Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10467
Peer-review started: May 30, 2022
First decision: August 21, 2022
Revised: August 31, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: October 16, 2022
Processing time: 122 Days and 3.7 Hours
Decompensated liver cirrhosis (DLC) is a stage in the progression of liver cirrhosis and has a high mortality.
To establish and validate a novel and simple-to-use predictive nomogram for evaluating the prognosis of DLC patients.
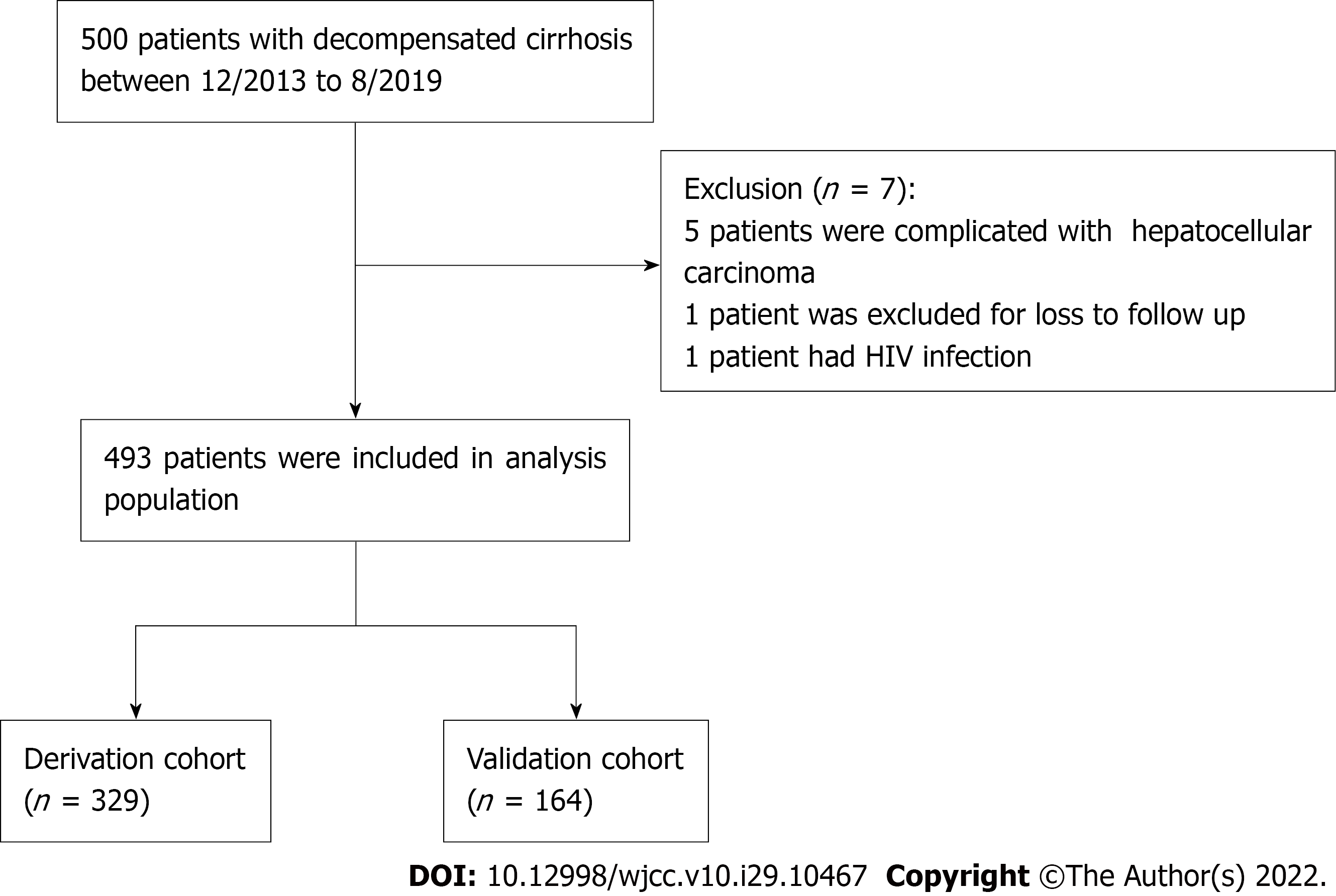
A total of 493 patients with confirmed DLC were enrolled from The First Affiliated Hospital of Nanchang University (Nanchang, Jiangxi Province, China) between December 2013 and August 2019. The patients were divided into two groups: a derivation group (n = 329) and a validation group (n = 164). Univariate and multivariate Cox regression analyses were performed to assess prognostic factors. The performance of the nomogram was determined by its calibration, discrimination, and clinical usefulness.
Age, mechanical ventilation application, model for end-stage liver disease (MELD) score, mean arterial blood pressure, and arterial oxygen partial pressure/inhaled oxygen concentration were used to construct the model. The C-indexes of the nomogram in the derivation and validation groups were 0.780 (95%CI: 0.670-0.889) and 0.792 (95%CI: 0.698-0.886), respectively. The calibration curve exhibited good consistency with the actual observation curve in both sets. In addition, decision curve analysis indicated that our nomogram was useful in clinical practice.
A simple-to-use novel nomogram based on a large Asian cohort was established and validated and exhibited improved performance compared with the Child-Turcotte-Pugh and MELD scores. For patients with DLC, the proposed nomogram may be helpful in guiding clinicians in treatment allocation and may assist in prognosis prediction.
Core Tip: The overall survival of decompensated liver cirrhosis (DLC) has been one of the main concerns of patients and clinicians. In this research, we established a simple and effective nomogram including age, application of mechanical ventilation, model for end-stage liver disease (MELD) score, mean arterial blood pressure and arterial oxygen partial pressure/inhaled oxygen concentration. This nomogram had better prognostic value than the Child-Turcotte-Pugh and MELD scores. Moreover, the nomogram was validated in the internal cohort, and it may be helpful in guiding clinicians in treatment allocation and in predicting the prognosis of DLC.
- Citation: Zhang W, Zhang Y, Liu Q, Nie Y, Zhu X. Development and validation of a prognostic nomogram for decompensated liver cirrhosis. World J Clin Cases 2022; 10(29): 10467-10477
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10467.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10467
Decompensated cirrhosis (DLC) is characterized by the development of cirrhosis-related complications, such as ascites, gastroesophageal variceal bleeding, hepatic encephalopathy (HE), and severe jaundice[1]. Less than 1% of patients with acute DLC do not have any evidence of systemic inflammation[2]. Patients with DLC have a dismal prognosis, with a median survival of approximately 2 years[3]. Variceal bleeding is one of the most feared complications in patients with cirrhosis due to its deleterious impact on prognosis. Severe hemorrhage may cause ischemic hepatitis, which causes the release of inflammatory stimuli and may precipitate acute-on-chronic liver failure (ACLF)[4]. The course of DLC is often abruptly accelerated by the development of ACLF, which has a high short-term mortality[5]. Therefore, early recognition and timely treatment are significantly important to improving survival in DLC patients. A previous study confirmed that the Child-Turcotte-Pugh (CTP) score and model for end-stage liver disease (MELD) score were predictors of mortality in DLC patients[6,7]; However, many studies have revealed the poor predictive performance of these two scores when compared with other scores or biomarkers[8,9].
Nomograms are easy to use and can facilitate management-related decision making. Nomograms are widely applied as statistical prognostic models in medicine and could offer more individualized and accurate predictions than other models for numerous illnesses[10,11]. The purpose of this study was to determine the risk factors for short-term death from DLC and to construct a clinically useful nomogram to estimate individual prognosis.
Patients were consecutively recruited from the Department of Gastroenterology, the First Affiliated Hospital of Nanchang University (Nanchang, Jiangxi Province, China), between December 2013 and August 2019. The inclusion criteria were as follows: (1) Age ≥ 18 years; (2) diagnosis of cirrhosis (on account of previous hepatic pathology or clinical signs, imaging examinations, or laboratory and endoscopic presentation results); and (3) presence of acute decompensated events, including HE, gastroesophageal varices hemorrhage, development of large ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, and any combination of these. Patients with the following were excluded: (1) Primary liver carcinoma or other malignant tumors; (2) concurrent pregnancy; (3) infection with human immunodeficiency virus; (4) liver operations such as liver transplantation; and (5) loss to follow-up. The Ethics Committee of The First Affiliated Hospital of Nanchang University approved this study protocol (No. 2013-1202). Three hundred twenty-nine patients who were enrolled between December 2013 and September 2016 were assigned to the derivation cohort, and the other 164 patients were assigned to the validation cohort between October 2016 and August 2019 (approximate ratio of 2:1). This study follows the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis reporting guidelines for the development and validation of prediction models (Supple
Demographic information, laboratory tests, and clinical features were collected from electronic medical records. All laboratory tests were performed within 24 h after admission. The primary outcome was defined as death at the 28-d, 3-mo and 6-mo follow-up durations. The CTP score was computed according to ascites, prothrombin time (PT), serum bilirubin, albumin, and HE[6]. The MELD score was calculated as follows: MELD =9.6 × ln[serum creatinine (mg/dL)] + 3.8 × ln[total bilirubin (mg/dL)] + 11.2 × ln[PT (international normalized ratio, INR)] + 6.43 × (etiology: 0 if cholestatic or alcoholic, 1 otherwise)[7].
For all patients, after the diagnosis of DLC was determined, standard medical therapies were used, such as endoscopic hemostasis therapy, antiviral treatment, liver-protecting therapy, albumin and plasma transfusion and prevention of complications such as HE and SBP.
Statistical analyses were performed with Statistical Product and Service Solutions (SPSS) software version 24.0 (SPSS Inc., Chicago, IL, United States). Continuous variables are expressed as the mean ± SD and were compared using an unpaired, 2-tailed t test or the Mann-Whitney test. Categorical variables were compared using the chi-squared test or Fisher’s exact test. Univariate risk factors that reached P < 0.2 were subjected to Cox regression analysis. The Age, mechanical ventilation application, MELD score, mean arterial blood pressure (MAP) and arterial oxygen partial pressure (PaO2)/inhaled oxygen concentration (FiO2) were used to construct the model. The final Cox regression model for fitness of the data and proportional hazards assumption was checked by the Schoenfeld residual test (the global test). The test revealed that the assumption was met with a P-value of the global test = 0.28.
The nomogram based on the results of previous Cox regression analyses was established using R version 3.3.2 (http://www.r-project.org/). The discrimination of the models was assessed according to the concordance index (C-index); the larger the C-index was, the more accurate the prognostic ability of the nomogram. The net reclassification index (NRI) quantifies the improvement in the predictive accuracy of the nomogram compared with the CTP and MELD scores. The integrated discrimination improvement index (IDI) evaluates different tangent lines that can be used to assess the overall improvement of the model and was calculated to assess the improvement of the nomogram compared with the CTP and MELD scores. In addition, NRI > 0 indicates that the new model is more accurate than the old model for prediction. The same is true for the IDI. Calibration curves were also drawn to evaluate the concordance between the predicted and observed probabilities. Decision curve analysis (DCA) was conducted to assess the clinical usefulness of the predictive nomogram by quantifying the net benefits at different threshold probabilities in both cohorts. In the present study, the R packages used included survival, survminer, rms, nricens, predictABEL, Hmisc, devtools, and ggDCA. All tests were two-sided, and differences were considered statistically significant when P < 0.05.
In total, 329 and 164 patients were included for analysis in the derivation and internal validation cohorts, respectively, and a flow diagram detailing the screening process is shown in Figure 1. The baseline characteristics of both cohorts are shown in Table 1. In the derivation and internal validation cohorts, the age ranges were 21-86 years (mean ± SD: 53.76 ± 11.64 years) and 31-91 years (mean ± SD: 57.38 ± 11.89 years), respectively. In the derivation and internal validation sets, the patients were predominantly men (253/329, 76.9% vs 116/164, 70.7%), the main etiology was hepatitis B virus (HBV) in 66.9% and 58.5% of patients, respectively, and the primary hospitalization reason was variceal bleeding (45.60% and 46.34%, respectively). When comparing the demographic and clinical characteristics between the derivation and validation sets, we found that the derivation set had shorter hospital stays (8.40 ± 5.88) and fewer patients with vasopressor use (88/329, 26.75%) and mechanical ventilation (MV) application (18/329, 5.47%) (P < 0.05). Moreover, the validation set had higher alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatinine, and INR levels and higher CTP and MELD scores (P < 0.05). The validation set had higher 28-d, 3-mo and 6-mo risks of death than the derivation set (P < 0.05).
| Derivation cohort (n = 329) | Internal validation cohort (n = 164) | P | |
| Age (yr), mean ± SD | 53.76 ± 11.64 | 57.38 ± 11.89 | 0.001a |
| Male sex, n (%) | 253 (76.90) | 116 (70.70) | 0.137 |
| Hospitalization period (d), mean ± SD | 8.40 ± 5.88 | 9.49 ± 4.69 | 0.026a |
| Liver cirrhosis etiology, n (%) | |||
| HBV | 220 (66.90) | 96 (58.50) | 0.07 |
| HCV | 13 (3.95) | 6 (3.66) | 0.87 |
| Alcoholic liver disease | 52 (15.80) | 30 (18.29) | 0.49 |
| Schistosomiasis cirrhosis | 14 (4.25) | 8 (4.88) | 0.75 |
| Primary biliary cirrhosis | 3 (0.90) | 5 (3.05) | 0.08 |
| Cryptogenic cirrhosis | 27 (8.21) | 18 (11.00) | 0.31 |
| Hospitalization reason, n (%) | |||
| Variceal bleeding | 150 (45.60) | 76 (46.34) | 0.875 |
| Hepatic encephalopathy | 86 (26.14) | 43 (26.22) | 0.985 |
| Infection | 45 (13.68) | 21 (12.80) | 0.789 |
| Ascites | 28 (8.50) | 13 (7.93) | 0.825 |
| Hepatorenal syndrome | 20 (6.08) | 11 (6.71) | 0.787 |
| Vasopressors | 88 (26.75) | 79 (48.17) | < 0.001a |
| HBeAg (+) | 35 (10.64) | 15 (9.15) | 0.605 |
| Mechanical ventilation | 18 (5.47) | 36 (21.95) | < 0.001a |
| Biochemical parameters | |||
| ALT, IU/L | 22.00 (16.00-40.75) | 29.00 (20.00-52.50) | < 0.001a |
| AST, IU/L | 44.00 (31.00-90.50) | 39.00 (28.00-74.00) | 0.085 |
| GGT, IU/L | 27.00 (15.00-74.50) | 29.00 (14.00-79.50) | 0.982 |
| ALP, IU/L | 73.70 (55.00-112.00) | 82.50 (56.25-138.75) | 0.037a |
| Serum sodium, mmol/L | 138.10 (135.00-141.00) | 137.85 (134.03-140.98) | 0.329 |
| Creatinine, μmol/L | 72.70 (59.35-93.20) | 79.70 (63.55-115.00) | 0.006a |
| INR | 1.32 (1.21-1.54) | 1.45 (1.24-1.74) | < 0.001a |
| Bilirubin, μmol/L | 24.70 (15.80-42.80) | 26.85 (16.03-58.25) | 0.157 |
| WBC, 10 × 9/L | 6.39 (3.94-9.61) | 6.81 (4.11-11.13) | 0.11 |
| Platelet, 10 × 9/L | 74.50 (47.50-111.50) | 73.80 (39.00-109.50) | 0.245 |
| PT, s | 15.00 (13.80-17.20) | 15.90 (13.40-19.20) | 0.086 |
| MAP, mmHg | 83.00 (77.67-89.33) | 82.17 (78.00-87.67) | 0.472 |
| PO2/FiO2 | 402.86 ± 118.57 | 388.28 ± 137.36 | 0.266 |
| Albumin, g/L | 28.41 ± 7.87 | 27.64 ± 6.18 | 0.256 |
| CTP score | 8 (7-10) | 9 (7-11) | 0.001a |
| MELD score | 9.44 (5.96-13.52) | 12.20 (7.77-16.67) | < 0.001a |
| Mortality, n (%) | |||
| 28 d | 41 (12.46) | 47 (28.66) | < 0.001a |
| 3 mo | 65 (19.76) | 69 (42.07) | < 0.001a |
| 6 mo | 75 (22.80) | 73 (44.51) | < 0.001a |
Univariate analysis showed that age, use of vasopressors, MV application, ALT, aspartate aminotransferase, γ-glutamyl transpeptidase, ALP, white blood cell count, MAP, PaO2/FiO2, CTP score and MELD score were significantly associated with the prognosis of DLC patients in the derivation set (P < 0.05, Table 2). Variables with significant differences by univariate analysis were subjected to multivariate Cox regression analyses. The results showed that only age, MV application and MELD score were inde
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.021 (1.001-1.040) | 0.037a | 1.028 (1.007-1.050) | 0.009a |
| Male sex | 1.133 (0.653-1.967) | 0.657 | ||
| Vasopressors | ||||
| No | Reference | |||
| Yes | 1.643 (1.027-2.628) | 0.038a | ||
| HBeAg | ||||
| negative | Reference | |||
| positive | 0.692 (0.301-1.594) | 0.387 | ||
| Mechanical ventilation | ||||
| No | Reference | |||
| Yes | 9.916 (5.486-17.922) | < 0.001a | 4.200 (1.975-8.932) | < 0.001a |
| ALT | 1.001 (1.000-1.002) | < 0.001a | ||
| AST | 1.001 (1.000-1.001) | < 0.001a | ||
| GGT | 1.001 (1.000-1.002) | 0.090a | ||
| ALP | 1.002 (1.001-1.003) | < 0.001a | ||
| Serum sodium | 1.003 (0.982-1.026) | 0.761 | ||
| WBC | 1.052 (1.030-1.073) | < 0.001a | ||
| Platelet | 1.002 (0.999-1.004) | 0.259 | ||
| MAP | 0.976 (0.967-0.996) | 0.019a | 0.979 (0.960-0.998) | 0.031a |
| PO2/FiO2 | 0.996 (0.994-0.998) | < 0.001a | 0.998 (0.995-1.000) | 0.038a |
| CTP score | 1.332 (1.214-1.462) | < 0.001a | ||
| MELD score | 1.142 (1.107-1.178) | < 0.001a | 1.080 (1.030-1.132) | 0.002a |
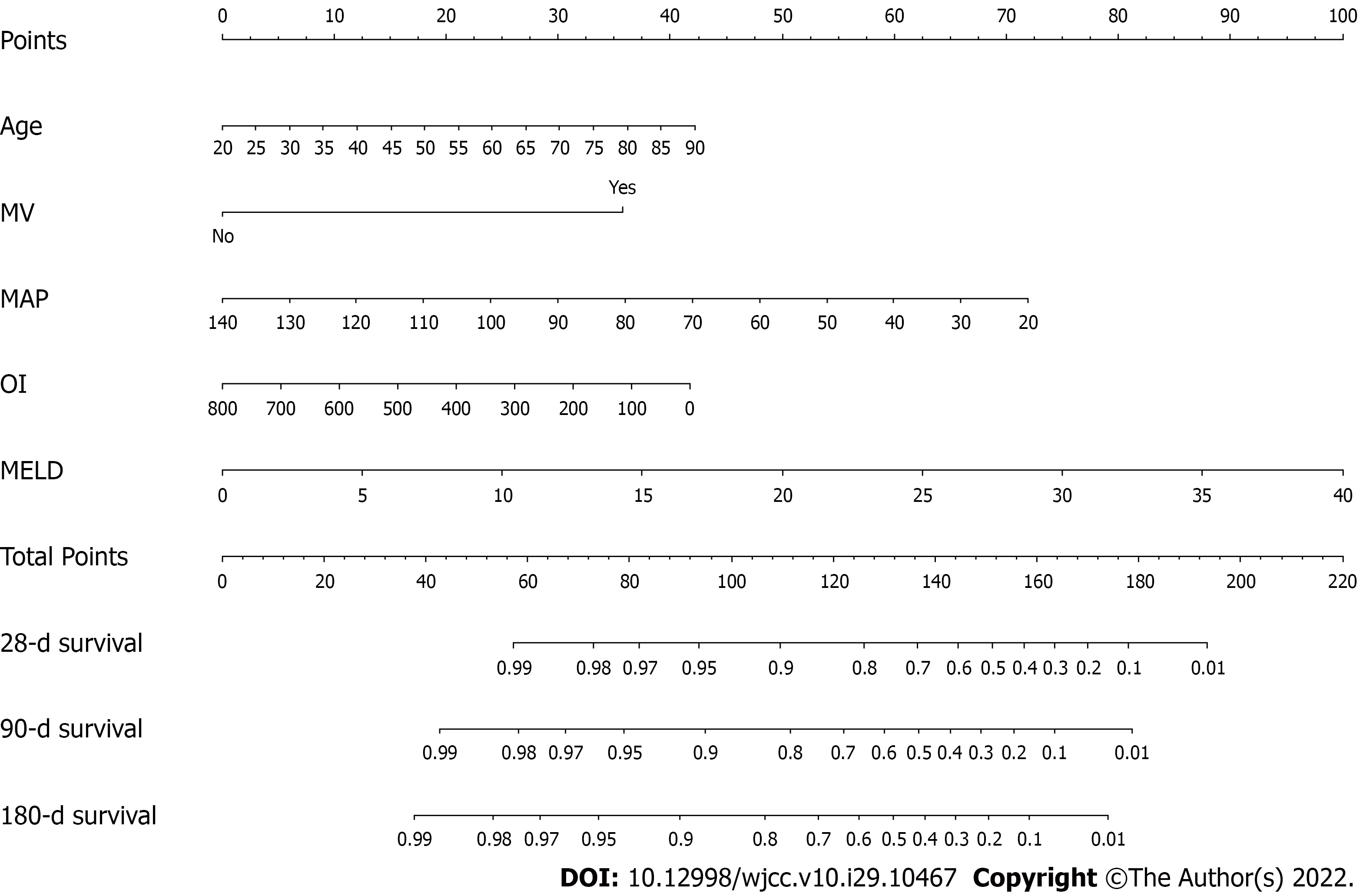
Based on the results of multivariable Cox regression analyses, a nomogram including age, MV application, MAP, oxygenation index (OI; PaO2/FiO2) and MELD score was established to predict 28-d, 3-mo and 6-mo mortality (Figure 2). By assigning a weighted score to each of the independent prognostic parameters and using that value in the total point scale axis, the total score could be easily computed to assign the probability of survival for individual patients. For instance, a DLC patient aged 45 (15 points) with MV application (36 points), a MAP of 100 (25 points), an OI of 400 (21 points), and a MELD score of 15 (37.5 points) had a total of 134.5 points for a predicted 28-d, 3-mo, and 6-mo survival probability of 72%, 55% and 48%, respectively.
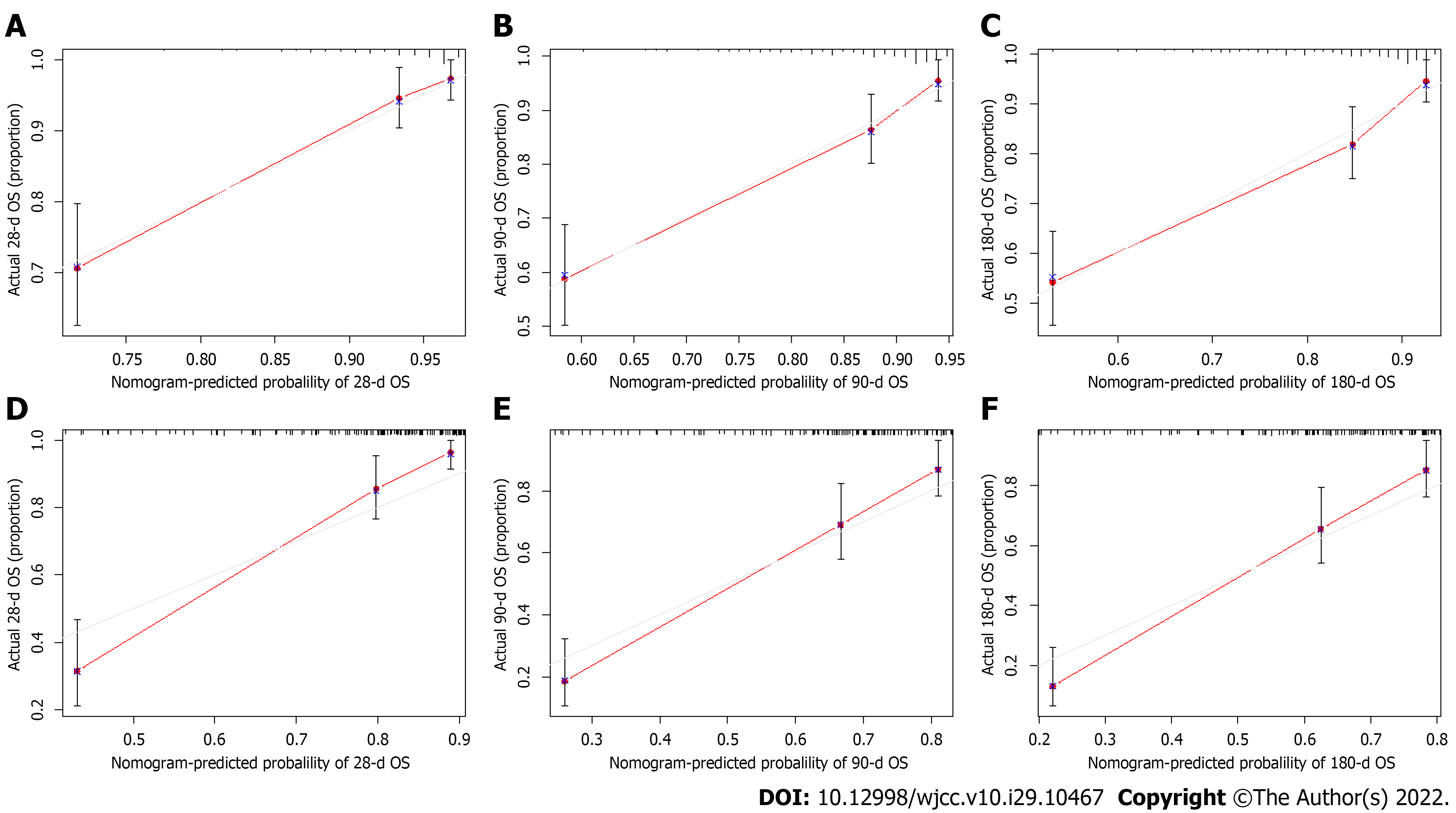
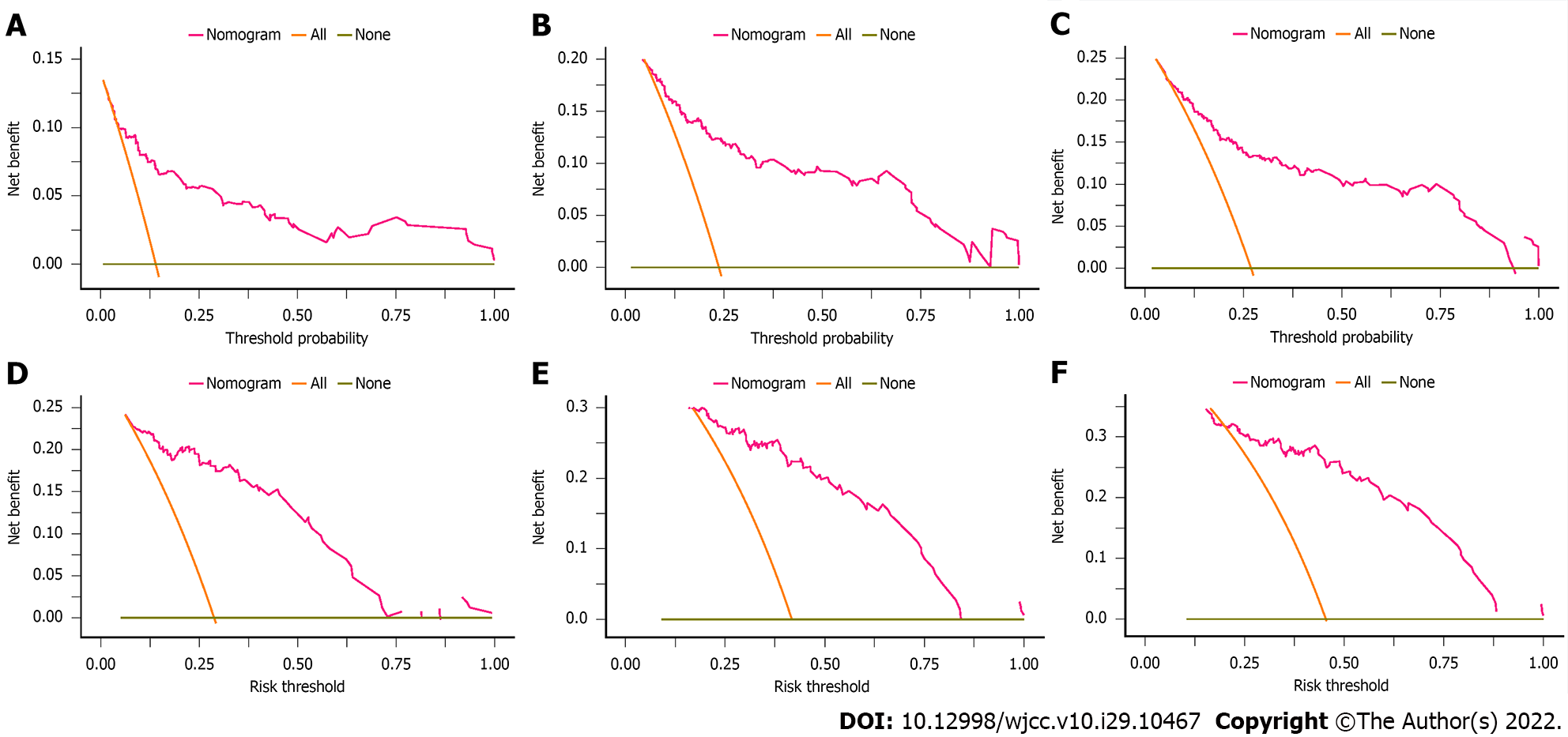
We assessed the discriminative ability of our final model by using the C-index, and the C-index of the nomogram was 0.780 (95%CI: 0.670-0.889) in the derivation cohort, showing superior predictive performance to that of the CTP score (0.708, 95%CI: 0.596-0.820) or the MELD score (0.735, 95%CI: 0.609-0.860). The NRI and IDI were also determined to compare the efficiency between the nomogram and the two scores (Table 3). In the derivation cohort, when compared with the CTP score, the NRI values for 28-d, 3-mo, and 6-mo mortality were 0.419 (95%CI: 0.192-0.606), 0.355 (95%CI: 0.134-0.488) and 0.319 (95%CI: 0.081-0.481), respectively, and the IDI values for 28-d, 3-mo, and 6-mo mortality were 0.262 (95%CI: 0.181-0.394), 0.193 (95%CI: 0.118-0.285) and 0.178 (95%CI: 0.098-0.270), respectively. When compared with the MELD score, the NRI values for 28-d, 3-mo, and 6-mo mortality were 0.395 (95%CI: 0.214-0.548), 0.305 (95%CI: 0.080-0.417) and 0.281 (95%CI: 0.077-0.402), respectively, and the IDI values for 28-d, 3-mo, and 6-mo mortality were 0.133 (95%CI: 0.070-0.247), 0.081 (95%CI: 0.026-0.151) and 0.064 (95%CI: 0.023-0.133), respectively. The calibration curve of the nomogram is shown in Figure 3A-C, and the calibration plot revealed an adequate fit of the nomogram for predicting the risk of death. As shown in Figure 4A-C, the DCA showed that across the entire range of threshold probability, using the nomogram model to predict 28-d, 3-mo, and 6-mo survival adds more net benefit in clinical decision making.
| Index | Derivation cohort | Validation cohort | ||||
| Estimate | 95%CI | P value | Estimate | 95%CI | P value | |
| NRI versus CTP | ||||||
| For 28-d mortality | 0.419 | 0.192-0.606 | < 0.001a | 0.395 | 0.061-0.662 | 0.020a |
| For 3-mo mortality | 0.355 | 0.134-0.488 | 0.007a | 0.241 | 0.005-0.490 | 0.040a |
| For 6-mo mortality | 0.319 | 0.081-0.481 | < 0.001a | 0.227 | 0.120-0.480 | < 0.001a |
| NRI vs MELD | ||||||
| For 28-d mortality | 0.395 | 0.214-0.548 | < 0.001a | 0.578 | 0.364-0.740 | < 0.001a |
| For 3-mo mortality | 0.305 | 0.080-0.417 | 0.013a | 0.388 | 0.233-0.547 | < 0.001a |
| For 6-mo mortality | 0.281 | 0.077-0.402 | 0.007a | 0.358 | 0.249-0.527 | < 0.001a |
| IDI vs CTP | ||||||
| For 28-d mortality | 0.262 | 0.181-0.394 | < 0.001a | 0.253 | 0.095-0.426 | < 0.001a |
| For 3-mo mortality | 0.193 | 0.118-0.285 | < 0.001a | 0.167 | 0.040-0.308 | 0.020a |
| For 6-mo mortality | 0.178 | 0.098-0.270 | < 0.001a | 0.152 | 0.013-0.294 | < 0.001a |
| IDI vs MELD | ||||||
| For 28-d mortality | 0.133 | 0.070-0.247 | < 0.001a | 0.322 | 0.195-0.473 | < 0.001a |
| For 3-mo mortality | 0.081 | 0.026-0.151 | < 0.001a | 0.220 | 0.122-0.343 | < 0.001a |
| For 6-mo mortality | 0.064 | 0.023-0.133 | < 0.001a | 0.212 | 0.132-0.298 | < 0.001a |
The established model was internally validated by using the bootstrap validation method. When the established nomogram was used in the validation set, the C-index of the nomogram was 0.792 (95%CI: 0.698-0.886), with better predictive value than the CTP score (0.714, 95%CI: 0.602-0.826) and the MELD score (0.695, 95%CI: 0.575-0.815), indicating that the nomogram is suitable for estimating the outcome of DLC patients. In addition, the NRI and IDI values of the validation set also indicated that the present nomogram had improved accuracy compared to the CTP and MELD scores for predicting mortality (Table 3). Furthermore, the promising performance of the nomogram was also identified by calibration curve and decision curve analyses (Figures 3D-F and 4D-F).
In the present study, an easy-to-use novel nomogram was constructed to assess the prognosis of DLC patients. The proposed nomogram was derived from clinical and laboratory data to improve its clinical generality and applicability. This nomogram had superior predictive ability and clinical usefulness compared to the current prognostic score models, including the CTP and MELD scores. In addition, we adopted the internal validation cohort to reduce the influence of patients of different ages and other factors on the results.
In our derivation and validation cohorts, HBV was the predominant etiology among the DLC patients, which was in line with that in a previous study[12], indicating that HBV infection is a critical global health threat, particularly in Asia. Furthermore, the results showed that 150 (45.60%) and 76 (46.34%) patients were hospitalized for variceal bleeding in the derivation and validation sets, respectively. Variceal hemorrhage is a severe complication of portal hypertension in patients with cirrhosis and is associated with significant mortality[13]. Patients with variceal bleeding may develop other complications of cirrhosis and are at increased risk of developing circulatory dysfunction and bacterial infections that may lead to ACLF[4], which has high short-term mortality.
The CTP and MELD scores are commonly used to predict adverse outcomes in DLC patients. However, CTP is a subjective variable, including the presence or absence of HE and ascites[6]. The MELD score contains three indicators, namely, creatinine, INR, and bilirubin, but it lacks indicators of organ failure and may have a lower prognostication accuracy[14]. The proposed nomogram included age, MV application, MELD score, MAP, and OI. A previous study indicated that age was closely related to patient prognosis and that old age was a crucial factor for poor prognosis in cirrhosis[15]. MV is required for patients who develop acute respiratory failure, and it was identified as an independent factor related to intensive care unit (ICU) mortality in cirrhotic patients or patients with liver cirrhosis[16]. The results of a prospective cohort study that enrolled 592 cirrhotic patients admitted to the ICU revealed that patients who required MV had a poor prognosis, which was associated with organ failure[17]. The MELD score is based on three readily available biochemical variables: creatinine, serum bilirubin, and INR. It has been confirmed as a highly reliable predictor of short- and mid-term mortality in patients with cirrhosis awaiting liver transplantation[18]. However, the MELD score does not consider other conditions that are related to poor prognosis, such as organ failure, especially in critically ill patients. MAP is a reliable marker of circulatory function in ICU patients, particularly in patients with hemodynamic instability[19]. In this setting, circulatory dysfunction is the key feature of DLC[20]. A previous study showed that higher MAP values were related to acute kidney injury recovery in patients with DLC[21]. Patidar et al[22] evaluated the associations between MAP and poor ICU outcomes in critically ill cirrhotic patients and found that the risk of mortality intensely increased in patients whose MAP was below a threshold of 65 mmHg. The OI is PaO2 divided by FiO2 and is widely to assess acute respiratory distress syndrome (ARDS); the normal range is 400-500 mmHg[23]. Campbell et al[24] suggested that the OI is an independent predictor of ICU mortality in patients with cirrhosis and validated prognostic scoring tools for predicting ICU mortality.
In fact, the developed nomogram had superior discrimination power (C-index of 0.780) compared with using the CTP or MELD score alone. Moreover, a slightly higher C-index (0.792) was observed while performing internal validation in another set of cirrhotic patients. Based on the calibration plots, good calibration was observed. As indicated by the positive NRI and IDI, this nomogram is more accurate in predicting mortality than the CTP and MELD scores. In addition, according to DCA, the nomogram provided better clinical benefits than the conventional scores. Overall, the rationale of the selected variables was confirmed.
Nevertheless, our study has several limitations. First, this was a retrospective study; therefore, some potential bias may exist. Second, patients were enrolled from a single center. Third, the model only underwent internal validation. In the future, a prospective study is needed to validate the sensitivity and specificity of the nomogram.
In summary, we developed and validated a nomogram for predicting the survival probability of DLC patients. This simple nomogram had an adequate calibration and discrimination ability and good clinical utility. This nomogram may be helpful for promoting the early diagnosis and prevention of DLC.
Decompensated liver cirrhosis (DLC) has high mortality, and there are some limitations when applying the common prognostic scores. Nomograms are widely used as prognostic models for many diseases.
Due to the worse prognosis, the overall survival of DLC has attracted much attention from clinicians. Thus, it is necessary to develop a prognostic model to evaluate the outcome of DLC patients.
This study aimed to develop and validate a novel and simple-to-use prognostic nomogram to assess the prognosis of DLC patients.
A total of 493 DLC patients were included in this study and divided into a derivation group (n = 329) and a validation group (n = 164). According to the results of univariate and multivariate Cox regression analyses, a nomogram model was developed to predict the prognosis of DLC.
The nomogram was developed based on age, mechanical ventilation application, model for end-stage liver disease (MELD) score, mean arterial blood pressure and PaO2/FiO2. The C-indexes, calibration curves and decision curve analysis revealed that the nomogram model is a valid tool.
We constructed a nomogram model that could accurately predict the prognosis of DLC patients and showed better prognostic performance than the CTP and MELD scores.
This research established a nomogram that could predict prognosis in DLC patients. In addition, the nomogram was precisely evaluated by internal validation, which may be helpful to clinicians in clinical decision making.
We would like to thanks the National Natural Science Foundation of China (grant number: 81960120), the “Gan-Po Talent 555” Project of Jiangxi Province (GCZ (2012)-1) and the Jiangxi Clinical Research Center for Gastroenterology (20201ZDG02007).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT, Brazil; Song MJ, South Korea S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2109] [Article Influence: 111.0] [Reference Citation Analysis (2)] |
| 2. | Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 275] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 3. | Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2018;15:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 417] [Article Influence: 83.4] [Reference Citation Analysis (2)] |
| 5. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2144] [Article Influence: 178.7] [Reference Citation Analysis (5)] |
| 6. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5709] [Article Influence: 109.8] [Reference Citation Analysis (2)] |
| 7. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3659] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 8. | Li FC, Fan YC, Li YK, Wang K. Plasma diamine oxidase level predicts 6-month readmission for patients with hepatitis B virus-related decompensated cirrhosis. Virol J. 2019;16:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yao Y, Yang D, Huang Y, Dong M. Predictive value of insulin-like growth factor 1-Child-Turcotte-Pugh score for mortality in patients with decompensated cirrhosis. Clin Chim Acta. 2020;505:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Touijer K, Scardino PT. Nomograms for staging, prognosis, and predicting treatment outcomes. Cancer. 2009;115:3107-3111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. 2006;24:3819-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 290] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 13. | Baiges A, Hernández-Gea V, Bosch J. Pharmacologic prevention of variceal bleeding and rebleeding. Hepatol Int. 2018;12:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Baldin C, Piedade J, Guimarães L, Victor L, Duarte J, Veiga Z, Alcântara C, Fernandes F, Pereira JL, Pereira G. CLIF-C AD Score Predicts Development of Acute Decompensations and Survival in Hospitalized Cirrhotic Patients. Dig Dis Sci. 2021;66:4525-4535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Schiødt FV, Chung RT, Schilsky ML, Hay JE, Christensen E, Lee WM; Acute Liver Failure Study Group. Outcome of acute liver failure in the elderly. Liver Transpl. 2009;15:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Levesque E, Hoti E, Azoulay D, Ichaï P, Habouchi H, Castaing D, Samuel D, Saliba F. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Levesque E, Saliba F, Ichaï P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1217] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 19. | Cesari M, Frigo AC, Tonon M, Angeli P. Cardiovascular predictors of death in patients with cirrhosis. Hepatology. 2018;68:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1021] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 21. | Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Patidar KR, Peng JL, Pike F, Orman ES, Glick M, Kettler CD, Nephew LD, Desai AP, Nair K, Khan BA, Buckley CA, Machado RF, Chalasani NP, Ghabril MS. Associations Between Mean Arterial Pressure and Poor ICU Outcomes in Critically Ill Patients With Cirrhosis: Is 65 The Sweet Spot? Crit Care Med. 2020;48:e753-e760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 23. | Bein T, Grasso S, Moerer O, Quintel M, Guerin C, Deja M, Brondani A, Mehta S. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016;42:699-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Campbell J, McPeake J, Shaw M, Puxty A, Forrest E, Soulsby C, Emerson P, Thomson SJ, Rahman TM, Quasim T, Kinsella J. Validation and analysis of prognostic scoring systems for critically ill patients with cirrhosis admitted to ICU. Crit Care. 2015;19:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |