Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10451
Peer-review started: May 22, 2022
First decision: July 14, 2022
Revised: July 21, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 129 Days and 19 Hours
The clinicopathological features and prognosis of gastric signet ring cell carci
To compare the pathological features of GSRC with those of gastric adenocarcinoma of different degrees of differentiation and the differences in survival prognosis between the different disease processes.
By screening gastric cancer patients from 2010 to 2015 in the database of Surveillance, Epidemiology and End Results, and collecting the clinicopathological and prognostic data of gastric cancer patients who underwent surgery from January 2014 to December 2016 in the Second Affiliated Hospital of Nanchang University, we analyzed the general pathological characteristics of GSRC by the chi-square test. Univariate and multivariate analyses were con
Analysis of pathological features revealed that signet ring-cell carcinoma (SRC) was more frequently seen in younger (< 60 years), female, and White patients compared to non-SRC patients. SRC was less commonly associated with early gastric cancer (EGC) (23.60% vs 39.10%), lower N0 (38.61% vs 61.03%), and larger tumour sizes > 5 cm (31.15% vs 27.10%) compared to the differentiated type, while the opposite was true compared to the undifferentiated type. Survival prognostic analysis found no significant difference in the prognosis of SRC patients among EGC patients. In contrast, among advanced gastric cancer (AGC) patients, the prognosis of SRC patients was correlated with age, race, tumour size, AJCC stage, T-stage, and postoperative adjuvant therapy. The predictive model showed that the 3-year AUC was 0.787, 5-year AUC was 0.806, and C-index was 0.766. Compared to non-SRC patients, patients with SRC had a better prognosis in EGC [hazard ratio (HR): 0.626, 95% confidence interval (CI): 0.427-0.919, P < 0.05] and a worse prognosis in AGC (HR: 1.139, 95%CI: 1.030-1.258, P < 0.05). When non-SRC was divided into differentiated and undifferentiated types for comparison, it was found that in EGC, SRC had a better prognosis than differentiated and undifferentiated types, while there was no significant difference between differentiated and undifferentiated types. In AGC, there was no significant difference in prognosis between SRC and undifferentiated types, both of which were worse than differentiated types. A prognostic analysis of postoperative adjuvant therapy for SRC in patients with AGC revealed that adjuvant postoperative radiotherapy or chemotherapy significantly improved patient survival (34.6% and 36.2% vs 18.6%, P < 0.05).
The prognosis of SRC is better than that of undifferentiated type, especially in EGC, and its prognosis is even better than that of differentiated type. SRC patients can benefit from early detection, surgical resection, and aggressive adjuvant therapy.
Core Tip: This observational study analysed the clinicopathological features and prognosis of gastric signet ring cell carcinoma (GSRC). We compared GSRC with differentiated gastric adenocarcinoma and found that GSRC had unique clinicopathological features, was more common in younger female patients, and was more aggressive, showing higher lymph node metastasis and tumour size. However, the prognosis of early GSRC was relatively good, even better than that of differentiated adenocarcinoma. GSRC should be diagnosed early, and radical surgical resection with adjuvant radiotherapy and chemotherapy can significantly improve the survival rate of patients, though it still needs more clinical data to verify.
- Citation: Tian HK, Zhang Z, Ning ZK, Liu J, Liu ZT, Huang HY, Zong Z, Li H. Clinicopathological characteristics and prognosis of gastric signet ring cell carcinoma. World J Clin Cases 2022; 10(29): 10451-10466
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10451.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10451
Gastric cancer, a kind of extremely common malignancy, ranked fifth in morbidity and fourth in mortality worldwide[1,2]. In accordance with the classification of the World Health Organization, gastric adenocarcinoma could be roughly divided into four histological types, mucinous adenocarcinoma, tubular adenocarcinoma, papillary adenocarcinoma, and signet ring cell carcinoma (SRC)[3]. In addition, it is divided into undifferentiated, poorly-differentiated, moderately-differentiated, and well-differentiated types depending on the degree of differentiation[4]. Gastric SRC (GSRC) is considered a special type of gastric adenocarcinoma and characterized by the accumulation of mucin in the cytoplasm and the displacement of the nucleus to the periphery of the cells[5]. GSRC is divided into diffuse type in the light of Lauren classification[6], infiltrating type by Ming staging[7], and undifferentiated by Nakamura staging[8].
GSRC is considered to be the histological type with the worst prognosis because of the low survival rate and high recurrence rate. However, with further research on GSRC, we found that it has unique clinicopathological characteristics and prognosis. It has been demonstrated that GSRC was highly aggressive, and once found, most of it had been in progressive stage with lymph node metastasis[9]. Other studies also showed that the histological characteristics of GSRC were weak cohesion, and early tumor cells mainly spread in the mucosa or submucosa[10]. Moreover, the survival prognosis and treatment options for GSRC were controversial. Early Western studies have shown that GSRC or diffuse gastric cancer (DGC) had a poor prognosis[11]. However, scholars in Asian had expressed doubts and believed that research should be carried out according to the different processes of tumors. Studies have showed that the prognosis of early SRC is good, while the prognosis of advanced stage SRC is poor[9,12]. Most recently, abundant evidence in the United States suggested that GSRC was not necessarily a risk factor affecting prognosis[13]. Combination of postoperative adjuvant radiotherapy, chemotherapy, and targeted therapy had an excellent effect in improving the survival rate of gastric cancer patients. However, a number of studies and retrospective analyses have shown that GSRC was resistant to chemotherapy, and patients could not benefit from postoperative adjuvant treatment[14]. For this conclusion, more research is needed to confirm.
In order to more accurately study the pathological features and survival prognosis of GSRC, the pathological features and prognosis of a large number of postoperative gastric cancer patients need to be analysed and compared with non-GSRC according to different tumour progression and different tumour differentiation types. Therefore, we explored the differences in pathological features and survival prognosis between GSRC and different differentiation types of gastric adenocarcinoma by analysing information related to pathological features and survival prognosis of surgically resected specimens from a large United States National Registry database [Surveillance, Epidemiology and End Results (SEER) database] and the Second Affiliated Hospital of Nanchang University.
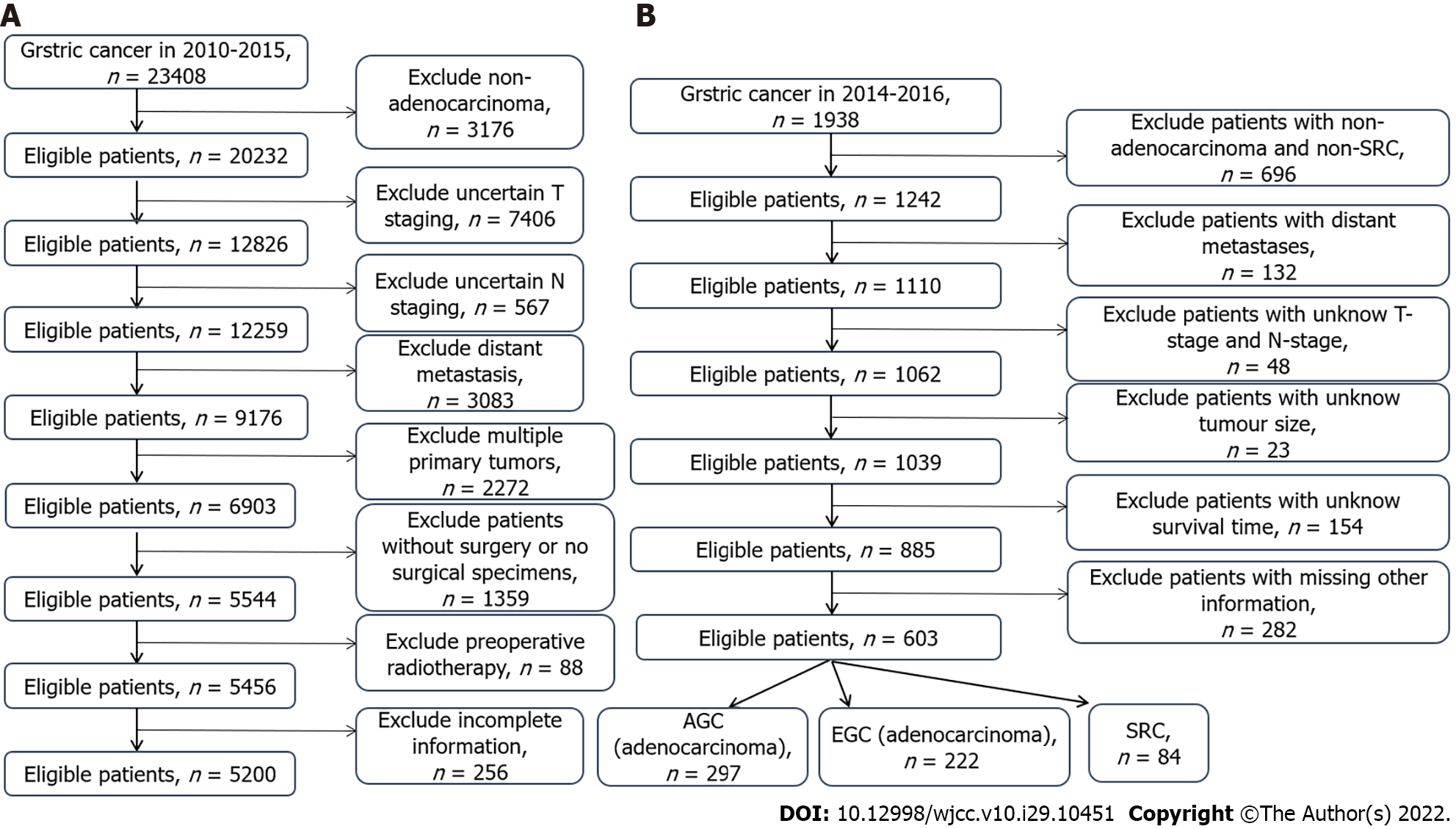
We applied the SEER database from the National Cancer Institute which recorded essential information of around 28% of United States cases. Since the database is available to the public, and we had achieved authorization from the database (account number: 12846-Nov2019), there is no need to acquire patients’ informed consent. Furthermore, the hospital ethics committee has approved the study to conduct. Some concerned information was obtained from the database, mainly including general characteristics, pathological characteristics, and clinical tumor characteristics, as well as treatment methods, survival, and prognosis. Meanwhile, we collected the clinical data of patients with gastric cancer who underwent surgery from January 2014 to December 2017 at the Second Affiliated Hospital of Nanchang University. The inclusion criteria were: (1) Postoperative diagnosis of gastric adenocarcinoma; (2) Complete survival information; and (3) Gastric cancer as the first primary tumor. The exclusion criteria were: (1) Suffering from multiple tumors in situ; (2) Incomplete tumor staging; (3) Distant metastasis; (4) Not undergoing surgical resection; and (5) Incomplete information. Tumor histology, site, and grade were classified based on the International Classification of Diseases for Oncology, version 3. Stage of tumor was identified on the basis of the AJCC tumor–node–metastasis staging system, 7th edition[15]. The details of screening is displayed in Figure 1.
Ages were divided into < 40 years old, 40-60 years old, 60-80 years old, and > 80 years old. Race included Blacks, Whites, Asian Pacific Islanders (API), and American Indians (AI). Size of tumor was classified into < 2 cm, 2-5 cm, > 5 cm, and not available (NA). The type of differentiation comprised SRC, differentiated type (highly differentiated and moderately differentiated), and undifferentiated (poorly differentiated and undifferentiated). T stage included T1a, T1b, T2, T3, T4a, and T4b. N stage included N0, N1, N2, and N3. AJCC stage included I, II, and III. Primary tumors could be located at different parts, divided into the fundus, gastric body, pylorus, antrum, greater curvature, lesser curvature, and overlapping/not otherwise specified (NOS). Tumor progression could be sorted into advanced gastric cancer (AGC) and early gastric cancer (EGC).
We applied the Fisher exact probability method or chi-square test to analyse categorical variables for descriptive statistics. Univariate and binary logistic regression was employed to conduct analysis of the risk factors for gastric cancer survival and prognosis, after which the result was suggested as 95% confidence intervals (CIs) and odds ratios (ORs). The R software (version 4.0.5) was adopted to establish the survival prognostic model for advanced GSRC. The area under curve (AUC) value and C-index were used to assess the accuracy of the model. The Kaplan-Meier method was used to conduct comparative analysis about the difference in survival [overall survival (OS)] and the efficacy of adjuvant therapy between early and advanced GSRC and gastric adenocarcinoma with different levels of differentiation, and the results were validated using the external data set. P < 0.05 was considered statistically significant.
According to the inclusion and exclusion criteria, finally 5200 patients were chosen in the SEER database, and 603 patients were collected from The Second Affiliated Hospital of Nanchang University finally. Table 1 summarizes the pathological and clinical variables of the two data sets.
| Variable | SEER data set (%), n = 5200 | Validation data set (%), n = 603 |
| Age (yr) | ||
| < 40 | 200 (3.85) | 41 (6.80) |
| 40-60 | 1389 (26.71) | 313 (51.91) |
| 60-80 | 2690 (51.73) | 236 (39.14) |
| > 80 | 921 (17.71) | 13 (2.15) |
| Sex | ||
| Male | 2933 (56.40) | 423 (70.15) |
| Female | 2267 (43.60) | 180 (29.85) |
| Race | ||
| White | 2947 (56.67) | |
| Black | 830 (15.96) | |
| Black-AI | 52 (1.00) | |
| Black-API | 1371 (26.37) | |
| T stage | ||
| T1a | 597 (11.48) | 247 (40.96) |
| T1b | 708 (13.62) | |
| T2 | 693 (13.33) | 70 (11.61) |
| T3 | 1697 (32.63) | 8 (1.33) |
| T4a | 1155 (22.21) | 278 (46.10) |
| T4b | 350 (6.73) | |
| N stage | ||
| N0 | 2233 (42.94) | 266 (44.11) |
| N1 | 951 (18.29) | 184 (30.52) |
| N2 | 855 (16.44) | 89 (14.76) |
| N3 | 1161 (22.33) | 64 (10.61) |
| AJCC stage | ||
| I | 1580 (30.38) | 262 (43.45) |
| II | 1449 (27.87) | 71 (11.77) |
| III | 2171 (41.75) | 270 (44.78) |
| Radiotherapy | ||
| Yes | 1416 (27.23) | 16 (2.66) |
| No | 3784 (72.77) | 587 (97.34) |
| Chemotherapy | ||
| Yes | 2599 (49.98) | 445 (73.80) |
| No | 2601 (50.02) | 158 (26.20) |
| Tumor size (cm) | ||
| < 2 | 820 (15.77) | |
| 2-5 | 2198 (42.27) | 94 (15.59) |
| > 5 | 1700 (32.69) | 393 (65.17) |
| NA | 482 (9.27) | 116 (19.24) |
| Histology | ||
| SRC | 1326 (25.50) | 84 (13.93) |
| Differentiated | 1550 (29.81) | 241 (39.97) |
| Undifferentiated | 2324 (44.69) | 278 (46.10) |
Compared with the differentiated type, GSRC was more common in young patients (< 60 years) (47.36% vs 16.39%, P < 0.05), and the same results were obtained when compared with the undifferentiated type (47.36% vs 30.42%, P < 0.05). In addition, GSRC was more frequent in female patients (52.87% vs 38.13% vs 41.95%, P < 0.05). In the case of race, compared with the differentiated type, GSRC was more common in Whites (61.74% vs 51.87%, P < 0.05), but less in While Blacks and API (Black: 14.19% vs 19.03%; API: 23.09% vs 28.07%, P < 0.05). Compared with the undifferentiated type, GSRC was also more common in Whites (61.74% vs 56.97%, P < 0.05), and less in API (23.09% vs 27.11%, P < 0.05).
At the initial diagnosis, 28.05% of GSRC patients had stage I disease, while 47.10% of differentiated patients and 20.57% of undifferentiated patients were diagnosed as stage I (P < 0.05). In terms of T stage and N stage, compared with the differentiated type, the proportion of EGC (23.60% vs 39.10%, P < 0.05) and N0 (38.61% vs 61.03%, P < 0.05) in GSRC patients was less; compared with the undifferentiated type, the proportion of EGC (23.60% vs 16.62%, P < 0.05) and N0 (38.61% vs 33.35%, P < 0.05) in GSRC patients was higher. Regarding tumor size, compared with the differentiated type, GSRC patients had more tumors > 5 cm (31.15% vs 27.10%, P < 0.05), and showed the opposite result when compared to the undifferentiated type (31.15% vs 37.31%, P < 0.05) (Table 2).
| Variable | Differentiated (%), n = 1550 | P value | SRC (%), n = 1326 | P value | Undifferentiated (%), n = 2324 |
| Age (yr) | |||||
| < 40 | 13 (0.84) | < 0.001 | 110 (8.30) | < 0.001 | 77 (3.31) |
| 40-60 | 241 (15.55) | < 0.001 | 518 (39.06) | < 0.001 | 630 (27.11) |
| 60-80 | 920 (59.35) | < 0.001 | 583 (43.97) | < 0.001 | 1187 (51.08) |
| > 80 | 376 (24.26) | < 0.001 | 115 (8.67) | < 0.001 | 430 (18.50) |
| Sex | < 0.001 | < 0.001 | |||
| Male | 959 (61.87) | 625 (47.13) | 1349 (58.05) | ||
| Female | 591 (38.13) | 701 (52.87) | 975 (41.95) | ||
| Race | |||||
| White | 804 (51.87) | < 0.001 | 819 (61.74) | 0.005 | 1324 (56.97) |
| Black | 295 (19.03) | 0.001 | 188 (14.19) | 0.536 | 347 (14.93) |
| Black-AI | 16 (1.03) | 0.890 | 13 (0.98) | 0.978 | 23 (0.99) |
| Black-API | 435 (28.07) | 0.002 | 306 (23.09) | 0.007 | 630 (27.11) |
| T stage | |||||
| T1a | 281 (18.13) | 0.004 | 187 (14.10) | < 0.001 | 129 (5.56) |
| T1b | 325 (20.97) | < 0.001 | 126 (9.51) | 0.140 | 257 (11.06) |
| T2 | 242 (15.61) | 0.002 | 155 (11.69) | 0.355 | 296 (12.74) |
| T3 | 450 (29.03) | 0.930 | 383 (28.89) | < 0.001 | 864 (37.17) |
| T4a | 171 (11.03) | < 0.001 | 385 (29.03) | 0.033 | 599 (25.77) |
| T4b | 81 (5.23) | 0.078 | 90 (6.78) | 0.309 | 179 (7.70) |
| N stage | |||||
| N0 | 946 (61.03) | < 0.001 | 512 (38.61) | 0.001 | 775 (33.35) |
| N1 | 278 (17.94) | 0.097 | 207 (15.61) | 0.001 | 466 (20.05) |
| N2 | 181 (11.68) | 0.010 | 198 (14.93) | < 0.001 | 476 (20.48) |
| N3 | 145 (9.35) | < 0.001 | 409 (30.85) | 0.002 | 607 (26.12) |
| AJCC stage | |||||
| I | 730 (47.10) | < 0.001 | 372 (28.05) | < 0.001 | 478 (20.57) |
| II | 452 (29.16) | < 0.001 | 305 (23.00) | < 0.001 | 692 (29.78) |
| III | 368 (23.74) | < 0.001 | 649 (48.95) | 0.679 | 1154 (49.65) |
| Primary site | |||||
| Fundus | 67 (4.32) | 0.084 | 41 (3.09) | 0.665 | 78 (3.36) |
| Body | 198 (12.77) | 0.527 | 180 (13.58) | 0.898 | 319 (13.73) |
| Antrum | 640 (41.29) | < 0.001 | 434 (32.73) | 0.061 | 832 (35.80) |
| Pylorus | 85 (5.48) | 0.879 | 71 (5.36) | 0.687 | 132 (5.68) |
| Lesser curve | 228 (14.71) | 0.694 | 202 (15.23) | 0.452 | 376 (16.18) |
| Lesser curve-Greater curve | 85 (5.48) | 0.294 | 85 (6.41) | 0.218 | 126 (5.42) |
| Lesser curve-Overlapping/NOS | 247 (15.95) | < 0.001 | 313 (23.60) | 0.007 | 461 (19.83) |
| Radiotherapy | < 0.001 | 0.410 | |||
| Yes | 304 (19.61) | 415 (31.30) | 697 (29.99) | ||
| No | 1246 (80.39) | 911 (68.70) | 1627 (70.01) | ||
| Chemotherapy | < 0.001 | 0.015 | |||
| Yes | 535 (34.52) | 785 (59.20) | 1279 (55.03) | ||
| No | 1015 (65.48) | 541 (40.80) | 1045 (44.97) | ||
| Tumor size (cm) | |||||
| < 2 | 354 (22.84) | < 0.001 | 212 (15.99) | < 0.001 | 254 (10.93) |
| 2-5 | 674 (43.48) | 0.008 | 512 (38.61) | 0.004 | 1012 (43.55) |
| > 5 | 420 (27.10) | 0.017 | 413 (31.15) | < 0.001 | 867 (37.31) |
| NA | 102 (6.58) | < 0.001 | 189 (14.25) | < 0.001 | 191 (8.21) |
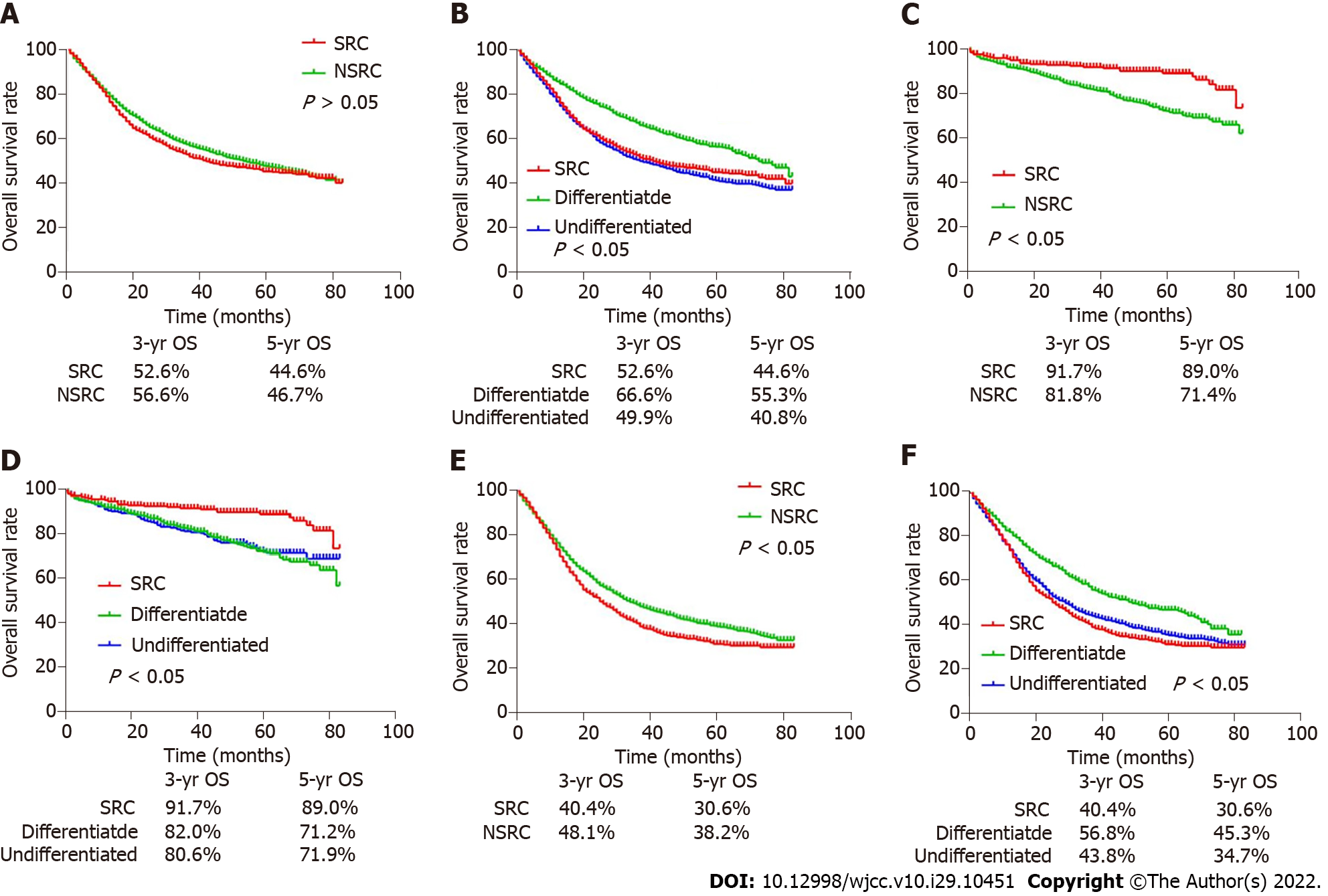
As shown in the survival curve of Figure 2, no significant difference existed between the 5-year OS of SRC and non-SRC patients (44.6% vs 46.7%, P > 0.05) (Figure 2A). Interestingly, when gastric cancer patients were divided into EGC and AGC, the 5-year OS of SRC patients was obviously higher than that of non-SRC patients in EGC (89.0% vs 71.4%, P < 0.05) (Figure 2C), while the result indicated the opposite conclusion in AGC patients (30.6% vs 38.2%, P < 0.05) (Figure 2E).
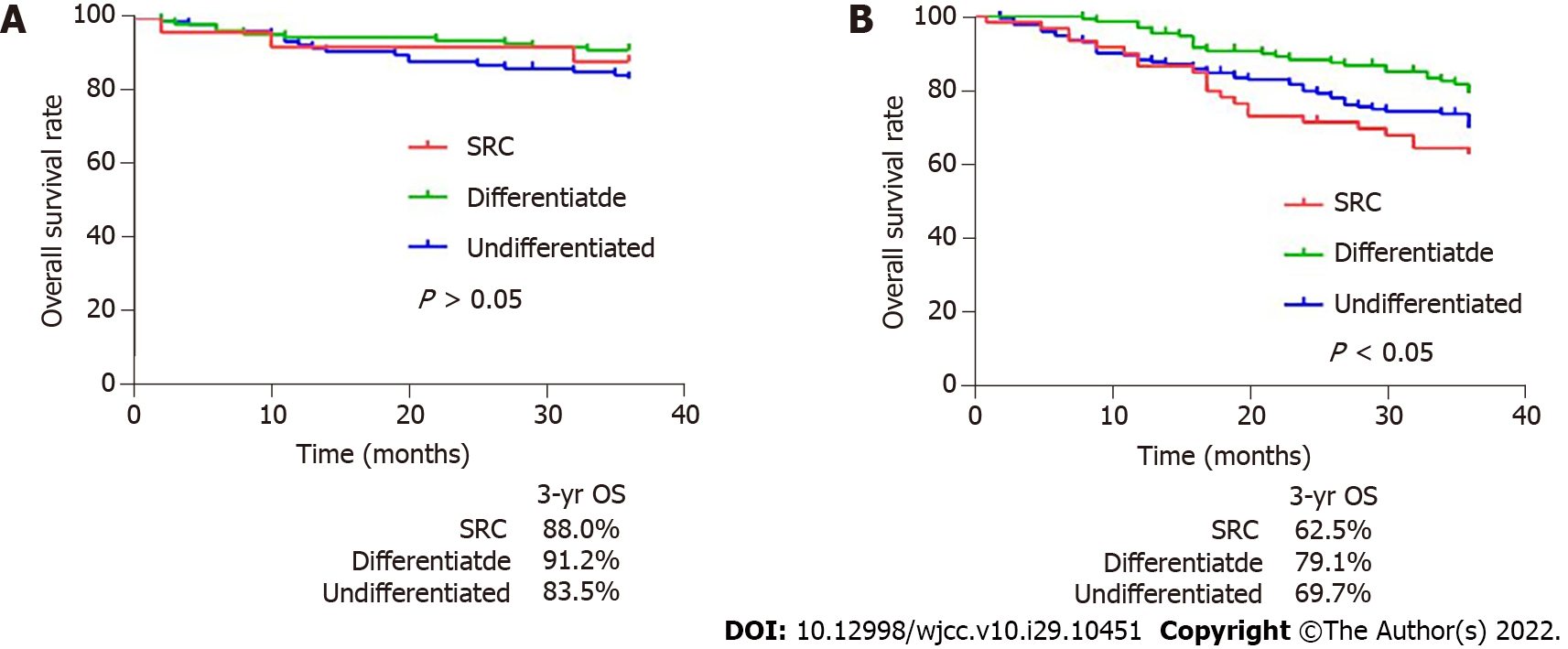
Afterwards, the comparison was conducted again when non-SRC was divided into differentiated type and undifferentiated type. We found that the 5-year OS of SRC was lower than that of differentiated patients (44.6% vs 55.3%, P < 0.05), but higher than that of undifferentiated patients (44.6% vs 40.8%) (Figure 2B). Interestingly, different results were obtained when gastric cancer patients were divided into EGC and AGC patients. In EGC patients, the 5-year OS of SRC was better than that of differentiated and undifferentiated types (89.0% vs 71.2% and 71.9%, P < 0.05), while there was no significant difference between differentiated and undifferentiated types (Figure 2D). And in the external data set, no obvious difference existed in survival rates between SRC and undifferentiated or differentiated types (Figure 3A). In AGC, the 5-year OS of SRC and undifferentiated type was worse than that of differentiated type (30.6% and 34.7% vs 45.3%, P < 0.05). However, in pairwise comparison, no obvious difference existed in survival rate between SRC and undifferentiated type (Figure 2F). It was the same as the verification result of the external data set (Figure 3B).
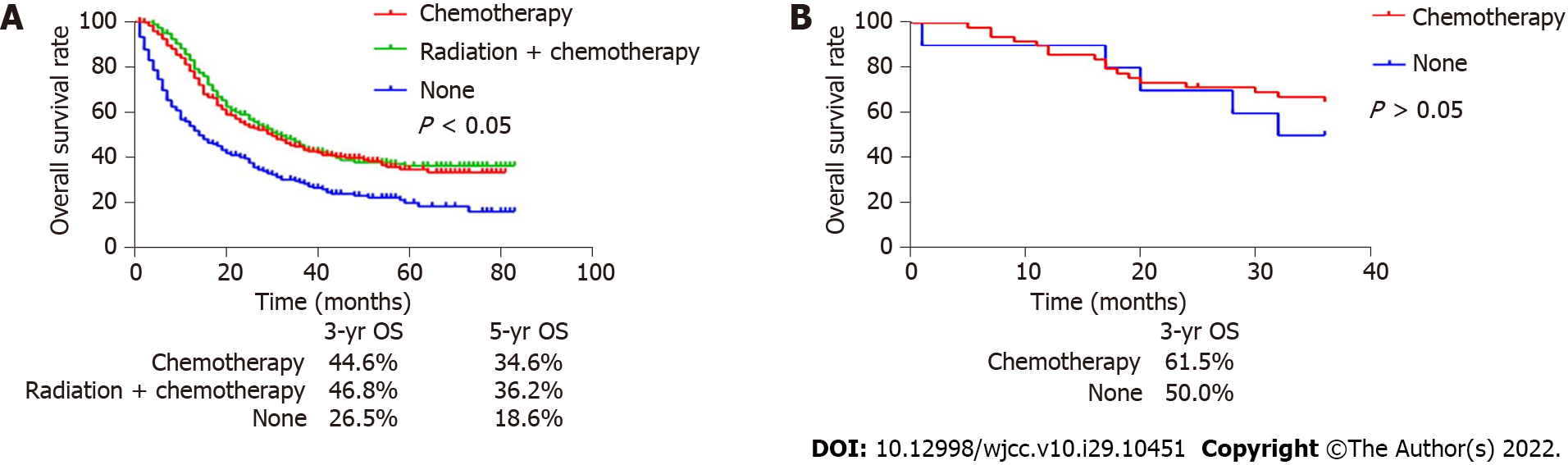
At the same time, our survival analysis of patients with advanced SRC after surgery and chemotherapy demonstrated that postoperative adjuvant chemotherapy or chemotherapy could significantly increase the 5-year OS of patients (34.6% and 36.2% vs 18.6%, P < 0.05) (Figure 4A). The external data set analysis showed that postoperative adjuvant chemotherapy had no effect in improving the survival rate of patients (P > 0.05) (Figure 4B).
In EGC, SRC was a favorable factor affecting patients’ prognosis, and suggested a better prognosis when compared to the differentiated type (HR: 0.636, 95%CI: 0.426-0.950, P < 0.05) and non-SRC (HR: 0.626, 95%CI: 0.427-0.919, P < 0.05). In AGC, SRC was an unfavorable factor, and the results showed the prognosis was worse when compared to the differentiated type (HR: 1.276, 95%CI: 1.117-1.458, P < 0.05) and non-SRC (HR: 1.139, 95%CI: 1.030-1.258, P < 0.05) (Tables 3 and 4).
| Variable | OS (EGC = 1305) or (95%CI) | P value | OS (SRC = 313) or (95%CI) | P value |
| Age (yr) | < 0.001 | 0.128 | ||
| < 40 | 1 (Reference) | 1 (Reference) | ||
| 40-60 | 1.189 (0.282-5.015) | 0.813 | 1.071 (0.128-8.947) | 0.950 |
| 60-80 | 2.246 (0.550-9.174) | 0.260 | 2.072 (0.268-16.012) | 0.485 |
| > 80 | 5.516 (1.337-22.753) | 0.018 | 4.204 (0.463-38.177) | 0.202 |
| Race | < 0.001 | 0.093 | ||
| White | 1 (Reference) | 1 (Reference) | ||
| Black | 1.459 (1.053-2.022) | 0.023 | 2.052 (0.878-4.793) | 0.097 |
| Black-AI | 1.947 (0.476-7.960) | 0.354 | 0.000 | 0.990 |
| Black-API | 0.497 (0.358-0.691) | < 0.001 | 0.471 (0.137-1.271) | 0.124 |
| Sex | ||||
| Male | 1 (Reference) | 1 (Reference) | ||
| Female | 0.610 (0.467-0.797) | < 0.001 | 0.791 (0.383-1.632) | 0.526 |
| Tumor size (cm) | 0.057 | 0.700 | ||
| < 2 | 1 (Reference) | 1 (Reference) | ||
| 2-5 | 1.317 (0.982-1.764) | 0.066 | 1.579 (0.707-3.525) | 0.265 |
| > 5 | 1.859 (1.161-2.978) | 0.010 | 0.000 | 0.974 |
| AJCC stage | ||||
| I | 1 (Reference) | 1 (Reference) | ||
| II | 1.836 (1.100-3.064) | 0.020 | 0.965 (0.231-4.027) | 0.961 |
| Depth | ||||
| T1a | 1 (Reference) | 1 (Reference) | ||
| T1b | 1.280 (0.962-1.703) | 0.090 | 1.786 (0.810-3.938) | 0.151 |
| LNM1 | ||||
| N0 | 1 (Reference) | 1 (Reference) | ||
| N1-3 | 1.271 (0.859-1.880) | 0.230 | 2.134 (0.883-5.154) | 0.151 |
| Histology | 0.045 | |||
| Differentiated | 1(Reference) | |||
| SRC | 0.636 (0.426-0.950) | 0.027 | ||
| Undifferentiated | 1.039 (0.780-1.384) | 0.794 | ||
| SRC vs non-SRC | 0.626 (0.427-0.919) | 0.017 |
| Variable | OS (AGC = 3895), or (95%CI) | P value | OS (SRC = 1013), or (95%CI) | P value |
| Age (yr) | < 0.001 | < 0.001 | ||
| < 40 | 1 (Reference) | 1 (Reference) | ||
| 40-60 | 1.130 (0.882-1.447) | 0.333 | 1.267 (0.898-1.788) | 0.178 |
| 60-80 | 1.543 (1.212-1.985) | < 0.001 | 1.578 (1.123-2.218) | 0.009 |
| > 80 | 2.936 (2.281-3.780) | < 0.001 | 2.290 (1.528-3.431) | < 0.001 |
| Race | < 0.001 | < 0.001 | ||
| White | 1 (Reference) | 1 (Reference) | ||
| Black | 1.096 (0.973-1.234) | 0.133 | 1.326 (1.057-1.663) | 0.015 |
| Black-AI | 1.382 (0.949-2.015) | 0.092 | 2.624 (1.423-4.838) | 0.002 |
| Black-API | 0.726 (0.651-0.810) | < 0.001 | 0.800 (0.867-1.205) | 0.041 |
| Sex | ||||
| Male | 1 (Reference) | 1(Reference) | ||
| Female | 0.964 (0.883-1.052) | 0.409 | 1.022 (0.867-1.205) | 0.791 |
| Tumor size (cm) | < 0.001 | 0.001 | ||
| < 2 | 1 (Reference) | 1 (Reference) | ||
| 2-5 | 1.026 (0.823-1.280) | 0.817 | 1.031 (0.680-1.565) | 0.885 |
| > 5 | 1.211 (0.971-1.511) | 0.089 | 1.415 (0.930-2.152) | 0.105 |
| AJCC stage | 0.019 | 0.028 | ||
| I | 1 (Reference) | 1 (Reference) | ||
| II | 1.227 (0.903-1.667) | 0.191 | 1.946 (0.923-4.102) | 0.080 |
| III | 1.584 (1.076-2.330) | 0.020 | 3.050 (1.247-7.458) | 0.015 |
| T stage | < 0.001 | < 0.001 | ||
| T2 | 1 (Reference) | 1 (Reference) | ||
| T3 | 1.384 (1.135-1.689) | 0.001 | 1.857 (1.189-2.899) | 0.006 |
| T4 | 2.025 (1.633-2.512) | < 0.001 | 2.615 (1.644-4.161) | < 0.001 |
| N stage | < 0.001 | 0.086 | ||
| N0 | 1 (Reference) | 1 (Reference) | ||
| N1 | 1.241 (1.056-1.459) | 0.009 | 1.061 (0.744-1.512) | 0.745 |
| N2 | 1.295 (1.050-1.597) | 0.016 | 1.034 (0.665-1.608) | 0.881 |
| N3 | 1.904 (1.549-2.341) | < 0.001 | 1.341 (0.882-2.037) | 0.170 |
| Histology | 0.002 | |||
| Differentiated | 1 (Reference) | |||
| SRC | 1.276 (1.117-1.458) | < 0.001 | ||
| Undifferentiated | 1.164 (1.037-1.306) | 0.010 | ||
| SRC vs non-SRC | 1.139 (1.030-1.258) | 0.011 | ||
| Adjuvant therapy | < 0.001 | |||
| Radiotherapy | 0.635 (0.367-1.099) | 0.105 | ||
| Chemotherapy | 0.481 (0.388-0.595) | < 0.001 | ||
| Radiotherapy + Chemotherapy | 0.414 (0.334-0.512) | < 0.001 | ||
| None | 1 (Reference) |
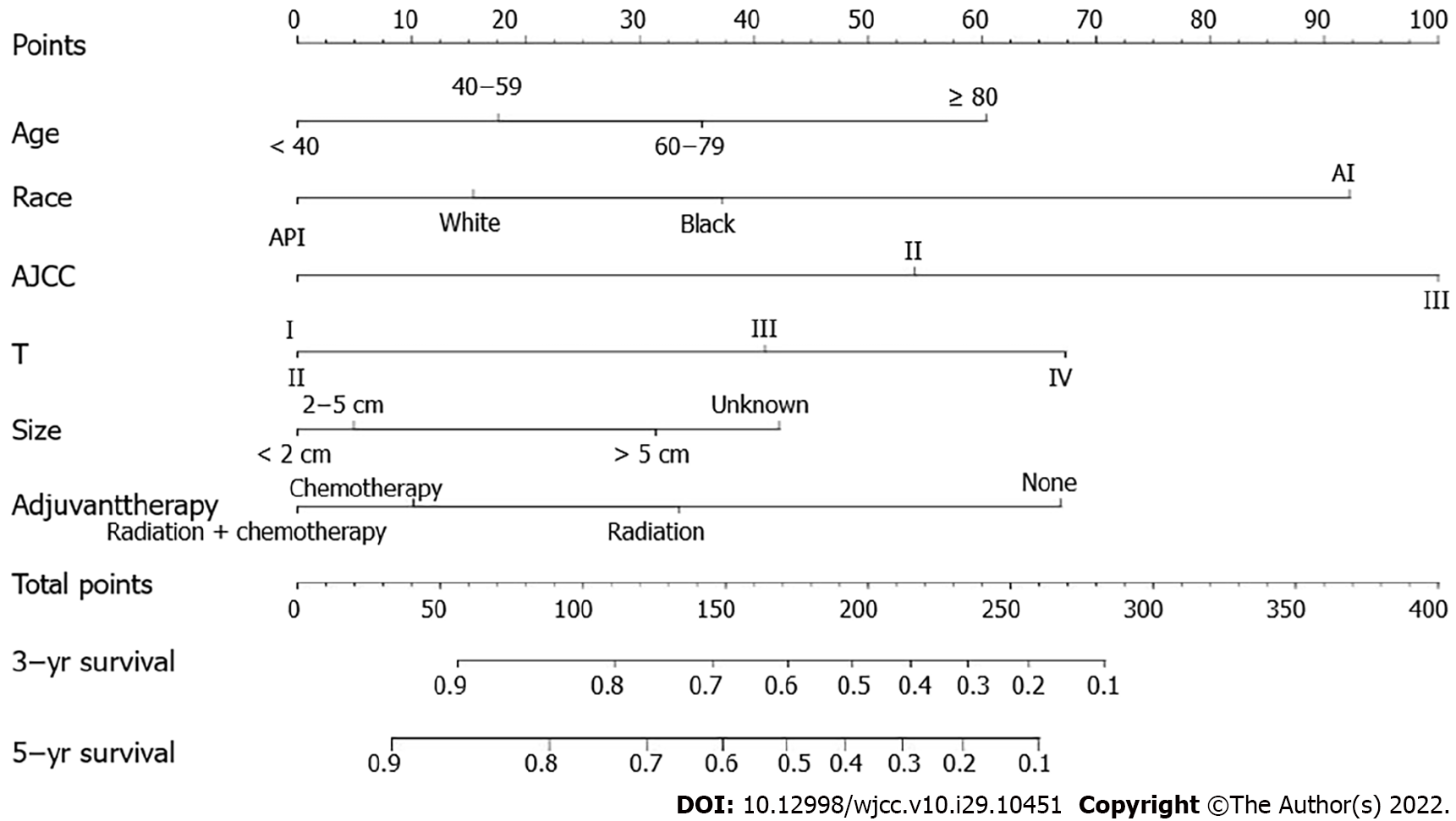
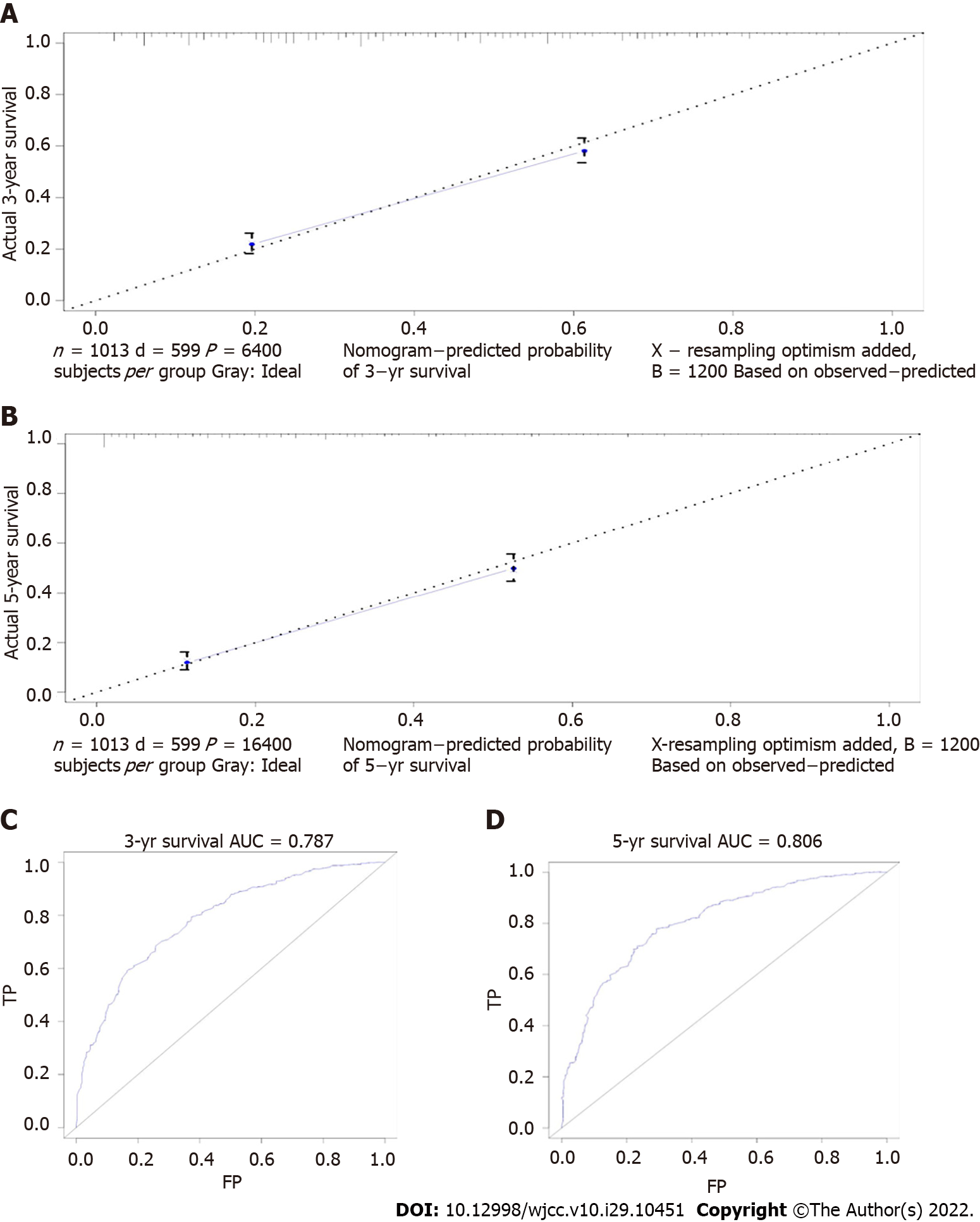
Among EGC, there was no obvious difference in SRC patients’ prognosis. In AGC, the prognosis of GSRC was related to tumor size, age, race, AJCC stage, T stage, and postoperative adjuvant therapy (Tables 3 and 4). Finally, we established a prognostic prediction model for advanced GSRC; the C-index of this model was 0.766, and the AUC values of the model for predicting 3-year and 5-year OS were 0.787 and 0.806, respectively (Figures 5 and 6). Table 5 shows the risk score of each factor.
| Variable | Points | Total points | 3/5-yr survival | Total points |
| Age (yr) | 0-56 | 0.9-1 | 0-33 | |
| < 40 | 0 | 56-111 | 0.8-0.9 | 33-88 |
| 40-60 | 18 | 111-146 | 0.7-0.8 | 33-123 |
| 60-80 | 36 | 146-172 | 0.6-0.7 | 123-149 |
| > 80 | 60 | 172-195 | 0.5-0.6 | 149-172 |
| Race | 195-215 | 0.4-0.5 | 172-192 | |
| White | 15 | 215-235 | 0.3-0.4 | 192-212 |
| Black | 37 | 235-257 | 0.2-0.3 | 212-234 |
| Black-AI | 92 | 257-283 | 0.1-0.2 | 234-260 |
| Black-API | 0 | > 283 | < 0.1 | > 260 |
| Tumor size (cm) | ||||
| < 2 | 0 | |||
| 2-5 | 5 | |||
| > 5 | 31 | |||
| NA | 42 | |||
| AJCC stage | ||||
| I | 0 | |||
| II | 54 | |||
| III | 100 | |||
| T stage | ||||
| T2 | 0 | |||
| T3 | 41 | |||
| T4 | 67 | |||
| Adjuvant therapy | ||||
| Radiotherapy | 33 | |||
| Chemotherapy | 10 | |||
| Radiotherapy + Chemotherapy | 0 | |||
| None | 67 |
The incidence of GSRC is 3.4%-39% in primary gastric cancer[16,17]. In this study, GSRC patients in the SEER data set accounted for 25.5% of all patients undergoing gastrectomy. GSRC patients in the external validation set accounted for 13.9%. The clinical characteristics and prognosis of GSRC are still controversial. Eastern researchers believed that GSRC was not necessarily an unfavorable prognostic factor, while Western researchers considered that the prognosis of GSRC was poor and the incidence rate continued to increase worldwide[9,12,18,19]. However, most previous studies only included a small number of heterogeneous patients, and did not distinguish distinct differentiation types. Comparing SRC and non-SRC together will inevitably cause a certain impact on the final results. Therefore, during our research, a large database was utilized to analyze, compare, and verify the GSRC patients undergoing surgical resection in terms of different progression and differentiation degrees, so as to obtain the pathological characteristics of GSRC and the survival differences between gastric adenocarcinoma with different differentiation degrees to provide more accurate guidance for clinical treatment.
Our research showed that GSRC, as a special pathological type of gastric adenocarcinoma, had different clinical characteristics from differentiated and undifferentiated adenocarcinoma. GSRC tended to occur in young and female patients, which was consistent with previous studies[9]. Although gastric cancer was considered to be a disease more frequently seen in men, a large number of studies had shown that the incidence of GSRC in women was higher[13]. At the same time, the age of onset of GSRC was significantly earlier than that of gastric adenocarcinoma. In terms of race, our research showed that GSRC was more frequent in Whites, while there were fewer patients in API. Part of the etiology of young patients may be attributed to genetic factors. Such patients should be diagnosed as hereditary diffuse gastric cancer (HDGC)[20]. The 2015 multidisciplinary symposium defined HDGC as "early-onset diffuse gastric cancer", where multiple generations of people in the family have a history of DGC or lobular breast cancer[21]. The gender differences of manifestations of GSRC may be related to the level of estrogen. Studies have shown that more frequent appearance of progesterone and estrogen receptors in the tissues of female patients suggests that they might be associated with the manifestations and progression of tumors[22]. The high-level CLDN18-ARHGAP26/6 fusion in GSRC results in genetic differences from other diffuse gastric cancer subtypes. These genetic types develop at a young age and are associated with a high proportion of females, high tumor stages, poor survival outcomes, and chemoresistance[23].
The microscopic features of GSRC are scattered malignant cells containing intracytoplasmic mucin, accounting for more than 50% of tumors[16,24]. GSRC is inert in the early stage and does not show strong invasiveness. When the tumor breaks through the submucosa, it shows strong aggression, rapidly invading the muscle layer, serosal layer, and surrounding lymph nodes[25]. Large-scale data studies have found that GSRC showed a higher proportion of serosal layer invasion and distant metastasis in the advanced stage, and it was prone to lymph node metastasis[25]. Our results found that compared to gastric adenocarcinoma, GSRC had a higher proportion of advanced stage, and showed larger tumor size and more lymph node metastases. This is consistent with the results of most studies.
The prognosis of GSRC is unanimously controversial. Previous studies have suggested that GSRC had a poorer prognosis than non-GSRC. In this study, we divided non-GSRC into differentiated gastric adenocarcinoma and undifferentiated gastric adenocarcinoma, and compared EGC and AGC separately. The results showed that in EGC, the prognosis of differentiated and undifferentiated gastric adenocarcinoma was not significantly different, and was both worse than that of SRC. The external validation indicated that the difference in the prognosis between the early gastric adenocarcinoma and GSRC was not obvious. Interestingly, the prognosis of patients with early GSRC had nothing to do with age, gender, and tumor size. In AGC patients, the prognosis of SRC and undifferentiated gastric cancer patients did not have much difference, and was both worse than that of differentiated type. The external validation set also reached the same conclusion. Through data analysis, we found that the prognosis of patients with advanced GSRC was related to tumor size, age, race, AJCC stage, T stage, and posto
Regarding the treatment of GSRC, surgical treatment methods are also controversial for different stages[26]. Endoscopic submucosal dissection is an optional treatment for EGC. On the basis of the guidelines of the Japanese Gastric Cancer Association, endoscopic therapy could be applied for the cases with well-differentiated and non-ulcerated carcinoma whose diameter is smaller than 2 cm, but the therapy might not be so feasible for ulcerated and undifferentiated submucosal carcinomas[27]. Research has demonstrated that tumor size and lymph node metastasis are important factors for not recommending endoscopic treatment in the early stage of GSRC[28]. According to the clinical characteristics of GSRC in our study, early GSRC showed a higher lymph node metastasis rate and larger tumor size, so endoscopic treatment of GSRC is also not recommended. For AGC, adjuvant radiotherapy and chemotherapy after surgical resection can significantly enhance the survival rate of patients. However, the specific treatment regimen for GSRC is still uncertain. Many studies have confirmed that GSRC was resistant to chemical agents. However, it is still controversial about the efficiency of chemotherapy for GSRC. Voron et al[29] showed that postoperative chemotherapy has no significant effect on the survival rate of GSRC patients. In multivariate analysis, GSRC is an independent poor prognostic factor[29]. Another study also proved that GSRC patients cannot benefit from postoperative chemotherapy[30]. Shi et al[31] indicated that postoperative chemotherapy could still be effective for the patients with stage Ⅳ GSRC[31]. A recent large-scale data study based on the SEER confirmed that surgical resection combined with adjuvant radiotherapy and chemotherapy provides a favorable prognosis for GSRC[32]. Our research showed that postoperative adjuvant radiotherapy and chemotherapy can improve the survival rate of advanced GSRC patients. However, the external validation set showed that posto
Certainly, our analysis was convincing, because we adopted a staged analysis method for GSRC and a large population-based study. Furthermore, we conducted reasonable and effective verification through an external validation set. However, certain limitations still exist in our research. First of all, the SEER database lacks information related to postoperative adjuvant treatment, and information about adjuvant chemotherapy, duration, and neoadjuvant treatment is not available. Second, the analysis of SRC patients with metastasis was not included in the research. Therefore, another study of the particular population may need to be conducted. Finally, although we have established a prognostic prediction model for the advanced GSRC, it cannot be effectively externally verified because the external validation set was insufficient.
In summary, our research analysis showed that GSRC is more common in young female patients, and the clinical characteristics of GSRC are significantly different from those of gastric adenocarcinoma. The prognosis of early GSRC is not worse, even better than that of differentiated gastric adenocarcinoma. GSRC should be diagnosed early, and radical surgical resection with adjuvant radiotherapy and chemotherapy can significantly improve the survival rate of patients, though it still needs more clinical data to verify.
The clinicopathological features and prognosis of gastric signet ring cell carcinoma (GSRC) remain controversial, particularly with regard to sensitivity to postoperative adjuvant therapy.
To our knowledge, this study is the first to analyse and compare the clinicopathological features and prognosis of GSRC with gastric adenocarcinoma of different degrees of differentiation and includes both Eastern and Western populations.
The aim of this study was to compare the pathological features of GSRC with those of gastric adenocarcinoma of different degrees of differentiation and the differences in survival prognosis between the different disease processes.
This study was first conducted by analysing the differences in clinicopathological features between GSRC and gastric adenocarcinoma in Western populations and comparing the survival prognosis of the different processes. Finally, validation was performed using an Eastern population.
GSRC was more commonly seen in younger female patients and was more aggressive in the progressive stage, showing more common lymph node metastasis and larger tumour size. However, the prognosis of early GSRC was relatively good, even better than that of differentiated adenocarcinoma. The prognosis of advanced GSRC was not significantly different from that of undifferentiated gastric adenocarcinoma and was worse than that of differentiated gastric adenocarcinoma. Postoperative adjuvant radiotherapy can improve the survival rate of GPC.
The prognosis of GSRC is better than that of undifferentiated type, especially in EGC, and its prognosis is even better than that of differentiated type. GSRC patients can benefit from early detection, surgical resection, and aggressive adjuvant therapy.
GSRC has unique clinicopathological features and prognosis, and early diagnosis and treatment can significantly improve survival rates. Patients may benefit from postoperative adjuvant radiotherapy, but further validation is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Kao JT, Taiwan; Saglam S, Turkey; Silano F, Brazil; Yu Y, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2744] [Article Influence: 548.8] [Reference Citation Analysis (5)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 62709] [Article Influence: 15677.3] [Reference Citation Analysis (172)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (3)] |
| 4. | Lai JF, Xu WN, Noh SH, Lu WQ. Effect of World Health Organization (WHO) Histological Classification on Predicting Lymph Node Metastasis and Recurrence in Early Gastric Cancer. Med Sci Monit. 2016;22:3147-3153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Börger ME, Gosens MJ, Jeuken JW, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Signet ring cell differentiation in mucinous colorectal carcinoma. J Pathol. 2007;212:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4301] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 7. | Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39:2475-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32 Suppl 2:329-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Chon HJ, Hyung WJ, Kim C, Park S, Kim JH, Park CH, Ahn JB, Kim H, Chung HC, Rha SY, Noh SH, Jeung HC. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg. 2017;265:946-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Wei Q, Gao Y, Qi C, Yuan X, Li J, Xu Q, Luo C, Chen L, Zhuo W, Xu Z, Ying J. Clinicopathological Characteristics and Prognosis of Signet Ring Gastric Cancer: A Population-Based Study. Front Oncol. 2021;11:580545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 14. | Charalampakis N, Nogueras González GM, Elimova E, Wadhwa R, Shiozaki H, Shimodaira Y, Blum MA, Rogers JE, Harada K, Matamoros A Jr, Sagebiel T, Das P, Minsky BD, Lee JH, Weston B, Bhutani MS, Estrella JS, Badgwell BD, Ajani JA. The Proportion of Signet Ring Cell Component in Patients with Localized Gastric Adenocarcinoma Correlates with the Degree of Response to Pre-Operative Chemoradiation. Oncology. 2016;90:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6423] [Article Influence: 428.2] [Reference Citation Analysis (0)] |
| 16. | Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, Jung SA. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (3)] |
| 18. | Peng J, Xiao P, Liao B, Ye J, He Y. [Analysis of clinicopathological features of 1879 cases of gastric cancer in Southern China: a single center experience]. Zhonghua Wai Ke Za Zhi. 2014;52:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 21. | van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, Bardram L, Benusiglio PR, Bisseling TM, Blair V, Bleiker E, Boussioutas A, Cats A, Coit D, DeGregorio L, Figueiredo J, Ford JM, Heijkoop E, Hermens R, Humar B, Kaurah P, Keller G, Lai J, Ligtenberg MJ, O'Donovan M, Oliveira C, Pinheiro H, Ragunath K, Rasenberg E, Richardson S, Roviello F, Schackert H, Seruca R, Taylor A, Ter Huurne A, Tischkowitz M, Joe ST, van Dijck B, van Grieken NC, van Hillegersberg R, van Sandick JW, Vehof R, van Krieken JH, Fitzgerald RC. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 392] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 22. | Liu S, He L, Wang Z, Wen G. [Expression of sex hormone receptor in gastric cancer with synchronous ovarian metastasis and its significance]. Zhonghua Yi Xue Za Zhi. 2014;94:1861-1865. [PubMed] [DOI] [Full Text] |
| 23. | Shu Y, Zhang W, Hou Q, Zhao L, Zhang S, Zhou J, Song X, Zhang Y, Jiang D, Chen X, Wang P, Xia X, Liao F, Yin D, Zhou X, Zhang D, Yin S, Yang K, Liu J, Fu L, Zhang L, Wang Y, Zhang J, An Y, Cheng H, Zheng B, Sun H, Zhao Y, Xie D, Ouyang L, Qiu M, Fu X, Dai L, He G, Yang H, Cheng W, Yang L, Liu B, Li W, Dong B, Zhou Z, Wei Y, Peng Y, Xu H, Hu J. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat Commun. 2018;9:2447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Fléjou JF. [WHO Classification of digestive tumors: the fourth edition]. Ann Pathol. 2011;31:S27-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Su WP, Wang WJ, Chang JY, Ho PC, Liu TY, Wen KY, Kuo HL, Chen YJ, Huang SS, Subhan D, Chen YA, Lu CY, Wu CY, Lin SR, Lee MH, Chiang MF, Sze CI, Chang NS. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs that Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Li Y, Zhu Z, Ma F, Xue L, Tian Y. Gastric Signet Ring Cell Carcinoma: Current Management and Future Challenges. Cancer Manag Res. 2020;12:7973-7981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1304] [Article Influence: 326.0] [Reference Citation Analysis (2)] |
| 28. | Yin X, Xia J, Sun Y, Zhang Z. CHCHD2 is a potential prognostic factor for NSCLC and is associated with HIF-1a expression. BMC Pulm Med. 2020;20:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Voron T, Messager M, Duhamel A, Lefevre J, Mabrut JY, Goere D, Meunier B, Brigand C, Hamy A, Glehen O, Mariette C, Paye F. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer. 2016;19:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Li Y, Ma FH, Xue LY, Tian YT. Neoadjuvant chemotherapy vs upfront surgery for gastric signet ring cell carcinoma: A retrospective, propensity score-matched study. World J Gastroenterol. 2020;26:818-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Shi T, Song X, Liu Q, Yang Y, Yu L, Liu B, Wei J. Survival benefit of palliative gastrectomy followed by chemotherapy in stage IV gastric signet ring cell carcinoma patients: A large population-based study. Cancer Med. 2019;8:6010-6020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Zhang S, Liu Y, Jiao Z, Li Z, Wang J, Li C, Qu X, Xu L. Development and Validation of a Prognostic Nomogram for Gastric Signet Ring Cell Carcinoma: A Multicenter Population-Based Study. Front Oncol. 2021;11:603031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |