Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12909
Peer-review started: July 10, 2022
First decision: August 4, 2022
Revised: September 12, 2022
Accepted: November 14, 2022
Article in press: November 14, 2022
Published online: December 16, 2022
Processing time: 156 Days and 17.3 Hours
Increased lipoprotein (a) [lp (a)] has proinflammatory effects, which increase the risk of coronary artery disease. However, the association between lp (a) variability and follow-up C-reactive protein (CRP) level in patients undergoing percutaneous coronary intervention (PCI) has not been investigated.
To explore the association between lp (a) variability and mean CRP levels within the 1st year post-PCI.
Results of lp (a) and CRP measurements from at least three follow-up visits of patients who had received PCI were retrospectively analyzed. Standard deviation (SD), coefficient of variation (CV), and variability independent of the mean (VIM) are presented for the variability for lp (a) and linear regression analysis was conducted to correlate lp (a) variability and mean follow-up CRP level. The relationship of lp (a) variability and inflammation status was analyzed by restricted cubic spline analysis. Finally, exploratory analysis was performed to test the consistency of results in different populations.
A total of 2712 patients were enrolled. Patients with higher variability of lp (a) had a higher level of mean follow-up CRP (P < 0.001). lp (a) variability was positively correlated with the mean follow-up CRP (SD: β = 0.023, P < 0.001; CV: β = 0.929, P < 0.001; VIM: β = 1.648, P < 0.001) by multivariable linear regression analysis. Exploratory analysis showed that the positive association remained consistent in most subpopulations.
Lp (a) variability correlated with mean follow-up CRP level and high variability could be considered an independent risk factor for increased post-PCI CRP level.
Core Tip: In this study, we determined the predictive value of lipoprotein (a) [lp (a)] variability for the C-reactive protein (CRP) level during the 1-year of follow-up after percutaneous coronary intervention (PCI). One of the main strengths is the large sample size of the study. Additionally we used multiple measures to methods to validate our results. In this multicenter retrospective study, the variability of lp (a) was closely correlated with the average follow-up CRP levels, and high lp (a) variability could be considered an independent risk factor for increased CRP levels during follow-up visits for patients treated with PCI.
- Citation: Zhang SS, Hu WY, Li YJ, Yu J, Sang S, Alsalman ZM, Xie DQ. Lipoprotein (a) variability is associated with mean follow-up C-reactive protein in patients with coronary artery disease following percutaneous coronary intervention. World J Clin Cases 2022; 10(35): 12909-12919
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12909.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12909
Coronary artery disease (CAD) is a major global public health problem, and percutaneous coronary intervention (PCI) to reconstruct coronary blood flow and improve prognosis has been of great benefit[1,2]. However, stent deformation, acute renal injury, stent thrombosis, and target lesion revascularization often occur post-PCI and lead to poor prognosis[3-5].
Elevated inflammatory biomarkers have been linked to a poor prognosis post-PCI and C-reactive protein (CRP), a critical indicator of inflammation status, is a predictor of cardiovascular disease risk[6,7]. CRP influences several pathogenic pathways associated with atherosclerosis and promotes the adhesion between monocytes and endothelial cells, low-density lipoprotein (LDL) accumulation in macrophages, platelet aggregation, and production of reactive oxygen species and reduces endothelial cell nitric oxide formation[8]. Such pathological mechanisms combine to induce arterial inflammation, formation of foam cells, and thrombosis[9-11]. Therefore, elevated CRP is considered an independent risk factor for cardiovascular events following PCI[12].
Atherosclerosis is a chronic disease characterized by disordered lipid metabolism and chronic inflammation, which are considered to be co-dependent processes. Indeed, aberrant lipid metabolism promotes both atherosclerotic progression and nonbacterial inflammatory reactions[13,14]. The lipid profile has also been closely related to coronary artery disease (CAD) prognosis[15].
Lipoprotein a [lp (a)] is an atherogenic lipoprotein, similar in structure to LDL cholesterol (LDL-C), which consists of an LDL-like particle and apolipoprotein B-100 (apo B100)[16]. By contrast with LDL-C, circulating lp (a) levels are primarily determined by activity of the LPA gene, without significant dietary or environmental influence, mediating CAD risk throughout a patient’s lifetime[17]. For these reasons, lp (a) variability has not received much attention but therapeutic agents targeting lp (a) are recently progressing through randomized clinical trials, such as proprotein convertase subtilisin/kexin type 9 serine protease inhibitors, IONIS-APO (a)Rx, etc, which makes lp (a) lowering therapy possible[18-20]. Circulating lp (a) levels are not as stable as they used to be. Higher lp (a) levels have been associated with elevated CRP, producing a greater risk of atherosclerotic cardiovascular disease[21]. However, neither the relationship between lp (a) and CRP in PCI-treated patients nor the association between lp (a) variability and CRP during PCI follow-up examinations has been explored.
The association between lp (a) variability and mean CRP level among patients participating in at least three follow-up visits within the 1st year post-PCI was examined during the current multicenter retrospective study.
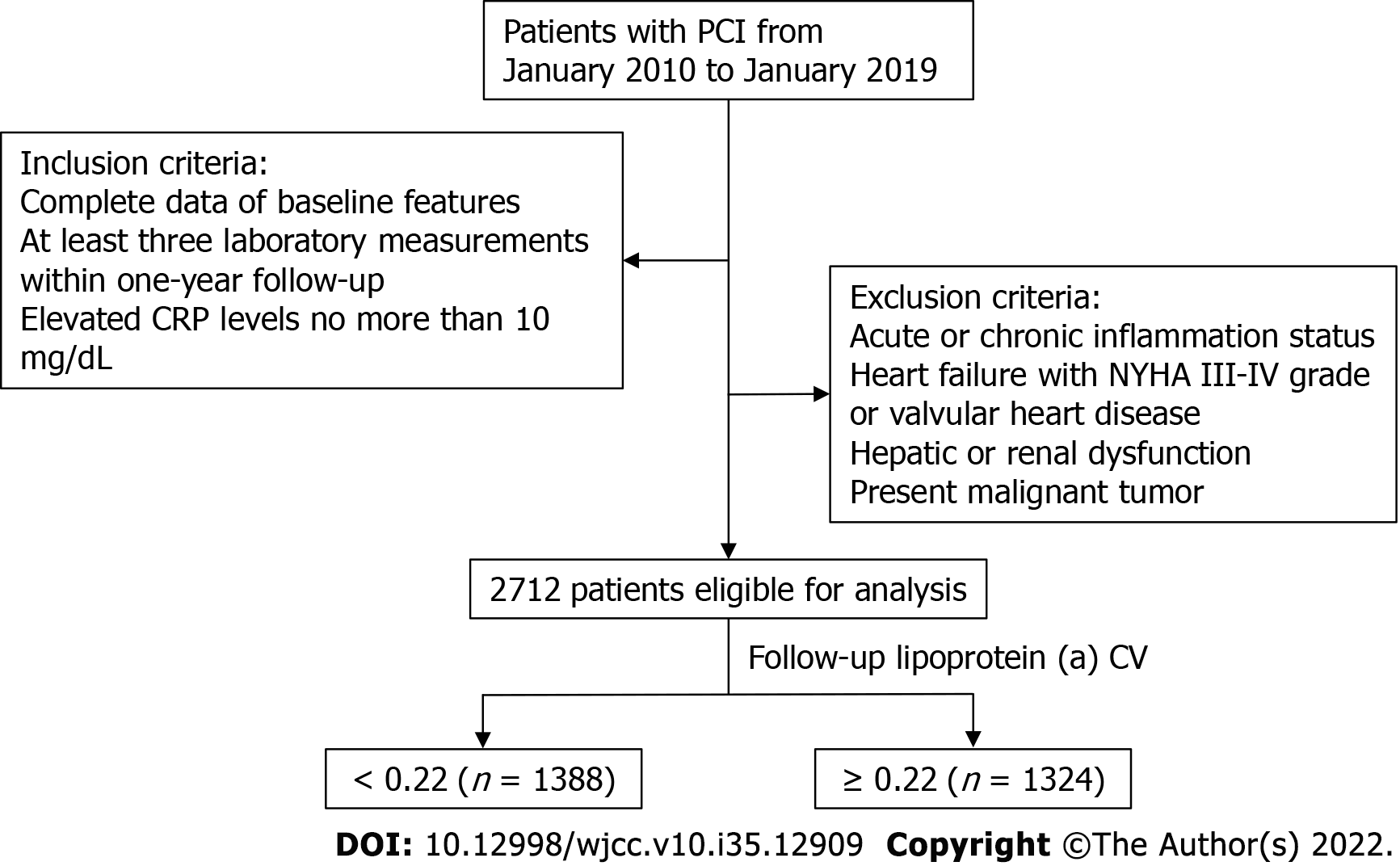
Data from patients treated with PCI between January 2010 and January 2019 at Sir Run Run Shaw Hospital and its medical consortium hospitals were retrospectively collated. Inclusion criteria were as follows: complete baseline data; at least three laboratory measurements within 1-year follow-up; and CRP levels elevated by no more than 10 mg/dL compared to baseline. Exclusion criteria were as follows: acute or chronic inflammatory status, concomitant heart failure (New York Heart Association Grade III-IV) or valvular diseases, severe hepatic or renal dysfunction, and active malignant tumor. All participants provided informed consent for use of data in scientific research and ethical approval was granted by the Ethics Review Committee of Sir Run Run Shaw Hospital (No. 20201217-36). The study was performed in accordance with the 1975 Declaration of Helsinki.
Demographic data (age, sex, height, weight, smoking status, and past medical history), laboratory measurements and medications at discharge for all eligible patients were collected from the Hospital Information System. Each patient in this study was asked to participate in at least three follow-up examinations within the 1st year after PCI. Routine biochemical analysis of fasting blood samples was performed by the local clinical laboratory at each of three follow-up visits. Total serum cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglyceride were measured enzymatically and the Friedewald equation was applied to estimate LDL-C concentrations. lp (a) measurements were performed using immunoturbidimetric assay (Denka Seiken Co. Ltd., Tokyo, Japan) and CRP by transmission turbidimetry on an automatic analyzer (Advia 2400; Siemens, Munich, Germany). White blood cell counts were measured by an automatic blood cell counter (XE-2100; Sysmex, Kobe, Japan).
Lp (a) variability was calculated and defined as the standard deviation (SD), coefficient of variation (CV), and variability independent of the mean (VIM) during follow-up visits. Calculations were performed as follows: SD: arithmetic square root of the square of the difference between measurements and the mean; CV: SD/mean × 100%; VIM: SD/meanβ × 100%, in which β is the regression coefficient based on the natural logarithm of the SD and mean.
Categorical variables are presented as numbers and percentages, and intergroup comparisons carried out using the Chi-square test. Continuous non-normally distributed variables are expressed as the median and interquartile range and comparisons were conducted with the Mann-Whitney U test. The patient cohort was divided into two groups based on the median value of lp (a) CV and baseline characteristics were compared. Potential risk factors linked to mean follow-up CRP level were identified by univariable linear regression between the baseline features and laboratory parameters. Factors with P < 0.10 were used for stepwise multivariable linear regression. A similar analysis was conducted for all three measurements of lp (a) variability. Restricted cubic spline (RCS) analysis of lp (a) variability and high inflammatory status using the median follow-up CRP level of 1.52 mg/L was performed. Exploratory analysis was conducted to determine whether the association between lp (a) variability and mean follow-up CRP was consistent across all subgroups. Subgroups were stratified by age, sex, diabetes, hypertension, and mean follow-up lp (a) concentration. P < 0.05 was considered statistically significant unless otherwise indicated. Statistical analyses were performed using SPSS 25.0 software (Chicago, IL, United States) and R 4.0.5 software (Vienna, Austria).
Baseline characteristics and follow-up laboratory measurements for the 2712 patients enrolled in the current study are shown in Table 1. Median age was 64.0 years and 72.2% were male. There was a tendency for patients with a high follow-up lp (a) CV to have diabetes but no intergroup differences in hypertension or previous PCI history were found. There were differences in mean HDL-C, triglyceride, CRP and lp (a) but none in total cholesterol, LDL-C or white blood cell (WBC). Interestingly, patients who had higher lp (a) CV had lower follow-up lp (a) but higher follow-up CRP (P < 0.001). Grouping by lp (a) SD or VIM shown in the Supplementary Tables 1 and 2, variables with between-group differences were similar to grouping by lp (a) CV (Figure 1).
| Follow-up lp (a) CV | ||||
| Parameter | Overall, n = 2712 | < 0.22, n = 1388 | ≥ 0.22, n = 1324 | P value |
| Demographics | ||||
| Age, yr | 64.0 (58.0, 72.0) | 64.0 (58.0, 72.0) | 64.0 (57.0, 72.0) | 0.593 |
| Male, n (%) | 1957 (72.2) | 982 (70.7) | 975 (73.6) | 0.102 |
| BMI, kg/m2 | 24.7 (22.7, 26.1) | 24.6 (22.6, 26.1) | 24.7 (22.8, 26.2) | 0.191 |
| Current smoking, n (%) | 710 (26.2) | 337 (24.3) | 373 (28.2) | 0.024a |
| Diabetes, n (%) | 692 (25.5) | 327 (23.6) | 365 (27.6) | 0.019a |
| Hypertension, n (%) | 1727 (63.7) | 869 (62.6) | 858 (64.8) | 0.251 |
| Previous PCI, n (%) | 172 (6.3) | 92 (6.6) | 80 (6.0) | 0.584 |
| Laboratory measurements | ||||
| Average TC level, mmol/L | 3.67 (3.23, 4.18) | 3.67 (3.24, 4.16) | 3.67 (3.21, 4.19) | 0.737 |
| Average LDL-C level, mmol/L | 1.80 (1.50, 2.18) | 1.80 (1.49, 2.17) | 1.79 (1.50, 2.18) | 0.968 |
| Average HDL-C level, mmol/L | 1.00 (0.86, 1.18) | 1.03 (0.88, 1.20) | 0.98 (0.84, 1.15) | < 0.001a |
| Average TG level, mmol/L | 1.34 (1.04, 1.83) | 1.32 (1.02, 1.75) | 1.37 (1.06, 1.91) | 0.002a |
| Average CRP level, mg/L | 1.52 (0.80, 2.83) | 1.40 (0.72, 2.60) | 1.65 (0.87, 3.00) | < 0.001a |
| Average WBC level, × 109/L | 6.38 (5.51, 7.25) | 6.30 (5.46, 7.25) | 6.43 (5.55, 7.26) | 0.089 |
| Average lp (a) level, mg/dL | 14.00 (7.32, 34.90) | 16.70 (7.16, 45.81) | 12.52 (7.36, 25.48) | < 0.001a |
| Lp (a) SD | 3.38 (1.61, 7.49) | 2.19 (0.95, 5.40) | 4.55 (2.61, 9.16) | < 0.001a |
| Lp (a) CV | 0.22 (0.14, 0.32) | 0.14 (0.10, 0.18) | 0.32 (0.26, 0.43) | < 0.001a |
| Lp (a) VIM | 0.17 (0.13, 0.23) | 0.13 (0.10, 0.15) | 0.24 (0.20, 0.29) | < 0.001a |
| Medication, n (%) | ||||
| Statin | 2675 (98.6) | 1379 (99.4) | 1296 (97.9) | 0.002a |
| Ezetimibe | 451 (16.6) | 218 (15.7) | 233 (17.6) | 0.204 |
| ACEI or ARB | 1588 (58.6) | 776 (55.9) | 812 (61.3) | 0.005a |
| Beta blocker | 1639 (60.4) | 823 (59.3) | 816 (61.6) | 0.228 |
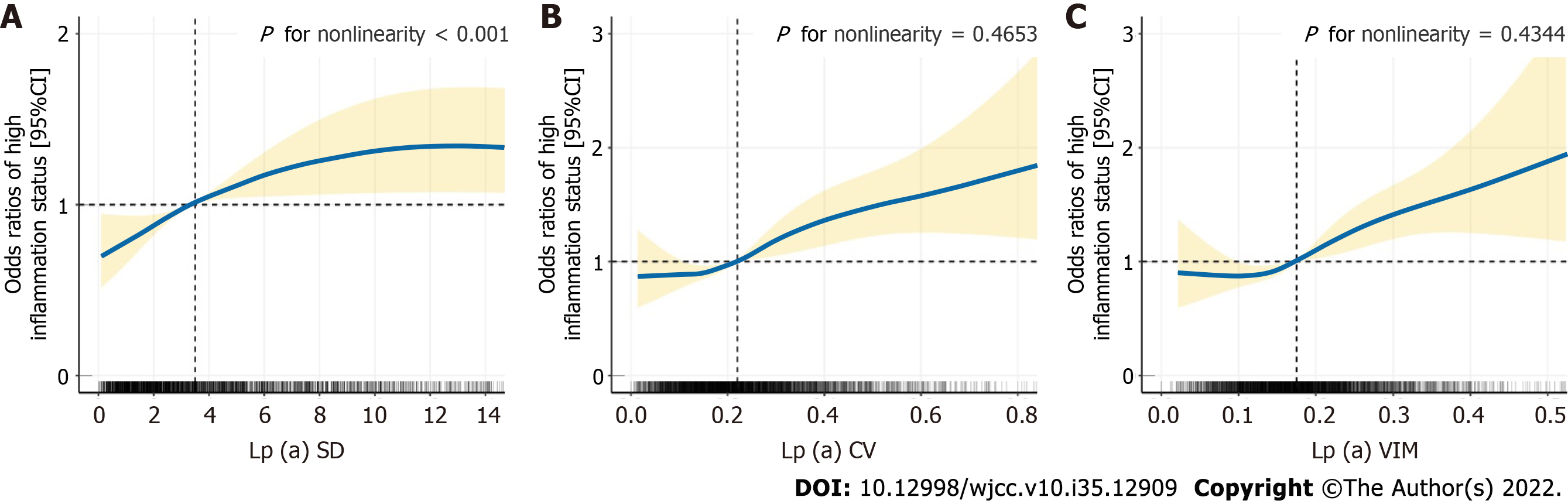
A follow-up CRP level higher than the median of 1.52 mg/L was considered to show high inflammatory status. RCS analysis suggested that the risk of hyperinflammation gradually increased with increasing lp (a) SD and then flattened out (Figure 2A) and initial shallow increases in lp (a) CV and VIM were followed by a rapid rate of increase (Figure 2B and C). A non-linear association was observed between lp (a) SD and hyperinflammation but linear trends between lp (a) CV or VIM and hyperinflammation (SD: P for nonlinearity< 0.001; CV: P for nonlinearity = 0.4653; VIM: P for nonlinearity = 0.4344).
Univariable linear regression of lp (a) variability at follow-up and other potential risk factors was performed to assess the impact on mean follow-up CRP level (Tables 2-4). The SD, CV, and VIM of lp (a) variability showed a positive relationship with mean follow-up CRP level (SD: β = 0.020, P < 0.001; CV: β = 0.961, P < 0.001; VIM: β = 1.692, P < 0.001). The positive relationship persisted after regression analysis of mean lp (a) level, age, diabetes, hypertension, BMI, average LDL-C level, average WBC count, statin and ezetimibe, regardless of the values of lp (a) SD, CV or VIM (SD: β = 0.023, P < 0.001; CV: β = 0.929, P < 0.001; VIM: β = 1.648, P < 0.001). In addition, age, diabetes, BMI, mean LDL-C level and mean WBC count were all independent risk factors for mean follow-up CRP while statin and ezetimibe were independent protective factors.
| Parameter | Univariable analysisb | Multivariable analysis | ||
| β coefficient (95%CI) | P value | β coefficient (95%CI) | P value | |
| Lp (a) SD | 0.020 (0.010, 0.029) | < 0.001 | 0.023 (0.009, 0.037) | 0.001a |
| Average lp (a) level, mg/dL | 0.002 (0.000, 0.004) | 0.059 | -0.002 (-0.005, 0.001) | 0.183 |
| Age in yr | 0.026 (0.020, 0.031) | < 0.001 | 0.030 (0.025, 0.036) | < 0.001a |
| Male | -0.017 (-0.152, 0.119) | 0.811 | ||
| Diabetes | 0.312 (0.173, 0.451) | < 0.001 | 0.201 (0.062, 0.340) | 0.005a |
| Hypertension | 0.314 (0.188, 0.440) | < 0.001 | 0.133 (0.003, 0.263) | 0.045a |
| BMI, kg/m2 | 0.029 (0.009, 0.049) | 0.004 | 0.028 (0.008, 0.048) | 0.007a |
| Current smoking | 0.104 (-0.034, 0.242) | 0.139 | ||
| Average LDL-C level, mmol/L | 0.215 (0.117, 0.312) | < 0.001 | 0.200 (0.098, 0.301) | < 0.001a |
| Average WBC count, × 109/L | 0.256 (0.207, 0.304) | < 0.001 | 0.264 (0.216, 0.312) | < 0.001a |
| Previous PCI | 0.041 (-0.208, 0.291) | 0.744 | ||
| Statin | -0.662 (-1.186, 0.139) | -0.013 | -0.706 (-1.229, 0.183) | -0.008a |
| Ezetimibe | -0.202 (-0.365, 0.039) | -0.015 | -0.334 (-0.497, -0.170) | < 0.001a |
| Univariable analysisb | Multivariable analysis | |||
| β coefficient (95%CI) | P value | β coefficient (95%CI) | P value | |
| Lp (a) CV | 0.961 (0.588, 1.334) | < 0.001 | 0.929 (0.554, 1.304) | < 0.001a |
| Average lp (a) level, mg/dL | 0.002 (0.000, 0.004) | 0.059 | 0.003 (0.001, 0.005) | 0.013a |
| Age in yr | 0.026 (0.020, 0.031) | < 0.001 | 0.031 (0.025, 0.036) | < 0.001a |
| Male | -0.017 (-0.152, 0.119) | 0.811 | ||
| Diabetes | 0.312 (0.173, 0.451) | < 0.001 | 0.201 (0.062, 0.340) | 0.005a |
| Hypertension | 0.314 (0.188, 0.440) | < 0.001 | 0.123 (-0.007, 0.253) | 0.064 |
| BMI, kg/m2 | 0.029 (0.009, 0.049) | 0.004 | 0.028 (0.008, 0.048) | 0.006a |
| Current smoking | 0.104 (-0.034, 0.242) | 0.139 | ||
| Average LDL-C level, mmol/L | 0.215 (0.117, 0.312) | < 0.001 | 0.200 (0.099, 0.301) | < 0.001a |
| Average WBC count, × 109/L | 0.256 (0.207, 0.304) | < 0.001 | 0.261 (0.214, 0.309) | < 0.001a |
| Previous PCI | 0.041 (-0.208, 0.291) | 0.744 | ||
| Statin | -0.662 (-1.186, 0.139) | -0.013 | -0.653 (-1.175, 0.130) | -0.014a |
| Ezetimibe | -0.202 (-0.365, 0.039) | -0.015 | -0.329 (-0.493, -0.166) | < 0.001a |
| Univariable analysisb | Multivariable analysis | |||
| β coefficient (95%CI) | P value | β coefficient (95%CI) | P value | |
| Lp (a) VIM | 1.692 (1.026, 2.359) | < 0.001 | 1.648 (0.977, 2.320) | < 0.001a |
| Average lp (a) level, mg/dL | 0.002 (0.000, 0.004) | 0.059 | 0.003 (0.001, 0.005) | 0.013a |
| Age in yr | 0.026 (0.020, 0.031) | < 0.001 | 0.031 (0.025, 0.037) | < 0.001a |
| Male | -0.017 (-0.152, 0.119) | 0.811 | ||
| Diabetes | 0.312 (0.173, 0.451) | < 0.001 | 0.201 (0.062, 0.340) | 0.005a |
| Hypertension | 0.314 (0.188, 0.440) | < 0.001 | 0.124 (-0.006, 0.254) | 0.062 |
| BMI, kg/m2 | 0.029 (0.009, 0.049) | 0.004 | 0.028 (0.008, 0.048) | 0.006a |
| Current smoking | 0.104 (-0.034, 0.242) | 0.139 | ||
| Average LDL-C level, mmol/L | 0.215 (0.117, 0.312) | < 0.001 | 0.200 (0.099, 0.301) | < 0.001a |
| Average WBC count, × 109/L | 0.256 (0.207, 0.304) | < 0.001 | 0.261 (0.213, 0.309) | < 0.001a |
| Previous PCI | 0.041 (-0.208, 0.291) | 0.744 | ||
| Statin | -0.662 (-1.186, 0.139) | -0.013 | -0.655 (-1.177, 0.132) | -0.014a |
| Ezetimibe | -0.202 (-0.365, 0.039) | -0.015 | -0.331 (-0.494, -0.167) | < 0.001a |
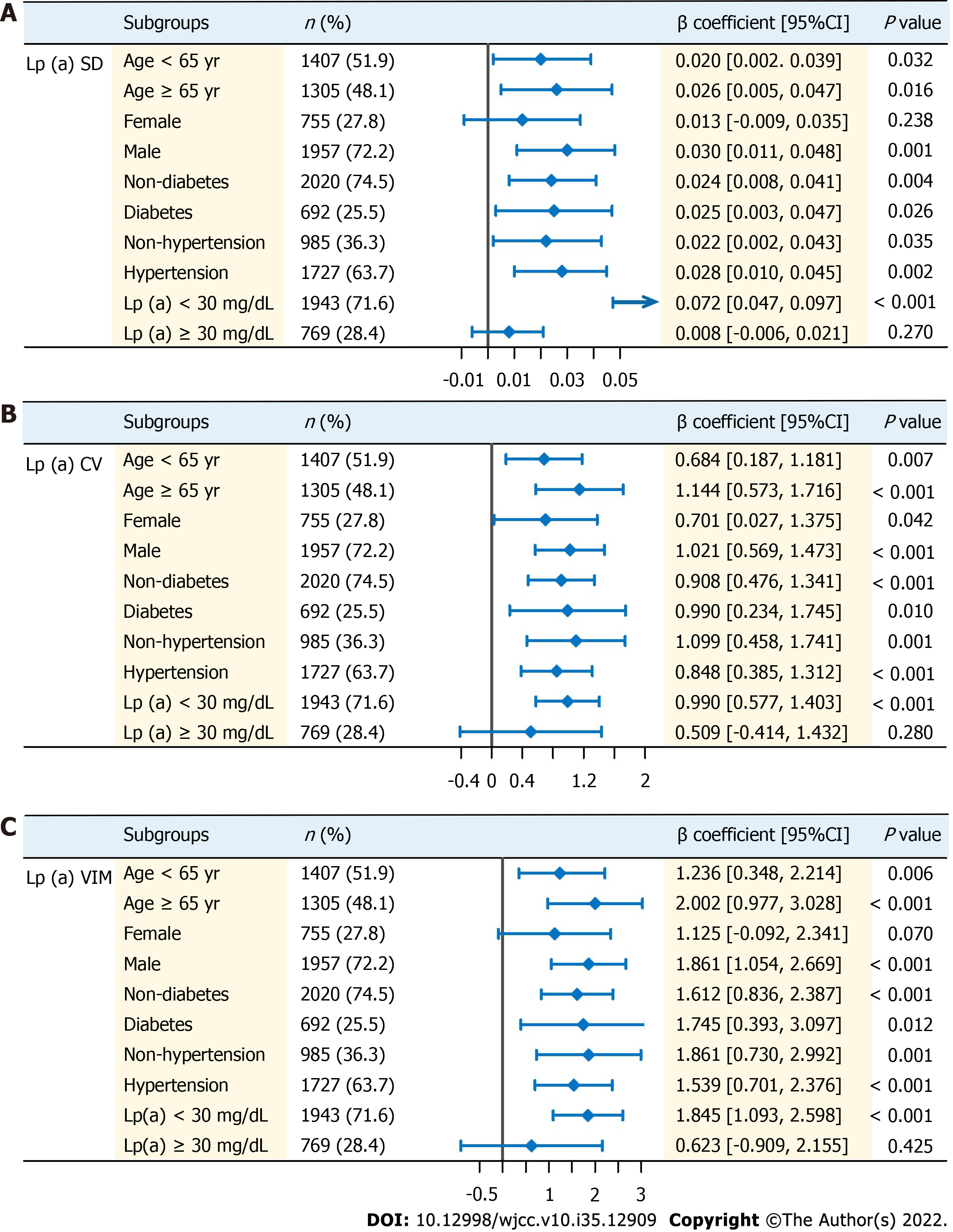
Exploratory analysis was conducted on the positive association between lp (a) variability and CRP among patients from different subgroups. Patients were divided into two groups according to age (< 65 years or ≥ 65 years), sex (female or male), presence of diabetes (yes or no), presence of hypertension (yes or no) and mean follow-up lp (a) levels (< 30 or ≥ 30 mg/dL; Figure 3). Multivariable linear regression was conducted using the same covariates from Tables 2-4. Significant positive associations were found for some subgroups regardless of age, presence of diabetes or presence of hypertension (P < 0.05). In particular, when studying the population with average follow-up lp (a) levels ≥ 30 mg/dL, the positive association no longer became significant in all measurements of variability of lp (a). Similar insignificant results were also found in female (in addition to lp (a) CV).
This study investigated correlations between lp (a) and CRP during 1-year follow-up in CAD patients who underwent PCI and found a significant relationship between metrics of lp (a) variability, SD, CV, VIM, and mean CRP levels. High lp (a) variability was found to be an independent risk factor for increased CRP and high inflammatory status. Interestingly, the relationship was not significant in the subgroup with lp (a) ≥ 30 mg/dL, perhaps due to the lipid-lowering medication prescribed.
The proatherogenic lipoprotein, lp (a), has been regarded as a promising biomarker for cardiovascular disease risk[22]. Lp (a) activates monocytes to cause accumulation of cholesterol in atherosclerotic plaques, inducing migration and proliferation of vascular smooth muscle cells and stimulating the secretion of endothelial adhesion molecules[23]. These pathological changes accelerate subendothelial foam cell formation. lp (a) has an additional thrombogenic action due to its content of apo (a), which competes with plasminogen receptors for binding sites[24]. Oxidation of LDL and LDL receptor 1 results in endothelial dysfunction, accelerating the pathogenesis of atherosclerosis[25]. Lp (a) is a LDL particle with the glycoprotein, apo (a), covalently bound to apo B-100 and has also been implicated in atheroma development due to its association with the oxidizable properties of LDLs[25]. Lp (a) exerts antifibrinolytic effects and increases platelet aggregation around the plaque, contributing to the pathogenesis and development of atherosclerosis[26,27].
Individual lp (a) expression is usually considered to be stable and 90% is determined by genetic phenotype. However, variable expression is observed in specific pathological conditions involving inflammation status such as renal dysfunction and diabetes mellitus[28-30]. Zhang et al[31] reported that lp (a) expression increased two-fold above the normal range in response to severe oxidative stress and it is acknowledged that lp (a) levels respond to changes in the internal environment, especially in inflammatory status. Recent studies have focused on the relationship between lp (a) levels and the mild chronic inflammatory status observed in patients with atherosclerosis[32]. Garrafa et al[33] found significantly increased levels of lp (a) and CRP in older persons, suggesting a relationship between the two biomarkers.
Visit-to-visit lipid profile variability has received increasing clinical attention as an independent risk factor for adverse cardiovascular events[34]. Kim et al[15] demonstrated that follow-up cholesterol variability is an independent predictor for the incidence of cardiovascular events and Zhao et al[35] found that blood lipid variability increased systemic inflammation. However, no previous study has focused on the impact of lp (a) variability on CRP level, acknowledged to be an inflammation indicator, in patients treated with PCI. The current study revealed that mean follow-up CRP was associated with lp (a) variability in post-PCI patients. Multivariate regression analysis confirmed high lp (a) variability as a risk factor for elevated mean follow-up CRP. This study suggests that lp (a) variability is a reliable indicator of inflammatory status during post-PCI follow-up, the knowledge of which may guide clinicians to prevent recurrence of cardiovascular events.
Most subgroups of the current cohort, including age < 65 years or ≥ 65 years, male, diabetes or not, hypertension or not and lp (a) < 30 mg/dL, gave results consistent with the main findings. However, no relationship emerged between lp (a) variability and CRP levels in the lp (a) ≥ 30 mg/dL subgroup. Lipid-lowering drugs, such as statins and ezetimibe, also have anti-inflammatory actions[36]. The lipid-lowering therapy which is the conventional treatment for post-PCI CAD patients with lp (a) > 30 mg/dL, who tend to have higher plasma lipid levels may have the indirect effect of decreasing CRP levels.
This study had some limitations. First, this was a multicenter retrospective observational study and additional prospective studies are required to verify the current findings. Second, other inflammatory markers such as procalcitonin, IL-6, and other cytokines were not measured and may be included in future research. Third, the link between lp (a) variability and CRP levels during follow-up was investigated but major adverse cardiovascular events (MACEs) were not. A future study focusing on the relationship between lp (a) and MACE is planned.
In this multicenter retrospective study, lp (a) variability was significantly associated with mean CRP levels during 1-year follow-up in post-PCI CAD patients. High lp (a) variability was found to be an independent risk factor for increased CRP levels following PCI treatment.
Lipoprotein a [lp (a)] variability is gaining growing attention in cardiovascular disease.
The association between lp (a) variability and follow-up C-reactive protein (CRP) level in patients undergoing percutaneous coronary intervention (PCI) has not been investigated.
To explore the association between lp (a) variability and mean CRP levels within the 1st year post-PCI.
This was a multicenter retrospective study.
Lp (a) variability was positively related to the mean follow-up CRP in patients undergoing PCI.
High variability could be considered an independent risk factor for increased post-PCI CRP level.
Prospective studies are further needed to verify the results of this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan; Moriyama K, Japan S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5243] [Article Influence: 524.3] [Reference Citation Analysis (0)] |
| 2. | Bhatt DL. Percutaneous Coronary Intervention in 2018. JAMA. 2018;319:2127-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Aijaz S, Ahmed N, Akhter Z, Sattar S, Lakhani S, Malik R, Pathan A. Clinical characteristics and in-hospital outcome in percutaneous coronary interventions with ST elevation myocardial infarction patients developing acute kidney injury. J Pak Med Assoc. 2019;69:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Giannini F, Candilio L, Mitomo S, Ruparelia N, Chieffo A, Baldetti L, Ponticelli F, Latib A, Colombo A. A Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2018;11:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O'Neil WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:e166-e286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | de Winter RJ, Heyde GS, Koch KT, Fischer J, van Straalen JP, Bax M, Schotborgh CE, Mulder KJ, Sanders GT, Piek JJ, Tijssen JG. The prognostic value of pre-procedural plasma C-reactive protein in patients undergoing elective coronary angioplasty. Eur Heart J. 2002;23:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Stancel N, Chen CC, Ke LY, Chu CS, Lu J, Sawamura T, Chen CH. Interplay between CRP, Atherogenic LDL, and LOX-1 and Its Potential Role in the Pathogenesis of Atherosclerosis. Clin Chem. 2016;62:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Hulthe J, Wikstrand J, Fagerberg B. Relationship between C-reactive protein and intima-media thickness in the carotid and femoral arteries and to antibodies against oxidized low-density lipoprotein in healthy men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin Sci (Lond). 2001;100:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Chu CS, Wang YC, Lu LS, Walton B, Yilmaz HR, Huang RY, Sawamura T, Dixon RA, Lai WT, Chen CH, Lu J. Electronegative low-density lipoprotein increases C-reactive protein expression in vascular endothelial cells through the LOX-1 receptor. PLoS One. 2013;8:e70533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Itabe H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: from atherosclerosis to periodontitis. J Clin Biochem Nutr. 2012;51:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 524] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6103] [Cited by in RCA: 6340] [Article Influence: 317.0] [Reference Citation Analysis (0)] |
| 14. | Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Kim MK, Han K, Kim HS, Park YM, Kwon HS, Yoon KH, Lee SH. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J. 2017;38:3560-3566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 16. | Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69:692-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 718] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 17. | Jin JL, Zhang HW, Liu HH, Zhu CG, Guo YL, Wu NQ, Xu RX, Dong Q, Li JJ. Lipoprotein(a) and Cardiovascular Outcomes in Patients With Coronary Artery Disease and Different Metabolic Phenotypes. Front Cardiovasc Med. 2022;9:870341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 18. | Lipoprotein(a): another emergent target for PCSK9 inhibitors? Eur Heart J. 2016;37:2966-2967. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 585] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 20. | Page MM, Watts GF. Contemporary perspectives on the genetics and clinical use of lipoprotein(a) in preventive cardiology. Curr Opin Cardiol. 2021;36:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Topçiu-Shufta V, Haxhibeqiri V, Begolli L, Baruti-Gafurri Z, Veseli S, Haxhibeqiri S, Miftari R, Kurti L, Avdiu D. Correlation of Inflammation and Lipoprotein (a) with Hypercoagulability in Hemodialysis Patients. Med Arch. 2015;69:232-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Kolski B, Tsimikas S. Emerging therapeutic agents to lower lipoprotein (a) levels. Curr Opin Lipidol. 2012;23:560-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Kaiser Y, Daghem M, Tzolos E, Meah MN, Doris MK, Moss AJ, Kwiecinski J, Kroon J, Nurmohamed NS, van der Harst P, Adamson PD, Williams MC, Dey D, Newby DE, Stroes ESG, Zheng KH, Dweck MR. Association of Lipoprotein(a) With Atherosclerotic Plaque Progression. J Am Coll Cardiol. 2022;79:223-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 24. | Vasquez N, Joshi PH. Lp(a): Addressing a Target for Cardiovascular Disease Prevention. Curr Cardiol Rep. 2019;21:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Hashimoto K, Akagi M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: A narrative review. J Int Med Res. 2020;48:300060520931609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, Im K, Lira Pineda A, Wasserman SM, Češka R, Ezhov MV, Jukema JW, Jensen HK, Tokgözoğlu SL, Mach F, Huber K, Sever PS, Keech AC, Pedersen TR, Sabatine MS. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 584] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 27. | Patel AP, Wang M, Pirruccello JP, Ellinor PT, Ng K, Kathiresan S, Khera AV. Lp(a) (Lipoprotein[a]) Concentrations and Incident Atherosclerotic Cardiovascular Disease: New Insights From a Large National Biobank. Arterioscler Thromb Vasc Biol. 2021;41:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 28. | Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta. 2006;364:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Lippi G, Braga V, Adami S, Guidi G. Modification of serum apolipoprotein A-I, apolipoprotein B and lipoprotein(a) levels after bisphosphonates-induced acute phase response. Clin Chim Acta. 1998;271:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Kronenberg F, Utermann G, Dieplinger H. Lipoprotein(a) in renal disease. Am J Kidney Dis. 1996;27:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Zhang Q, Ai Y, Dong H, Wang J, Xu L. Circulating Oxidized Low-Density Lipoprotein is a Strong Risk Factor for the Early Stage of Coronary Heart Disease. IUBMB Life. 2019;71:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Ugovšek S, Šebeštjen M. Lipoprotein(a)-The Crossroads of Atherosclerosis, Atherothrombosis and Inflammation. Biomolecules. 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Garrafa E, Casnici N, Squazzoni F, Uberti D, Marengoni A. C-reactive protein, lipoprotein (a) and cystatin C levels increase with multimorbidity in older persons. Eur J Intern Med. 2017;42:e25-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Clark D 3rd, Nicholls SJ, St John J, Elshazly MB, Kapadia SR, Tuzcu EM, Nissen SE, Puri R. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur Heart J. 2018;39:2551-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Zhao L, Xu T, Li Y, Luan Y, Lv Q, Fu G, Zhang W. Variability in blood lipids affects the neutrophil to lymphocyte ratio in patients undergoing elective percutaneous coronary intervention: a retrospective study. Lipids Health Dis. 2020;19:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Tuñón J, Badimón L, Bochaton-Piallat ML, Cariou B, Daemen MJ, Egido J, Evans PC, Hoefer IE, Ketelhuth DFJ, Lutgens E, Matter CM, Monaco C, Steffens S, Stroes E, Vindis C, Weber C, Bäck M. Identifying the anti-inflammatory response to lipid lowering therapy: a position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc Res. 2019;115:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |