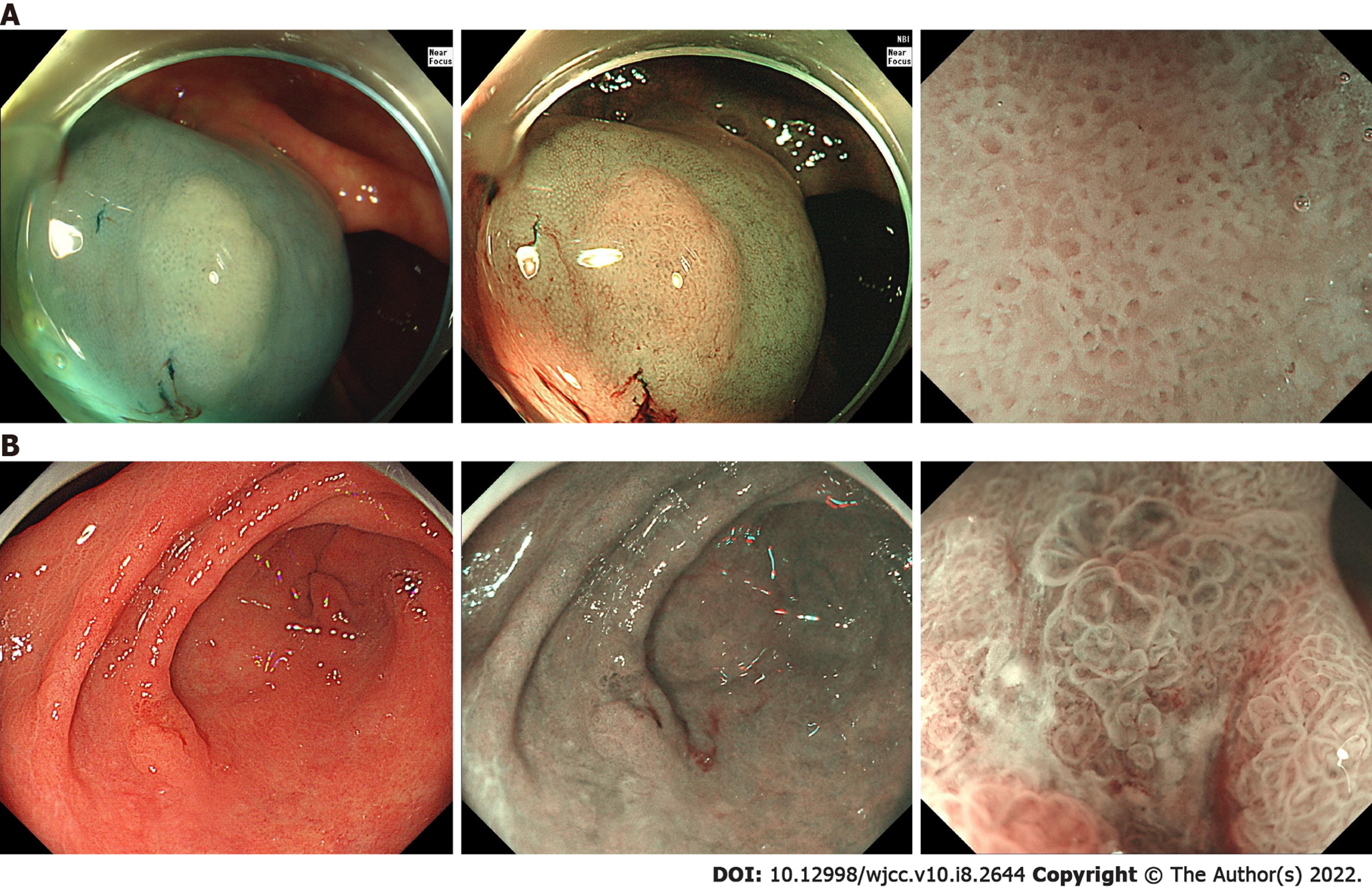
The patient underwent colonoscopy and found multiple flat and sessile polyps located throughout different segments of the colon and ranging from 5 to 20 mm in diameter. More than 10 polyps were removed and pathological examination confirmed most polyps to be sessile serrated lesions (SSLs) and 4 as tubular adenoma, all without severe dysplasia (Figure 1A). The diagnosis of SPS was established. Half a year later, a gastroscopy was performed during the postoperative re-examination to screen for other lesions of the upper gastrointestinal tract. An elevated lesion was detected in the anterior wall of the gastric antrum (Figure 1B).
Total genome DNA from peripheral blood was extracted using the cetrimonium bromide/sodium dodecyl sulfate method. Gene libraries were constructed and paired-end sequencing was performed using the Illumina® HiSeq platform. Statistics was mapped with a reference genome using Burrows-Wheeler Alignment software (parameters: mem-t4-k32-M) and the duplicates were removed by Picard. Individual single nucleotide polymorphism (SNP) variations were detected using the Genome Analysis Toolkit. Subsequently, annotation of the detected SNPs was performed using SnpEff.
To explore the molecular characteristics of the patient, sequencing analysis was performed. Exome sequencing identified 3111 nonsynonymous single nucleotide variants in the exon region. These genes were filtered by the mutation data in ClinVar, COSMIC v90 and previous genome-wide association study reports. Five GC-associated variants (methylenetetrahydrofolate reductase [MTHFR], metaxin 1 [MTX1], coiled-coil domain containing 6 [CCDC6], glutamate ionotropic receptor delta type subunit 1 [GRID1], and aldehyde dehydrogenase 1 [ALDH2]) were identified, as shown in Table 1. Additionally, a cross check for genes that has been reported as causative of SPS or relating to the serrated pathway was performed. The BRAF V600E and KRAS G12D mutations, common hotspot mutations in SPS, were not found.
| Gene | Chr | Mutation | SIFT/Polyphen_2/MT | Pathways |
| MTHFR | 1 | exon5:c.C788T:p.A263V | D/D/P | Folate metabolism |
| exon5:c.C665T:p.A222V | ||||
| MTX1 | 1 | exon1:c.T187A:p.S63T | - | Metabolism of proteins |
| CCDC6 | 10 | exon9:c.C1408A:p.P470T | D/D/P | DNA damage response, Cell cycle, Apoptosis |
| GRID1 | 10 | exon11:c.G1585A:p.V529I | D/D/D | Peptide ligand-binding receptors |
| ALDH2 | 12 | exon11:c.G1369A:p.E457K | D/D/P | Ethanol degradation, Cytochrome P450 |
| exon12:c.G1510A:p.E504K | ||||
The pathological result of the lesion in the gastric antrum was high-grade dysplasia with focal intramucosal carcinoma.
Curative en bloc resection of the lesion was achieved via endoscopic submucosal dissection (ESD).
The lesion in gastric antrum was considered to be curatively resected. No recurrence was observed on her last esophagogastroduodenoscopy surveillance 1 year after surgery.
SL/Ps include hyperplastic polyps, traditional serrated adenoma, and SSLs. SPS was redefined by World Health Organization (WHO) in 2019 and its diagnosis is based on the cumulative number of serrated lesions in a patient who meets one of the two following WHO criteria: ≥ 5 SL/Ps proximal to the rectum, all ≥ 5 mm in size and including ≥ 2 Larger than 10 mm; or > 20 SL/Ps of any size distributed throughout the colon, with ≥ 5 proximal to the rectum[1]. The true prevalence of SPS is likely under-recognized and not diagnosed because of the need to keep track of the cumulative lifetime number of SL/Ps in a patient[4]. To monitor for risk of malignant progression, endoscopic surveillance is recommended for all patients every 1 year to 3 years[5]; however, suitable monitoring schedules remain controversial.
SL/Ps are currently recognized as the precursors of CRC and SPS has been considered a high-risk condition for CRC. However, there are only a few reported cases of SPS patients having extracolonic malignancies and the association between SPS and extracolonic cancer risk in various studies are not consistent. In their American cohort, Jasperson et al[6] found 12 of 51 SPS patients (24%) had a history of extracolonic tumors, but none were found to have gastric lesions. Hazewinkel et al[7] reported 9 of 105 SPS patients (8.6%) from five medical centers in Europe, which did not significantly differ from the expected number of the general population, but the cancer-specific risk was not estimated. A Korean study[8] reported the diagnosis of stomach cancer in 2 of 30 SPS patients (6.7%) via esophagogastroduodenoscopy, suggesting that Asian patients with SPS require screening of the upper gastrointestinal tract. The lack of data makes it difficult to determine whether patients with SPS are at increased risk of extracolonic cancers or whether these tumors were unrelated to SPS.
In the present case, the stomach lesion was detected in the postoperative re-examination 6 mo after the diagnosis of SPS. As gastroscopy was not performed when the sessile serrated lesions were removed from the colon, the condition of any GC at that time cannot be confirmed. This emphasizes the importance of upper gastrointestinal tract screening in SPS patients.
To date, some molecular signatures of the serrated pathway of CRC formation have been described, including BRAF and KRAS mutations, microsatellite instability and CpG island methylator phenotype. However, the molecular processes of tumorigenesis are still largely unknown, let alone the molecular characteristics of synchronized cancers. Having sequenced the exosome of the patient’s peripheral blood, five variants (MTHFR, MTX1, CCDC6, GRID1, and ALDH2), which are reportedly related to GC, were identified. MTHFR encodes a key enzyme in the folate metabolism pathway, with MTHFR polymorphisms having a functional impact on metabolism[9]. ALDH2, encoding tissue alcohol metabolizing enzymes, can influence acetaldehyde levels in the stomach, which increase the risk of GC through a variety of mechanisms[10]. MTX1 encodes metaxin-1, a mitochondrial protein involved in tumor necrosis factor-induced cell death[11]. MTX1 is overexpressed in GC tissue compared with paired normal tissues, and patients with higher MTX1 expression experience a poorer prognosis[12]. CCDC6, which is recognized as the target gene of microRNA-149-5p (miR-149-5p) and miR-19b-3p[13], inhibits cell proliferation and the epithelial-mesenchymal transition and facilitates cell apoptosis[14]. Although the glutamate receptor GRID1 exclusively functions in the central nervous system, recent evidence suggests that GRID1 may also be involved in multiple kinds of malignant processes during the progression of cancer[15]. As the annotation information of SPS-related genes is limited, we could only first identify the mutations that are reportedly closely related to GC. Considering the characteristics of this patient, these five mutations are presumably associated with both GC and SPS. The mechanism by which these genes affect the pathogenesis of GC and SPS remains to be determined.
Here, exome sequencing was performed for a patient with SPS and synchronized early GC. Although a single patient is not sufficient to identify potential genetic characteristics of SPS, the findings still add to the body of knowledge on the molecular mechanism underpinning SPS with synchronized GC. Further validation experiments using resected specimen are necessary to clarify the effect of mutations on GC and SPS.
In conclusion, this paper reports a case that presented with both SPS and early GC. Genetic mutations that were potentially responsible for this condition were identified by exome sequencing. Further studies are needed regarding the extracolonic cancer risk of SPS patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Lu WS, Muguruma N S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2430] [Article Influence: 486.0] [Reference Citation Analysis (3)] |
| 2. | Fousekis FS, Mitselos IV, Christodoulou DK. Diagnosis, epidemiology and management of serrated polyposis syndrome: a comprehensive review of the literature. Am J Transl Res. 2021;13:5786-5795. [PubMed] |
| 3. | Koeppel F, Bobard A, Lefebvre C, Pedrero M, Deloger M, Boursin Y, Richon C, Chen-Min-Tao R, Robert G, Meurice G, Rouleau E, Michiels S, Massard C, Scoazec JY, Solary E, Soria JC, André F, Lacroix L. Added Value of Whole-Exome and Transcriptome Sequencing for Clinical Molecular Screenings of Advanced Cancer Patients With Solid Tumors. Cancer J. 2018;24:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | van Herwaarden YJ, Verstegen MH, Dura P, Kievit W, Drenth JP, Dekker E, IJspeert JE, Hoogerbrugge N, Nagengast FM, Nagtegaal ID, Bisseling TM. Low prevalence of serrated polyposis syndrome in screening populations: a systematic review. Endoscopy. 2015;47:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1090] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 6. | Jasperson KW, Kanth P, Kirchhoff AC, Huismann D, Gammon A, Kohlmann W, Burt RW, Samadder NJ. Serrated polyposis: colonic phenotype, extracolonic features, and familial risk in a large cohort. Dis Colon Rectum. 2013;56:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Hazewinkel Y, Reitsma JB, Nagengast FM, Vasen HF, van Os TA, van Leerdam ME, Koornstra JJ, Dekker E. Extracolonic cancer risk in patients with serrated polyposis syndrome and their first-degree relatives. Fam Cancer. 2013;12:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Kim ER, Jeon J, Lee JH, Lee YJ, Hong SN, Chang DK, Kim YH. Clinical characteristics of patients with serrated polyposis syndrome in Korea: comparison with Western patients. Intest Res. 2017;15:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Petrone I, Bernardo PS, Dos Santos EC, Abdelhay E. MTHFR C677T and A1298C Polymorphisms in Breast Cancer, Gliomas and Gastric Cancer: A Review. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Na HK, Lee JY. Molecular Basis of Alcohol-Related Gastric and Colon Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | Wang X, Ono K, Kim SO, Kravchenko V, Lin SC, Han J. Metaxin is required for tumor necrosis factor-induced cell death. EMBO Rep. 2001;2:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Sung H, Hu N, Yang HH, Giffen CA, Zhu B, Song L, Su H, Wang C, Parisi DM, Goldstein AM, Taylor PR, Hyland PL. Association of high-evidence gastric cancer susceptibility loci and somatic gene expression levels with survival. Carcinogenesis. 2017;38:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Jin D, Huang K, Peng L, Xu P, Dang Y, Yang J, Chen M, Zhu X, Wei S, Yan J, Zhang G. Circular RNA circDNA2 upregulates CCDC6 expression to promote the progression of gastric cancer via miR-149-5p suppression. Mol Ther Nucleic Acids. 2021;26:360-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Tang Y, Yang J, Wang Y, Tang Z, Liu S, Tang Y. MiR-19b-3p facilitates the proliferation and epithelial-mesenchymal transition, and inhibits the apoptosis of intrahepatic cholangiocarcinoma by suppressing coiled-coil domain containing 6. Arch Biochem Biophys. 2020;686:108367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Wang H, Ma X, Liu J, Wan Y, Jiang Y, Xia Y, Cheng W. Prognostic value of an autophagy-related gene expression signature for endometrial cancer patients. Cancer Cell Int. 2020;20:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |