Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5551
Peer-review started: December 10, 2021
First decision: January 8, 2022
Revised: January 20, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Processing time: 180 Days and 9.8 Hours
Biliary obstruction is a relatively common condition that affects approximately 5 in 1000 people annually. Malnutrition is very common in patients with biliary obstruction and since it is associated with significant morbidity and mortality, it is important to identify factors and mechanisms involved in its development.
To determine the influence of obstructive jaundice on the hormones controlling appetite and nutritive status.
This was a prospective case control study performed in a tertiary center in Zagreb, Croatia. Patients with biliary obstruction undergoing internal biliary drainage from September 2012 until August 2013 were enrolled. After excluding patients who developed procedure related complications or were lost in the follow-up, out of initial 73 patients, 55 patients were included in the analysis, including 34 with benign and 21 with malignant disease. Meanwhile, 40 non-jaundiced controls were also included. Appetite, nutritional status, and serum ghrelin, cholecystokinin (CCK), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) were determined at admission, 48 h and 28 d after internal biliary drainage. Chi square test was used for categorical variables. Continuous variables were analysed for normality by Kolmogorov–Smirnov test and relevant non-parametric (Mann-Whitney, Kruskal-Wallis, and Friedman) or parametric (t-test and analysis of variance) tests were used.
Patients with obstructive jaundice were significantly malnourished compared to controls, regardless of disease etiology. Plasma ghrelin and CCK levels were significantly higher in patients with obstructive jaundice. Serum bilirubin concentrations were negatively correlated with ghrelin levels and positively correlated with TNF-α, but had no correlation with CCK concentrations. After internal biliary drainage, a significant improvement of nutritional status was observed although serum concentrations of ghrelin, IL-6, and TNF-α remained significantly elevated even 28 d after the procedure. CCK levels in patients without malnutrition remained elevated 28 d after the procedure, but in patients with malnutrition, CCK levels decreased to levels comparable with those in the control group. We have not established any correlation between appetite and serum levels of ghrelin, CCK, IL-6, and TNF-α before and after biliary drainage.
Possible abnormalities in ghrelin and CCK regulation may be associated with the development of malnutrition during the inflammatory response in patients with biliary obstruction.
Core Tip: Biliary obstruction is a common condition with subsequent malnutrition that contributes to patient’s high morbidity and mortality. Therefore, it is important to better understand the interaction of hormonal and inflammatory parameters on nutritional status. We conducted a case control study to determine the influence of obstructive jaundice on the hormones controlling appetite and nutritional status and showed that patients with obstructive jaundice had worse nutritional status compared to controls, regardless of disease etiology. Plasma ghrelin and cholecystokinin levels were significantly higher in patients with obstructive jaundice, which may be associated with the development of malnutrition during the inflammatory response.
- Citation: Pavić T, Pelajić S, Blažević N, Kralj D, Milošević M, Mikolasevic I, Lerotic I, Hrabar D. Gut peptide changes in patients with obstructive jaundice undergoing biliary drainage: A prospective case control study. World J Clin Cases 2022; 10(17): 5551-5565
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5551.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5551
Biliary obstruction is a relatively common condition that affects approximately 5 in 1000 people annually[1]. It can be caused by a variety of malignant and benign conditions[2-4]. Regardless of the underlying pathology, the blockage of bile ducts causes accumulation of bilirubin and bile salts in the blood and can lead to complications such as acute cholangitis, sepsis, liver dysfunction, renal failure, and cardiovascular impairment[1,3]. Malnutrition is common in patients with biliary obstruction with the prevalence of malnutrition in this population being up to 80% according to some studies[5-7]. Since malnutrition is associated with significant morbidity and mortality[8-10], it is important to identify factors and mechanisms involved in development of malnutrition in patients with biliary obstruction.
Tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) are inflammatory cytokines associated with negative energy balance and cachexia[11,12]. Previous studies have found increased levels of both TNF-α and IL-6 in patients with biliary obstruction[1,2,5]. Cholecystokinin (CCK) is a gut derived hormone that inhibits food intake in humans[13,14]. CCK regulates gallbladder and small intestinal motility through CCK1-1 receptor (CCK-1R) found in various locations throughout the digestive system[15]. A study by Padillo et al[4] found that increased CCK concentration can be predictive of malnutrition in patients with biliary obstruction. Ghrelin is a 28 amino acid peptide hormone produced mostly by X/A cells found in the neck to the base of the oxyntic gland in the gastric fundus. It is the only known peripherally produced hormone with central orexigenic effect to date[16] and it exhibits a functional antagonism with CCK[14,17,18]. It also has anti-inflammatory effects and studies have shown that it can antagonize the effects of TNF-α and IL-6 by limiting the activation of NF-κB signaling[19,20]. The few studies performed so far have not given us clear answers about factors that regulate appetite and nutritional status of patients before and after resolution of biliary obstruction. Food intake and energy homeostasis are tightly linked by a complex system of nervous structures and humoral factors that form the brain-gut axis[21]. In biologically important signaling systems, multiple repetitive components are often encountered in order to be able to compensate in the event of failure of some parts of the signaling network[22]. Food intake is one of the vital functions, so it is not surprising that there are a multitude of regulatory molecules networked by complex interactions. Moreover, many of the primary regulatory molecules controlling body weight are deployed at multiple physiological levels, as an example of evolutionary adjustment[22]. CCK was the first intestinal hormone to be shown to reduce appetite in humans[23]. This was followed by the discovery of a multitude of peptides from the stomach and intestines that have a similar effect, but to date the only known peripheral peptide with central action that stimulates appetite is ghrelin[24]. Many researchers believe that the example of ghrelin illustrates the importance of the functional interdependence of the stomach and hypothalamus and that it is one of the missing links in understanding the regulation of energy balance, growth, and the functioning of the digestive system[24]. There is evidence to support a functional antagonism of ghrelin and CCK on food intake, but their exact interaction in specific clinical situations has not yet been proven[25]. Since the presence of bile in the duodenum is an important modulator of postprandial CCK secretion, the aim of this study was to examine the effect of biliary obstruction and consequent decreased bile acid content in the duodenum on serum CCK concentration and its influence on ghrelin, CCK, TNF-α, and IL-6, as well as nutritional parameters and appetite.
We recruited 73 patients with biliary obstruction who underwent biliary drainage via endoscopic retrograde cholangiopancreatography (ERCP) at University Hospital Center Sestre Milosrdnice from September 2012 until December 2013 and 40 sex and age matched healthy controls. The inclusion criteria were bilirubin levels > 35 µmol/L and intrahepatic and/or extrahepatic biliary dilation on transabdominal ultrasound. Patients who developed complications such as acute pancreatitis, acute cholangitis, or biliary sepsis as well as patients with other conditions that are known to lead to malnutrition or inadequate oral intake were excluded from the study. The study was approved by the ethics committees of University Hospital Center Sestre Milosrdnice and University of Zagreb School of Medicine.
This was a prospective case control study carried out at the University Hospital Center Sestre Milosrdnice, Zagreb, Croatia. After obtaining informed consent, patients had blood drawn for laboratory studies including complete blood count, C-reactive protein (CRP), sedimentation rate, liver enzymes, bilirubin, creatinine, blood glucose, electrolytes, calcium, amylase in blood and urine, protein electrophoresis, and lipid status and coagulation parameters. As a standard pre-ERCP procedure, esophagogastroduodenoscopy (EGD) was performed and biopsies for Helicobacter pylori (H. pylori) were taken. On the day of the procedure, after 10 h of fasting, anthropometric measurements were obtained, including mass, height, body mass index (BMI), and body fat percentage. Nutritional risk screening 2002 scoring (NRS 2002) was performed[26,27]. Blood samples for concentration of CRP, CCK, TNF–α, and IL-6 measurements were drawn. Pain and appetite were assessed using validated visual analog scales. ERCP was performed in intravenous anesthesia and obstruction was managed depending on underlying pathology either by stone extraction or biliary stent implantation. Nutritive assessment, laboratory studies and hormone levels were obtained 48 h and 28 d after successful biliary drainage. Patients at high nutritional risk received oral nutritional supplements (720-880 kcal/d) for 28 d or more. After a follow-up period of 6 mo, all the available patients were clinically reassessed. Healthy controls underwent the same initial laboratory studies and anthropometric measurement, but without EGD, so the testing for H. pylori was done using stool antigen test.
Ten milliliters of venous blood were collected in chilled test tubes with ethylenediaminetetraacetic acid and 1000 units of aprotinin per milliliter of blood. Plasma was separated by centrifugation (1000 revolutions per min, 15 min, 4 °C) within 30 min of collection and stored at -70 °C until thawed for analysis.
CCK was measured quantitatively by enzyme-linked immunosorbent assay method using a commercial CCK kit (Cusabio). It is an in vitro quantitative assay that uses antibodies specific for human CCK in a double sandwich technique. Cross-reactivity with other similar molecules has not been found. The minimal detectable level was 20 pg/mL.
Ghrelin concentration was measured by radioimmunoassay technique using a commercial kit (DIAsource ImmunoAssays, South Africa) that uses 125I-labeled recombinant ghrelin as a tracer and a polyclonal antibody raised in rabbits against the C-terminal end of human ghrelin. No cross-reactivity with any relevant molecules such as insulin and growth hormone has been found. The measuring range for ghrelin using this method was 200-6400 pg/mL.
IL-6 concentration was determined by electrochemiluminescence immunoassay using a commercial kit (Roche Diagnostics GmbH, D-68305 Mannheim, Germany) and Roche Cobas e411 automatic analyzer. The method uses IL-6 specific monoclonal antibodies labeled with biotin and ruthenium that produce chemiluminesence when exposed to electrodes. The measuring range is 1.5-5000 pg/mL.
TNF-α was measured by chemiluminescence immunoassay using a commercially available kit (IMMULITE 1000 System, Siemens Medical). It is a quantitative assay that uses TNF-α antibodies as a solid phase and alkaline phosphatase as a reagent and substrate for chemiluminescent enzyme. The measuring range for this method is 1.7-1000 pg/mL.
For the patients who underwent EGD and had standard biopsies taken, presence of H. pylori infection was assessed histologically after Giemsa staining. Patients taking proton pump inhibitors (PPI) and healthy controls were tested for H. pylori antigen in stool using a commercially available test (Diaquick, Dialab) after at least 2 wk of PPI therapy cessation.
Other routine blood tests were performed using the automatic analyzer AU 2700 (Beckman 40 Coulter International, South Africa) and Beckman Coulter HmX automatic hematologic analyzer.
Analysis of test power for analysis of variance (ANOVA) (3 repeated measurements) with the following parameters: Test power of 90%, α significance level of 0.05, and sample size effect of 0.2 with an assumed correlation between measurements of 0.5, estimated the total sample size to be 55. Additional analysis for the independent t-test (difference between test and control groups), under the same conditions, found that the control group should consist of 40 healthy subjects. After testing for normality by Kolmogorov-Smirnov test, relevant parametric and nonparametric tests were used. Quantitative data are expressed as the arithmetic mean and their standard deviation if they were normally distributed, or as medians with interquartile ranges if they did not fit a normal distribution. Continuous variables were compared by Mann-Whitney and Kruskal-Wallis tests. Differences in quantitative values between measurements were analyzed using repeated measurement ANOVA and Friedman’s test. Logistic regression was used to make a multivariate model for the impact of clinical features, inflammatory predictors, as well as ghrelin and CCK concentrations on appetite and nutritional status. P values < 0.05 were considered significant. The statistical analyses were done using G*Power for windows version 3.1.2. and MedCalc for windows version 11.3.1.
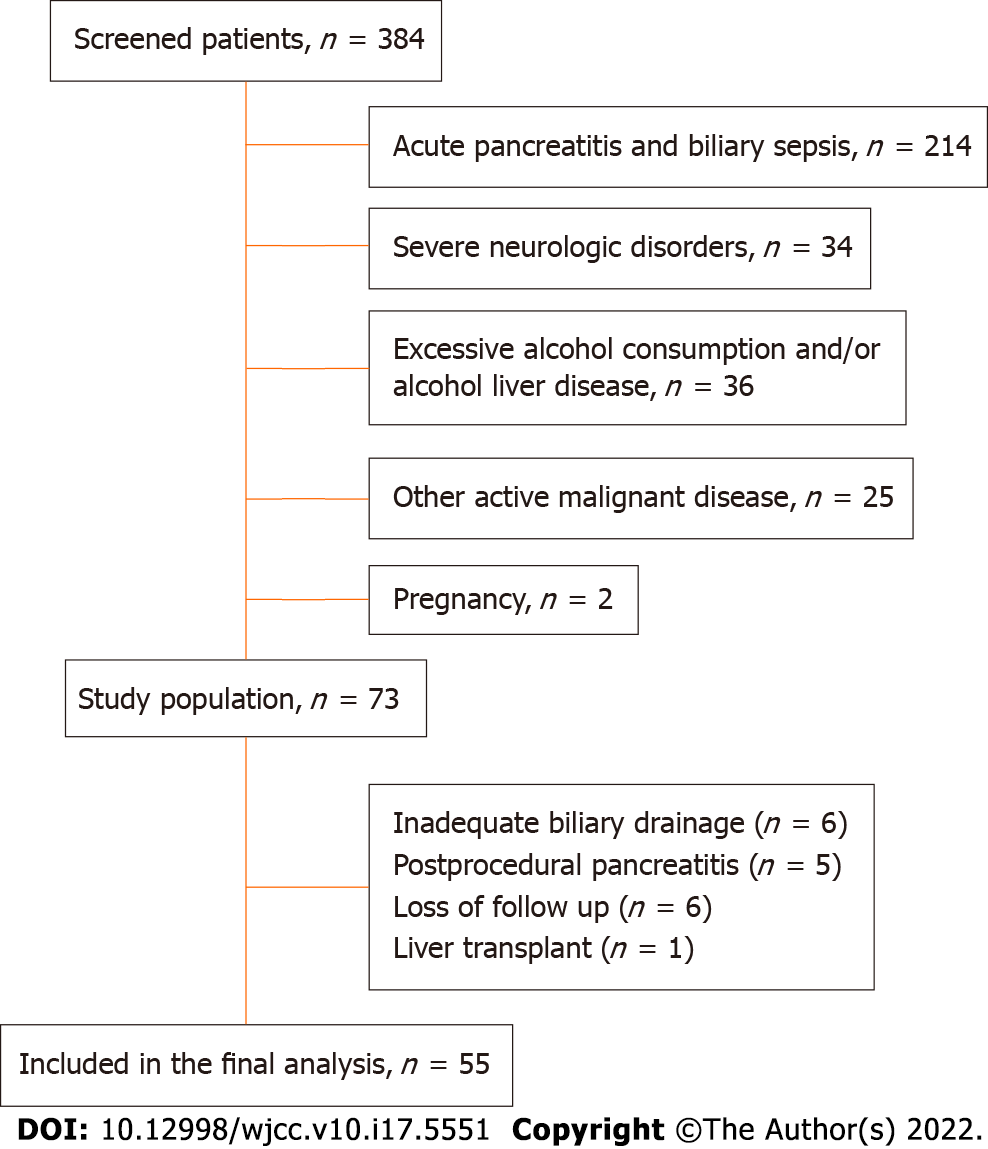
We initially enrolled 73 patients with biliary obstruction out of which 55 were included in the final analysis, as well as 40 healthy sex and age matched controls. Reasons for exclusion of the 18 patients were inadequate biliary drainage in six patients, postprocedural pancreatitis in five patients, loss to follow-up in six, and one patient underwent a liver transplant during the study period in one (Figure 1). Patients with biliary obstruction were further divided into two groups based on etiology of the obstruction; 65% had benign and 57% malignant biliary obstruction. Various clinical characteristics were compared among the groups, and the results are summarized in Table 1. Compared to the control group, patients with biliary obstruction had worse nutritional parameters, as expected, but there was no significant difference in appetite levels (P = 0.641). Patients with malignant biliary obstruction were on average older, and had longer duration of symptoms and worse nutritional parameters compared to those with benign etiology. There was no difference in appetite, pain levels, or prevalence of H. pylori colonization (P = 0.834), while smoking was far more prevalent in patients with benign etiology of obstruction (P = 0.001).
| Benign etiology (n = 34) | Malignant etiology (n = 21) | Control (n = 40) | P value | ||
| Gender | Female | 12 (35%) | 9 (43%) | 13 (32%) | |
| Male | 22 (65%) | 12 (57%) | 27 (68%) | ||
| Age (yr) | Median (Q1-Q3) | 55.0 (43.0-67.0) | 66.0 (60.5-83.0) | 55.0 (43.0-67.0) | |
| Min-max | 33-84 | 55-88 | 39-90 | ||
| Biliary obstruction etiology | Biliary stones | 28 (82%) | |||
| Chronic pancreatitis | 5 (15%) | ||||
| Stenosing papillitis | 1 (3%) | ||||
| Tumor of pancreatic head | 15 (72%) | ||||
| Malignant lymphadenopathy | 4 (19%) | ||||
| Cholangiocarcinoma | 2 (9%) | ||||
| Duration of biliary obstruction (d) | 10.0 (5.0-20.2) | 14.0 (10.0-21.0) | NA | 0.0451 | |
| Body weight (kg) | 80.5 (73.5-92.0) | 61.0 (46.0-75.8) | 72.5 (63.0-75.4) | 0.041 | |
| BMI (kg/m2) | 26.9 (23.7-28.7) | 21.8 (17.0-24.0) | 25.8 (22.9-27.9) | 0.0011 | |
| Weight loss (%) | 3.8 (0.0-7.6) | 12.0 (8.1-18.0) | 0.0 (0.0-0.0) | 0.0022 | |
| Body fat (%) | 24.0 (22.0-25.3) | 19.0 (13.0-24.0) | 29.5 (27.0-32.0) | < 0.0012 | |
| NRS 2002 | 2.5 (0.0-3.3) | 4.0 (3.5-5.0) | 0.0 (0.0-0.0) | < 0.0012 | |
| NRS 2002 | < 3 | 17 (50%) | 2 (9.5%) | 38 (95%) | < 0.0012 |
| ≥ 3 | 17 (50%) | 19 (90.5%) | 2 (5%) | ||
| Appetite (VAS) | 4.0 (2.8-6.3) | 3.0 (2.0-6.0) | 4.0 (2.0-5.0) | 0.6412 | |
| Pain (VAS) | 0.0 (0.0-1.3) | 1.0 (0.0-2.5) | 0.0 (0.0-0.0) | 0.0012 | |
| Helicobacter pylori infection | 12 (35.2%) | 8 (38.1%) | 3 (7.5%) | 0.0052 | |
| Smoking | 15 (44.1%) | 0 (0%) | 7 (17.5%) | < 0.0012 |
Table 2 presents measured laboratory parameters and their comparison between the groups. In patients with biliary obstruction all inflammatory markers (CRP, IL-6, and TNF-α), except leukocyte count, were significantly higher compared to controls. Patients with biliary obstruction also had significantly higher levels of ghrelin and CCK. Regarding etiology, there was no significant difference for most of the measured laboratory parameters between the two groups, except CRP levels which were higher in patients with benign etiology, while bilirubin levels were higher in patients with malignant etiology. There was also no significant difference regarding ghrelin or CCK levels.
| Parameter | Benign etiology (n = 34) | Malignant etiology (n = 21) | P value1 | Controls (n = 40) | P value2 |
| CRP (mg/L) | 13.1 (6.6-19.6) | 6.3 (2.9-11.3) | 0.001 | 2.3 (0.9-3.4) | < 0.001 |
| ES (mm/h) | 25 (13.0-31.5) | 25 (12.0-41.0) | NS | 6.5 (4-13.2) | < 0.001 |
| Leukocytes (× 109) | 6.7 (4.9-8.9) | 6.7 (3.9-7.0) | NS | 5.9 (5.5-7.2) | NS |
| Hemoglobin (g/L) | 141 (123.8-147.0) | 125 (113.5-134.5) | 0.002 | 140 (135.0-156.5) | < 0.001 |
| Thrombocytes (× 109) | 220 (197.2-248.3) | 178 (113.5-289.5) | NS | 241 (188.5-289) | 0.042 |
| Bilirubin (mmol/L) | 85.3 (51.9-183.3) | 270 (145-359.8) | < 0.001 | 12.1 (10.7-14.0) | < 0.001 |
| AST (U/L) | 123 (84.0-212.3) | 155.0 (78.0-186.0) | NS | 19.0 (18.0-23.8) | < 0.001 |
| ALT (U/L) | 337.5 (84.8-547.5) | 232 (114-355.5) | NS | 16.0 (14.0-21.8) | < 0.001 |
| GGT (U/L) | 448 (333.8-602.8) | 396.0 (243.5-892.5) | NS | 18.0 (16.0-25.8) | < 0.001 |
| ALP (U/L) | 287.5 (261.8-418.5) | 281.0 (243.5-434.0) | NS | 83.5 (63.5-94.3) | < 0.001 |
| Blood glucose (mmol/L) | 5.8 (5.2-6.7) | 6.7 (6.3-7.6) | 0.006 | 5.3 (4.8-5.6) | < 0.001 |
| Creatinine (mmol/L) | 89.0 (82.0-110.5) | 105.0 (80.5-123.5) | NS | 84.5 (76.0-104.0) | 0.032 |
| Calcium (mmol/L) | 2.3 (2.2-2.5) | 2.4 (2.3-2.4) | NS | 2.4 (2.3-2.4) | NS |
| LDH (U/L) | 187.5 (152.8-213.5) | 196.0 (175.0-232.5) | NS | 158.0 (147.3-188.0) | 0.004 |
| Cholesterol (U/L) | 5.2 (4.9-6.8) | 5.4 (4.7-8.4) | NS | 6.0 (5.5-6.6) | 0.033 |
| Triglycerides (mmol/L) | 1.9 (1.1-2.3) | 2.4 (1.9-3.1) | 0.005 | 1.3 (1.1-1.6) | < 0.001 |
| Albumin (g/L) | 37.8 (32.9-41.0) | 36.0 (33.1-39.3) | NS | 43.0 (42.0-44.1) | < 0.001 |
| Ghrelin (pg/mL) | 1449.5 (1124.5-2398.8) | 1646.0 (1010.0-4179.0) | NS | 533.5 (368.5-931.5) | < 0.001 |
| CCK (pg/mL) | 291.7 (160.9-411.7) | 191.0 (142.6-218.5) | NS | 153.1 (72.3-262.4) | < 0.001 |
| IL-6 (pg/mL) | 7.0 (5.0-18.3) | 9.0 (80-11.0) | NS | 2.5 (2.0-4.0) | < 0.001 |
| TNF-α (pg/mL) | 11.0 (8.0-12.0) | 12.0 (10.5-14.5) | NS | 6.0 (5.0-7.0) | < 0.001 |
The intensity of biliary obstruction was negatively correlated to ghrelin (r = -0.424; P = 0.001), and positively correlated to TNF-α (r = 0.486, P < 0.001), loss of body weight, and nutritional risk. There was no correlation between the intensity of biliary obstruction and CCK levels and appetite. We did not observe a correlation between duration of biliary obstruction and tested hormones, inflammatory markers, and appetite. We also did not observe the effect of H. pylori colonization on the concentrations of appetite-regulating hormones (ghrelin: τb = - 0.152, P = 0.267, CCK: τb = 0.136, P = 0.323), but appetite levels correlated positively with H. pylori colonization (τb = 0.356, P = 0.008). Of all the inflammatory markers, only TNF-α showed a positive correlation in the case of colonization with H. pylori (τb = 0.396, P = 0.003).
There was a positive correlation between age and CCK levels (r = 0.354, P = 0.008), while no effect was observed for ghrelin (r = -0.104, P = 0.451). Also, there was no statistically significant correlation between age and tested inflammatory markers (IL-6: r = 0.230, P = 0.091; TNF-α: r = 0.180, P = 0.189; CRP: r = -0.105, P = 0.444). As expected, age was associated with higher nutritional risk measured by NRS 2002 (r = 0.454, P = 0.001), greater loss of body weight (r = 0.304, P = 0.024), lower fat percentage (r = -0.309, P = 0.022), and lower albumin levels (r = -0.346, P = 0.010).
A negative correlation between ghrelin and CRP levels was observed (r = -0.37, P = 0.005), while no significant correlation was found between CCK levels and all tested inflammatory markers.
We found that the concentrations of tested humoral parameters were sex-dependent-male gender was associated with higher ghrelin levels (τb = -0.100, P = 0.467), and higher concentrations of IL-6 (τb = -0.371, P = 0.005), TNF-α (τb = -0.341, P = 0.011), and CRP (τb = -0.502, P = 0.001).
Smoking had no effect on the concentration of any of the appetite-regulating hormones, but lower concentrations of TNF-α were observed in smokers (τb = -0.379, P = 0.004). Interestingly, a lower percentage of weight loss was also registered in smokers (τb = -0.521, P < 0.001). Since there were no smokers in the group of patients with malignant biliary obstruction, a subanalysis was performed only for patients with benign etiology, which confirmed that the percentage of weight loss was significantly higher in non-smokers (median 6.3% vs. 1.9%, P = 0.047).
Ghrelin and CCK concentrations did not differ significantly between malnourished patients and those with normal nutritional status, but significantly higher CCK levels were observed in malnourished patients compared to the control group (P = 0.017). Again, TNF-α levels were significantly increased in patients with a high nutritional risk compared to those with normal nutritional status (P = 0.001), although both groups had higher TNF-α levels compared to the control group, as stated earlier. Furthermore, the correlation of inflammatory markers and appetite-regulating hormones with various nutritional parameters was analyzed and the results are summarized in Table 3. The data shows that patients with a higher concentrations of TNF-α experienced significantly higher weight loss. Interestingly, patients with higher BMI and adipose tissue values had significantly higher serum CRP concentrations. Likewise, a positive correlation of BMI and adipose tissue was observed with serum IL-6 and CCK concentrations, while a negative correlation existed with serum ghrelin concentration. Again, we could not establish a significant correlation between the levels of tested inflammatory and hormonal markers and appetite, but a negative correlation was observed between appetite and pain level (r = -0.306, P = 0.023).
| Spearman's correlation coefficient | CRP (mg/L) | IL-6 (pg/mL) | TNF-α (pg/mL) | Ghrelin (pg/mL) | CCK (pg/mL) | |
| Weight loss (%) | rho | -0.21 | 0.263 | 0.438 | 0.12 | 0.242 |
| P | 0.124 | 0.052 | 0.001 | 0.385 | 0.075 | |
| BMI (kg/m2) | rho | 0.638 | 0.31 | 0.024 | -0.342 | 0.411 |
| P | 0.001 | 0.021 | 0.861 | 0.011 | 0.002 | |
| NRS2002 | rho | -0.236 | 0.193 | 0.355 | -0.057 | 0.142 |
| P | 0.083 | 0.157 | 0.008 | 0.682 | 0.302 | |
| Adipose tissue (%) | rho | 0.313 | 0.136 | 0.069 | -0.348 | 0.289 |
| P | 0.02 | 0.324 | 0.617 | 0.009 | 0.033 | |
| Albumin (g/L) | rho | 0.002 | -0.384 | -0.461 | -0.024 | -0.026 |
| P | 0.987 | 0.004 | 0.001 | 0.861 | 0.848 | |
| Cholesterol (U/L) | rho | -0.162 | 0.054 | -0.212 | 0.209 | 0.355 |
| P | 0.238 | 0.696 | 0.121 | 0.125 | 0.008 | |
| Apetite (VAS) | rho | 0.047 | 0.039 | 0.037 | 0.027 | -0.121 |
| P | 0.733 | 0.776 | 0.786 | 0.847 | 0.379 | |
The tested parameters were repeated after successful biliary drainage and differences across multiple measurements are summarized in Table 4.
| During biliary obstruction (n = 55) | 48 h after drainage (n = 55) | 28 d after drainage (n = 55) | P value1 | |
| Bilirubin (mmol/L) | 145 (63.9-230.7) | 88.5 (38.75-190.5) | 25 (18.85-38.7) | < 0.001 |
| ALT (U/L) | 133 (78-194) | 91.5 (63.75-144.25) | 30 (23-46.5) | < 0.001 |
| GGT (U/L) | 278 (103-421) | 194 (103.25-267.25) | 36 (28-64) | < 0.001 |
| ALP (U/L) | 448 (288.5-612.5) | 382.5 (184-606.5) | 85 (61-111) | < 0.001 |
| AST (U/L) | 284 (260.5-382) | 281.5 (231.75-429.25) | 123 (103.5-166) | < 0.001 |
| CRP (mg/L) | 10.0 (5.8-14.1) | 17.9 (5.9-26.8) | 8.3 (2.5-17.3) | 0.009 |
| IL-6 (pg/mL) | 9 (6-13) | 11 (7-22) | 9 (5-21.5) | 0.332 |
| TNF-α (pg/mL) | 11 (8.5-13) | 10 (9-12) | 10 (8-12.5) | 0.088 |
| Ghrelin (pg/mL) | 1549 (1079.5-2400.5) | 1648.5 (1137.25-2767) | 1612 (891.5-2962) | 0.552 |
| Cholecystokinin (pg/mL) | 213.0 (161.0-380.7) | 266.0 (203.0-400.5) | 235.0 (158.0-433.0) | 0.03 |
| Albumin (g/L) | 37.1 (33.0-40.8) | 35.0 (32.4 -38.0) | 38.0 (35.0-42.0) | < 0.001 |
| Appetite (VAS) | 4 (2-6) | 6 (4-8) | 7 (6-9) | < 0.001 |
| BMI (kg/m2) | 24.5 (21.3-28.2) | 24.4 (20.6-27.9) | 24.9 (21.2-26.8) | < 0.001 |
| Body fat (%) | 23 (17-25) | 23 (17-24) | 23 (19-25) | 0.002 |
| NRS 2002 | 3.0 (2.0-4.0) | 3.5 (2.0-4.0) | 0.0 (0.0-4.0) | < 0.001 |
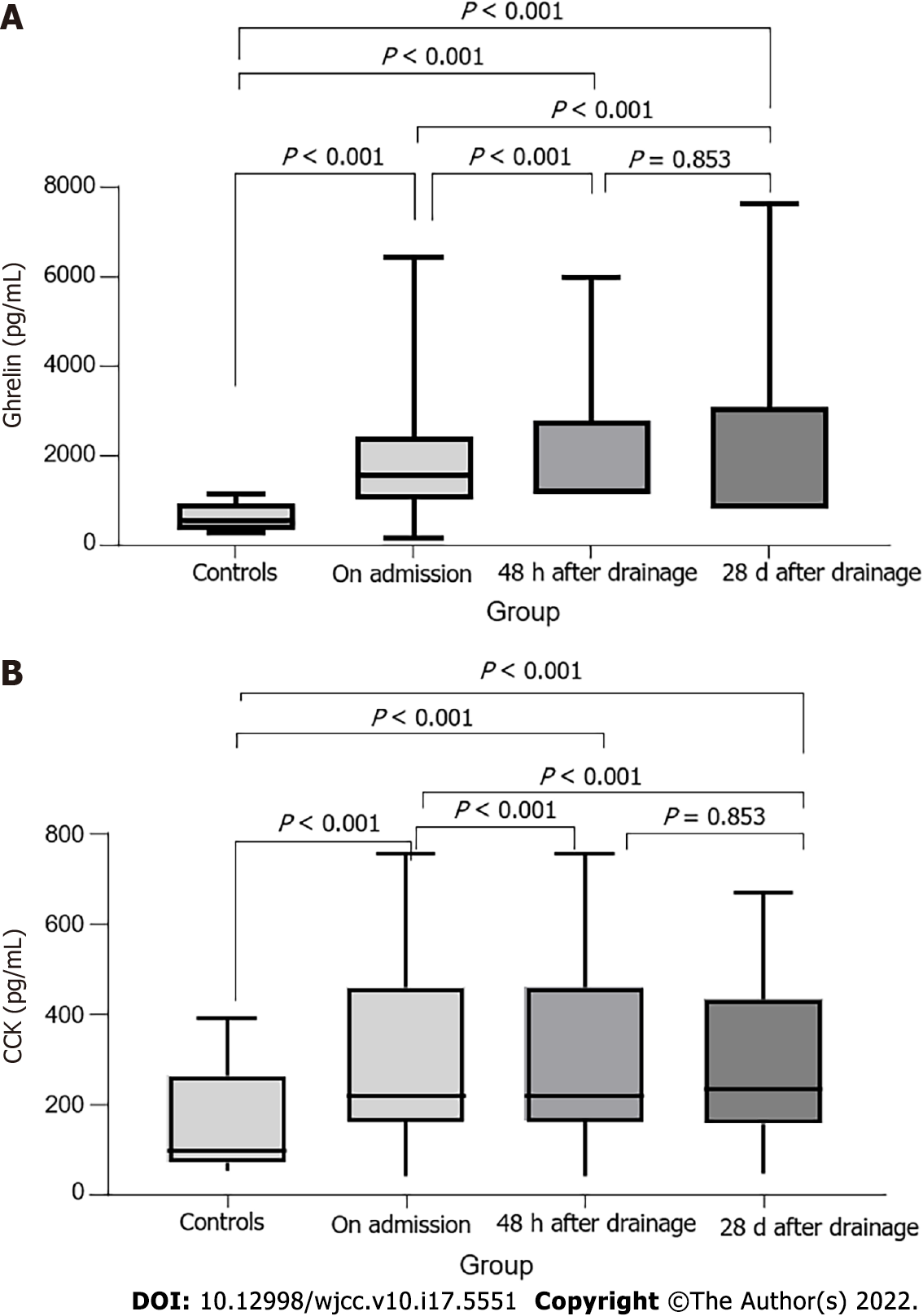
As expected, biliary drainage had a positive effect on the reduction of all cholestatic parameters. CRP levels increased significantly 48 h after biliary drainage and resolved to lower than initial values after 28 d. IL-6, TNF-α, and ghrelin concentrations did not significantly change after biliary drainage. CCK concentration showed significant dynamics, 48 h after biliary drainage it increased, but after 28 d it dropped to a value which was significantly higher than initial measurement, but lower than the one 48 h after intervention (Figure 2). There was significant improvement in appetite after biliary drainage as well as in relevant nutritional parameters.
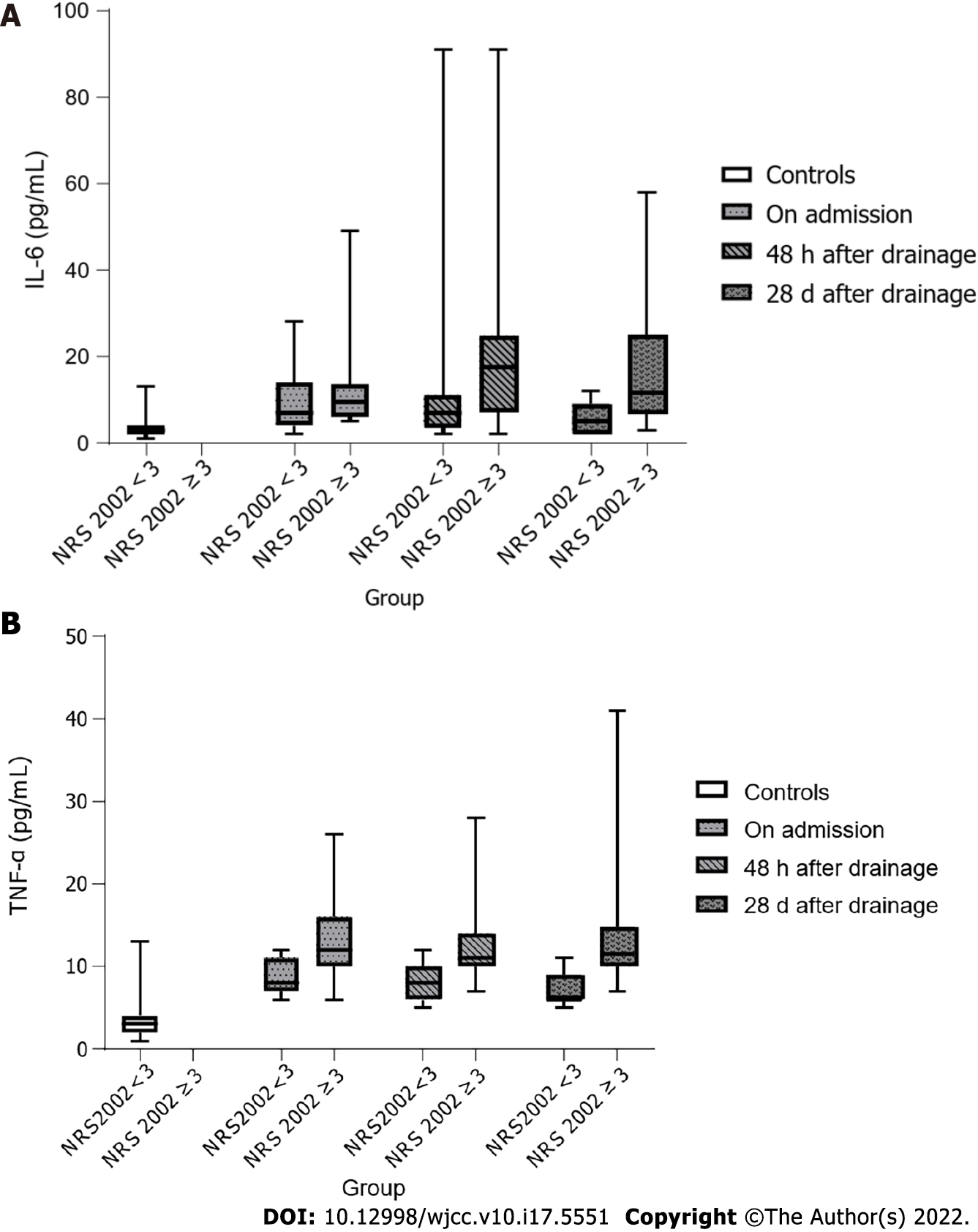
Ghrelin levels remained elevated in both patients with and without nutritional risk even 28 d after resolution of biliary obstruction (P < 0.001). CCK concentrations also did not show a difference between malnourished patients and those with normal nutritional status, but significantly higher CCK concentrations were now observed in both patient groups compared to the controls. IL-6 and TNF-α concentrations were significantly higher in the serum of malnourished patients, while there were no longer significant differences in TNF-α concentration between the control group and patients with normal nutritional status (Figure 3).
Predictive factors associated with higher nutritional risk were increased TNF-α at the initial measurement [odds ratio (OR): 2.03, 95% confidence interval (CI)] and increased IL-6 and TNF-α concentrations 48 h after biliary drainage (OR: 1.88, 95%CI: 1.12-3.15 and OR: 5.01, 95%CI: 1.19-21.12, respectively). Higher concentration of CCK 28 d after successful biliary drainage significantly reduced the chance of nutritional risk by 1.01 times. The regression model for predicting the influence of ghrelin, CCK, and serum inflammatory markers on appetite did not show statistical significance during the biliary obstruction and 28 d after its resolution. However, 48 h after resolution of the obstruction, higher IL-6 concentration (OR: 1.07, 95%CI: 1.00-1.14, P = 0.043) increased the chance of having better appetite, while higher CCK concentration (OR: 0.99, 95%CI: 0.99-1.00, P = 0.032) and leukocyte count (OR: 0.62, 95%CI: 0.38-0.99, P = 0.045) decreased that chance.
During the follow-up period of 6 mo, eight patients with malignant biliary obstruction died-the cause of death was pneumonia in one patient and pulmonary embolism in three, one patient died within 7 d after hepatectomy, and for three patients the cause of death was unknown. The remaining 47 patients were clinically stable. Patients who died at the time of the initial measurement had greater loss in body weight, lower appetite, higher NRS 2002 scores, and higher concentrations of TNF-α and CRP, and 28 d after biliary drainage, they also had worse NRS 2002 scores and appetite levels, reported higher pain levels, and had higher concentrations of TNF-α and IL-6, as well as lower CCK and albumin concentrations. In a sub-group analysis, in patients with malignant etiology higher concentrations of TNF-α (P = 0.018) and IL-6 (P = 0.003) in initial measurement and after 28 d were significantly correlated with death. We observed significant dynamics in CCK levels across the measurements in the surviving group (P = 0.003), while no changes have been noted for the group with a negative outcome. There were no significant changes in ghrelin levels regardless of the outcome. TNF-α (P = 0.004) levels were higher at initial measurement in the group with a negative clinical outcome, while IL-6 levels did not significantly differ. The percentage of body fat was higher in surviving patients, and it also showed a significant increase across the measurements (P < 0.001), while there was no significant dynamic in deceased patients. Surviving patients had better appetite, although both groups showed a significant increase in appetite across measurements.
Our results confirmed observations made in previous studies[1,2,4,5] that patients with biliary obstruction, and especially those with malignant etiology, generally have worse nutritional indicators compared to healthy controls. To the best of our knowledge, this is the first study to prove elevated ghrelin concentrations in patients with biliary obstruction. At the same time, CCK concentrations were significantly elevated in malnourished patients compared to controls. There was a positive correlation of CCK with BMI and percentage of body fat, while the same parameters correlated negatively with ghrelin. These results are in accordance with studies suggesting that ghrelin is a clinical marker of catabolism, since elevated ghrelin levels are found in serum of patients with cachexia associated with malignant diseases and chronic kidney failure[28]. Our results also support the theory that circulating ghrelin reflects changes in body weight over a longer period of time and that it could be an adiposity signal[29-31]. Contrary to expectations, in the correlation analysis we found a negative association of biliary obstruction severity with ghrelin concentrations, while no association was found with serum CCK concentration. This confirms the results of Koop and associates that a chronic decrease in the amount of bile in the duodenum does not have an effect on serum CCK concentration[32]. However, in a study by Padillo et al[4], which had a similar design to our study, serum bilirubin showed a clear correlation to CCK concentration. Different findings could be partially explained by higher inclusion criterion regarding bilirubin values (> 85 μmol/L compared to > 35μmol/L in our study); their study also had a higher proportion of patients with pancreatic malignancies who had significantly higher bilirubin concentrations. Our results did show a tendency toward positive correlation between CCK and bilirubin, but this was only borderline significant, so these inconsistent findings might be due to a small sample size along with high variations among subjects.
All tested inflammatory markers, including IL-6, TNF-α, and CRP, were significantly elevated in patients with biliary obstruction. When comparing the etiology of the obstruction, only CRP values were significantly elevated in patients with benign biliary obstruction (P = 0.001), while there was no difference in TNF-α or IL-6 concentrations. However, TNF-α showed a positive correlation with both biliary obstruction severity and nutritional risk. Furthermore, TNF-α proved to be a prognostic factor of malnutrition in a binary logistic model. Interestingly, CRP correlated positively with body mass index and body fat, while simultaneously having a negative correlation with ghrelin. This is another indirect proof of ghrelin as a marker of catabolism and signal of changes in the amount of adipose tissue.
We have not established any significant correlation between appetite and appetite regulating hormones or inflammatory markers. The only two parameters for which we found correlation with appetite were pain level and H. pylori colonization. Appetite expectedly correlated negatively with pain intensity. Interestingly, although H. pylori colonization was more prevalent among subjects with biliary obstruction compared to the control group, H. pylori colonization was associated with better appetite. H. pylori colonization did not show correlation with ghrelin concentration. Most studies suggest that H. pylori colonization negatively affects ghrelin production and that H. pylori eradication leads to an increase in ghrelin levels, appetite and BMI[33-35]. However, a clear mechanism of these effects is not yet understood[36].
Our study showed a positive correlation between age and CCK concentration, but not for ghrelin. We have also found that patients older than 65 years had significantly higher CCK levels, while there was no difference in ghrelin, IL-6, or TNF-α concentrations. These results are in accordance with previous studies[12,37,38] and indicate that CCK is a potential mediator of anorexia of aging. Appetite level was not significantly different regarding the age of subjects, but older patients had significantly worse nutritional parameters, including loss of body weight, albumin concentration, and percentage of body fat. However, BMI did not significantly differ between older and younger patients, which confirms that it is not a precise tool and should only be used in context with other nutritional parameters[7].
All relevant nutritional parameters expectedly showed significant improvement after resolution of biliary obstruction. Regarding inflammatory factors, only CRP concentration showed significant changes after biliary drainage (first increasing and then decreasing to lower than initial values). A possible explanation for these results is transient asymptomatic bacteremia after ERCP, which, according to some studies, may occur in up to 27% of therapeutic procedures[39,40]. It is important to note that patients who had clinical complications after the procedure were excluded from the analysis. Although IL-6 and TNF-α concentrations did not show significant changes, their concentrations after biliary drainage were higher in malnourished patients and patients with malignant obstruction. Previous studies done in patients with malignant obstruction also have not found significant changes after biliary drainage in IL-6 and TNF-α levels, even after a prolonged follow-up[41,42]. One of the possible explanations for this phenomenon can be found in the study of Rosen et al[43] in which they investigated biliary concentration of IL-6 and TNF-α in patients undergoing ERCP; these cytokines have been shown to be sensitive markers of subclinical biliary infection. One can assume that aside from the tumor role in activation of acute-phase reactants, the increased levels of TNF-α and IL-6 might be a host response to contamination and biliary tree infection after loss of Oddi sphincter control due to biliary sphincterotomy.
After resolution of biliary obstruction, both ghrelin and CCK levels were not significantly different between malnourished and patients with normal nutritional status. Higher ghrelin levels persisted after biliary drainage, without changing significantly between the measurements. Ghrelin levels also did not significantly differ regarding the etiology of the obstruction. Contrary to ghrelin, we observed a significant increase in CCK concentration 48 h after intervention, followed by a significant decrease after 28 d. The increase in CCK concentration might be explained by a transient postprocedural bacteremia[40] and subsequent lipopolysaccharide and IL-1 production, which are known stimulators for CCK secretion[44]. Furthermore, we showed that patients with malignant obstruction who died had lower CCK levels initially, and a more pronounced decrease in CCK levels after 28 d compared to patients who survived. The same dynamic was also observed for malnourished patients. Both malnourished and deceased patients had higher IL-6 and TNF-α levels both initially and after 28 d. There is evidence in animal models that prolonged IL-6 secretion leads to a decrease in IL-1 activity through increased secretion of soluble IL-1R[44]. We hypothesize that in patients with advanced malignant disease and malnourishment prolonged inflammation, secretion of IL-6 and TNF-α leads to suppression of CCK secretion, possibly through lower IL-1 activity[44], but this requires further studies. Logistic regression model also showed that higher CCK levels after 28 d reduce chances of malnutrition. Although there was no significant difference in appetite level between the control group and patients with biliary obstruction, we observed a significant increase in appetite levels after a successful biliary drainage. During biliary obstruction and 28 d after its resolution, ghrelin and CCK levels were not predictive of appetite levels. However, higher CCK levels 48 h after biliary drainage were predictive of decreased appetite.
Among other investigated parameters, higher loss of body weight, malnutrition, lower appetite, and lower CRP concentration and higher concentration of TNF-α in serum were associated with negative clinical outcome after 6 mo. Factors correlating with negative outcome after biliary obstruction resolution were higher values of IL-6 and TNF-α, as well as lower CCK concentration, poorer appetite and lower albumin concentrations. This confirms previous findings of lower albumin concentration as a predictor of mortality in hospitalized patients[8].
Ghrelin can have an anti-inflammatory effect by inhibiting the expression of IL-6 and TNF-α in humans[45]. High ghrelin levels possibly represent a compensatory mechanism due to persistent inflammation considering that, along with ghrelin, both TNF-α and IL-6 levels were elevated 28 d after resolution of biliary obstruction. We have demonstrated a correlation between TNF-α and weight loss, as well as increased TNF-α levels in malnourished patients, which is in accordance with previous studies suggesting that TNF-α and IL-6 are mediators of cachexia and increased catabolism[12,46]. Increased plasma ghrelin concentration could therefore reflect a compensatory mechanism for negative energy balance. The fact that appetite level was not increased despite increased plasma ghrelin concentration could suggest ghrelin resistance. Moreover, the increase in appetite after biliary drainage, despite increased TNF-α and IL-6 levels, suggests the existence of additional factors of appetite regulation. Des-acyl ghrelin is a ghrelin isoform that does not activate growth hormone secretagogue receptor-1a, and was previously thought to be a byproduct of ghrelin degradation, but recent research indicates it is a distinct hormone with a wide variety of biological activity. It has been extensively studied in feeding disorders, obesity, and cardiovascular diseases[16,25,47-49]. Although specific pathways are not yet understood, studies suggested that des-acyl ghrelin might exhibit opposite effects to those that of acylated ghrelin in peripheral tissues[16,47,50,51], so measuring des-acyl ghrelin concentration and its relation to acylated ghrelin in future studies might lead to better interpretation of the results.
Previous research indicated functional antagonism between ghrelin and CCK regarding food intake[52], but their interaction on secretion of both peptides is not well understood. An animal study showed that intraduodenal ghrelin infusion increases CCK concentration[52], and human research indicated that CCK can suppress ghrelin secretion[14,17]. Nonetheless, in our study group, both high CCK and ghrelin concentrations persisted. A previous study showed that application of ghrelin after CCK infusion does not induce feeding and vice versa, and CCK application after ghrelin infusion does not reduce food intake[53]. This suggests that the efficacy of ghrelin and CCK signaling depends on their mutual balance. Disruption of that balance, such as that being able to be seen in patients with biliary obstruction, can lead to dysfunction in appetite regulation which, according to our results, can be restored, but through some other mechanisms. This shows that changes in concentrations of appetite regulating hormones and inflammatory factors play only a small part in feeding regulation. More studies are needed to gain a better grasp of underlying mechanisms involved in this complex process.
The overall small sample size was a constraint in this study. Despite the initial power analysis, we have observed some differences between patients with malignant and benign etiology of biliary obstruction, but the separate subgroup analysis of the effects of biliary drainage could not be performed due to the lack of power. Unacylated ghrelin was not measured due to the biochemical reagent being unavailable at the time of the study. Since we had very limited data on CCK and ghrelin changes in this specific population, we have decided to increase a follow-up period bearing in mind the results of the only available study (6 d, Padillo et al[5]), but our results have shown that even a period of 28 d follow-up was too short to observe the complete dynamics of these hormones following a resolution of biliary obstruction.
Patients with biliary obstruction have higher plasma concentrations of ghrelin, as well as increased concentrations of TNF-α, IL-6, and CRP and worse nutritional status. Resolution of biliary obstruction via endoscopic drainage leads to improvement in nutritional status and appetite, but levels of ghrelin and CCK remain increased even 28 d after drainage. Biliary drainage has no effect on the dynamics of TNF-α and IL-6 concentrations, with higher levels noted in patients with malignant disease. Concentration of CCK is higher in patients with biliary obstruction and worse nutritional status. Appetite does not correlate with ghrelin, CCK, IL-6, or TNF-α serum concentrations before or after resolution of biliary obstruction. In patients with malignant obstruction, negative clinical outcome after 6 mo is associated with greater loss of body weight, lower appetite levels, and higher TNF-α concentrations.
The basic assumption of this study is based on the observation from everyday clinical practice that obstructive jaundice has a negative effect on the nutritional status of patients regardless of the obstruction etiology. Nutritional parameters play an important role in the treatment outcome of these patients.
Given the conflicting results of a very small number of studies determining the concentrations of appetite-regulating hormones in patients with biliary obstruction and their changes after cholestasis resolution, we considered that it is of scientific interest to investigate key mediators regulating the appetite and nutritional status of patients before and after biliary obstruction resolution.
The research objectives were to determine the levels of ghrelin, cholecystokinin and inflammatory markers in patients with obstructive jaundice, and to analyze their effect on appetite and nutritional status; to investigate the influence of the severity, duration and etiology of biliary obstruction, Helicobacter pylori infection and general characteristics of patients on the concentrations of hormones and inflammatory markers and the impact of endoscopic internal biliary drainage on the investigated parameters.
This was a prospective case control study performed in a tertiary center in Zagreb, Croatia. Fifty-five patients (34 with benign and 21 with malignant disease) with biliary obstruction undergoing internal biliary drainage, along with 40 healthy controls, were enrolled. Appetite, nutritional status, serum ghrelin, cholecystokinin (CCK), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) were determined at admission, 48 h and 28 d after internal biliary drainage. Chi square test was used for categorical variables. Continuous variables were analyzed for normality by Kolmogorov–Smirnov test and relevant non-parametric (Mann-Whitney, Kruskal-Wallis, and Friedman) or parametric (t-test and ANOVA) tests were used.
Plasma ghrelin, IL-6, TNF-α, and C-reactive protein (CRP) were significantly higher in patients with obstructive jaundice. An increase in CCK was observed only in malnourished patients with obstructive jaundice. TNF-α was a predictive factor for malnutrition in obstructive jaundice. After internal biliary drainage, a significant improvement of nutritional status was observed in spite of the fact that concentrations of ghrelin, CCK, IL-6, and TNF-α remained significantly elevated even 28 d after procedure. We have not established any correlation between appetite and serum levels of ghrelin, CCK, IL-6, and TNF-α before and after biliary drainage. Malnutrition, lower appetite, lower serum CRP and higher TNF-α in patients with obstructive jaundice were associated with long-term mortality.
The efficacy of ghrelin and CCK signaling depends on their mutual balance. Disruption of that balance, such as it that be able to be seen in patients with biliary obstruction, can lead to dysfunction in appetite regulation which, according to our results, can be restored, but through some other mechanisms. This shows that changes in concentrations of appetite regulating hormones and inflammatory factors play only a small part in feeding regulation.
Changes in the concentration of appetite-regulating hormones and inflammatory markers are only a part of the feeding regulation process, which will certainly continue to be the subject of numerous future studies due to its complexity.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdalla MMI, Malaysia; Tan X, China S-Editor: Guo XR L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Pavlidis ET, Pavlidis TE. Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int. 2018;17:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Boulay BR, Birg A. Malignant biliary obstruction: From palliation to treatment. World J Gastrointest Oncol. 2016;8:498-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Gondal B, Aronsohn A. A Systematic Approach to Patients with Jaundice. Semin Intervent Radiol. 2016;33:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Padillo FJ, Andicoberry B, Muntane J, Lozano JM, Miño G, Sitges-Serra A, Pera-Madrazo C. Factors predicting nutritional derangements in patients with obstructive jaundice: multivariate analysis. World J Surg. 2001;25:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Padillo FJ, Andicoberry B, Pera-Madrazo C, Sitges-Serra A. Anorexia and malnutrition in patients with obstructive jaundice. Nutrition. 2002;18:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | La Torre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol. 2013;107:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Pavic T, Ljubicic N, Stojsavljevic S, Krznaric Z. Nutritional screening model in tertiary medical unit in Croatia. Ann Nutr Metab. 2012;61:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983;24:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 430] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 937] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 11. | Langhans W, Hrupka B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides. 1999;33:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Patel HJ, Patel BM. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017;170:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 13. | Di Francesco V, Fantin F, Omizzolo F, Residori L, Bissoli L, Bosello O, Zamboni M. The anorexia of aging. Dig Dis. 2007;25:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Degen L, Drewe J, Piccoli F, Gräni K, Oesch S, Bunea R, D'Amato M, Beglinger C. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1391-R1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Wang HH, Portincasa P, Wang DQ. Update on the Molecular Mechanisms Underlying the Effect of Cholecystokinin and Cholecystokinin-1 Receptor on the Formation of Cholesterol Gallstones. Curr Med Chem. 2019;26:3407-3423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Yanagi S, Sato T, Kangawa K, Nakazato M. The Homeostatic Force of Ghrelin. Cell Metab. 2018;27:786-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 17. | Brennan IM, Otto B, Feltrin KL, Meyer JH, Horowitz M, Feinle-Bisset C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides. 2007;28:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev. 2017;97:411-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 466] [Cited by in RCA: 407] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 19. | Qu R, Chen X, Hu J, Fu Y, Peng J, Li Y, Chen J, Li P, Liu L, Cao J, Wang W, Qiu C, Guo L, Vasilev K, Zhou G, Li W, Zhao Y. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci Rep. 2019;9:1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Himmerich H, Sheldrick AJ. TNF-alpha and ghrelin: opposite effects on immune system, metabolism and mental health. Protein Pept Lett. 2010;17:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Ellacott KL, Halatchev IG, Cone RD. Interactions between gut peptides and the central melanocortin system in the egulation of energy homeostasis. Peptides. 2006;27:340-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Meyer-Gerspach AC, Häfliger S, Meili J, Doody A, Rehfeld JF, Drewe J, Beglinger C, Wölnerhanssen B. Effect of L-Tryptophan and L-Leucine on Gut Hormone Secretion, Appetite Feelings and Gastric Emptying Rates in Lean and Non-Diabetic Obese Participants: A Randomized, Double-Blind, Parallel-Group Trial. PLoS One. 2016;11:e0166758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Pi-Sunyer X, Kissileff HR, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol Behav. 1982;29:627-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 131] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Wisser AS, Habbel P, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Interactions of gastrointestinal peptides: ghrelin and its anorexigenic antagonists. Int J Pept. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Padillo F, Rodríguez Mf, Hervas A, Miño G, Sitges-Serra A, Pera-Madrazo C. Nutritional assessment of patients with benign and malignant obstructions of the biliary tract. Rev Esp Enferm Dig. 1999;91:622-629. [PubMed] |
| 27. | Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1900] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 28. | Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 270] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Al Massadi O, López M, Tschöp M, Diéguez C, Nogueiras R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017;40:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Ma X, Lin L, Yue J, Wu CS, Guo CA, Wang R, Yu KJ, Devaraj S, Murano P, Chen Z, Sun Y. Suppression of Ghrelin Exacerbates HFCS-Induced Adiposity and Insulin Resistance. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Mihalache L, Gherasim A, Niţă O, Ungureanu MC, Pădureanu SS, Gavril RS, Arhire LI. Effects of ghrelin in energy balance and body weight homeostasis. Hormones (Athens). 2016;15:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Koop I, Koop H, Gerhardt C, Schafmayer A, Arnold R. Do bile acids exert a negative feedback control of cholecystokinin release? Scand J Gastroenterol. 1989;24:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014;20:12781-12808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 188] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (3)] |
| 34. | Jeffery PL, McGuckin MA, Linden SK. Endocrine impact of Helicobacter pylori: focus on ghrelin and ghrelin o-acyltransferase. World J Gastroenterol. 2011;17:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S. Influence of H pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol. 2007;13:1595-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Boltin D, Niv Y. Ghrelin, Helicobacter pylori and body mass: is there an association? Isr Med Assoc J. 2012;14:130-132. [PubMed] |
| 37. | Moss C, Dhillo WS, Frost G, Hickson M. Gastrointestinal hormones: the regulation of appetite and the anorexia of ageing. J Hum Nutr Diet. 2012;25:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Akimoto S, Miyasaka K. Age-associated changes of appetite-regulating peptides. Geriatr Gerontol Int. 2010;10 Suppl 1:S107-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Kullman E, Borch K, Lindström E, Anséhn S, Ihse I, Anderberg B. Bacteremia following diagnostic and therapeutic ERCP. Gastrointest Endosc. 1992;38:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Kovaleva J, Peters FT, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26:231-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 41. | Ballinger AB, Woolley JA, Ahmed M, Mulcahy H, Alstead EM, Landon J, Clark ML, Farthing MJ. Persistent systemic inflammatory response after stent insertion in patients with malignant bile duct obstruction. Gut. 1998;42:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Rosen HR, Winkle PJ, Kendall BJ, Diehl DL. Biliary interleukin-6 and tumor necrosis factor-alpha in patients undergoing endoscopic retrograde cholangiopancreatography. Dig Dis Sci. 1997;42:1290-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Cross-Mellor SK, Kent WD, Ossenkopp KP, Kavaliers M. Differential effects of lipopolysaccharide and cholecystokinin on sucrose intake and palatability. Am J Physiol. 1999;277:R705-R715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 362] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 47. | Nishida T, Tsubouchi H, Hamada T, Imamura N, Hiyoshi M, Yano K, Kangawa K, Nakazato M, Nanashima A. Plasma desacyl ghrelin-to-acyl ghrelin ratio is a predictor of postoperative complications and prognosis after pancreaticoduodenectomy. Oncol Lett. 2019;18:4974-4983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, Ortolano S, Pani G, Athanasopoulou S, Gonos ES, Schosserer M, Grillari J, Peterson P, Tuna BG, Dogan S, Meyer A, van Os R, Trendelenburg AU. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 335] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 49. | Stark R, Santos VV, Geenen B, Cabral A, Dinan T, Bayliss JA, Lockie SH, Reichenbach A, Lemus MB, Perello M, Spencer SJ, Kozicz T, Andrews ZB. Des-Acyl Ghrelin and Ghrelin O-Acyltransferase Regulate Hypothalamic-Pituitary-Adrenal Axis Activation and Anxiety in Response to Acute Stress. Endocrinology. 2016;157:3946-3957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D'Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4:437-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 775] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 52. | Stengel A, Taché Y. Interaction between gastric and upper small intestinal hormones in the regulation of hunger and satiety: ghrelin and cholecystokinin take the central stage. Curr Protein Pept Sci. 2011;12:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518-3525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |