Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3401
Peer-review started: October 27, 2021
First decision: December 27, 2021
Revised: January 8, 2022
Accepted: February 17, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 163 Days and 1 Hours
Previous studies have found that hyperuricaemia (HUA) is closely related to intestinal flora imbalance.
The current study investigated the effects and safety of washed microbiota transplantation (WMT) on serum uric acid (SUA) levels in different populations.
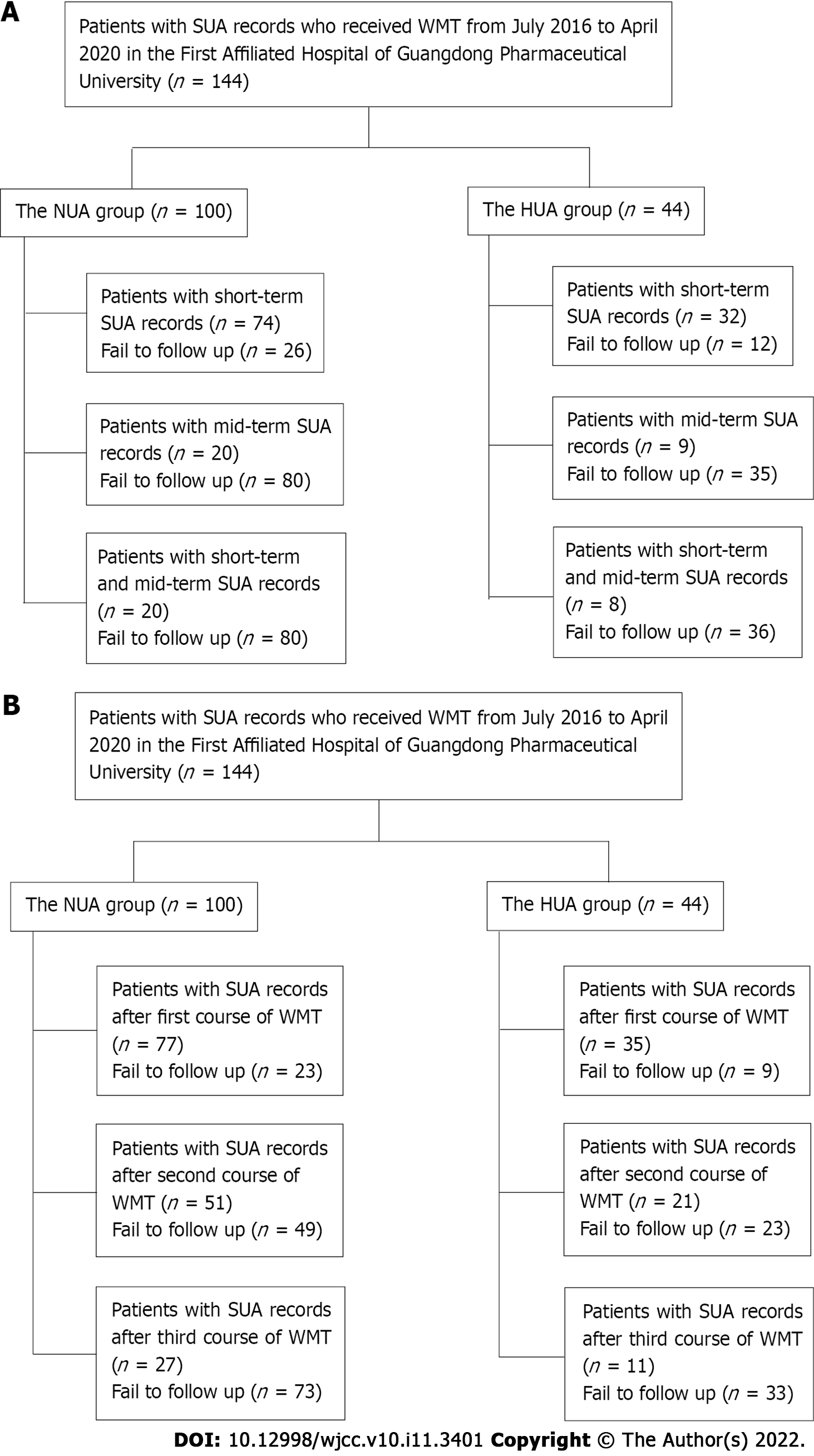
A total of 144 patients who received WMT from July 2016 to April 2020 in the First Affiliated Hospital of Guangdong Pharmaceutical University and had SUA data before treatment were selected. Changes in SUA levels before and after treatment were retrospectively reviewed based on short-term and mid-term effects of WMT regimens. SUA levels measured in the last test within 3 mo after the first WMT represented the short-term effect, and SUA levels measured in the last test within 3-6 mo after the first WMT represented the mid-term effect. The patients were divided into an HUA group (SUA > 416 μM) and a normal uric acid (NUA) group (SUA ≥ 202 μM to ≤ 416 μM) based on pretreatment SUA levels.
Average short-term SUA levels in the HUA group decreased after WMT (481.00 ± 99.85 vs 546.81 ± 109.64 μM, n = 32, P < 0.05) in 25/32 patients and returned to normal in 10/32 patients. The short-term level of SUA reduction after treatment moderately correlated with SUA levels before treatment (r = 0.549, R² = 0.300, P < 0.05). Average SUA levels decreased after the first and second courses of WMT (469.74 ± 97.68 vs 540.00 ± 107.16 μM, n = 35, and 465.57 ± 88.88 vs 513.19 ± 78.14 μM, n = 21, P < 0.05). Short-term and mid-term SUA levels after WMT and SUA levels after the first, second and third courses of WMT were similar to the levels before WMT in the NUA group (P > 0.05). Only 1/144 patients developed mild diarrhea after WMT.
WMT reduces short-term SUA levels in patients with HUA with mild side effects but has no obvious effect on SUA levels in patients with NUA.
Core Tip: In this study, we demonstrate that washed microbiota transplantation (WMT) can lower serum uric acid (SUA) levels in patients with hyperuricaemia in the short term with only mild side effects but that WMT has no obvious effect on the SUA levels of people with normal uric acid levels.
- Citation: Cai JR, Chen XW, He YJ, Wu B, Zhang M, Wu LH. Washed microbiota transplantation reduces serum uric acid levels in patients with hyperuricaemia. World J Clin Cases 2022; 10(11): 3401-3413
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3401.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3401
Nutrition-related diseases caused by changes in people’s diet are gradually increasing, and hyperuricaemia (HUA) has gradually developed into a global public health problem. HUA is a common disease in China. The prevalence of HUA in the Chinese population is 13%[1]. HUA is a metabolic syndrome related to obesity, insulin resistance, diabetes, coronary artery disease and hypertension, but it is also a pathological state that causes uric acid (UA) crystal deposition in bones, joints and kidneys, which may lead to gout, urolithiasis and UA nephropathy[2,3].
The traditional treatment methods for HUA include maintaining a healthy lifestyle, UA-lowering drugs, alkalizing urine, and traditional Chinese medicine. Existing drugs for the treatment of HUA include xanthine oxidase inhibitors (alopurinol) to inhibit UA production, benzbromarone, which promotes UA excretion, and UA enzymes that promote UA decomposition[4,5]. However, these drugs have adverse reactions, such as gastrointestinal discomfort, diarrhoea, and rash, and some patients have poor tolerance. Patients also need to take drugs regularly for a long time, which causes a long-term burden for patients and their families because the existing UA-lowering drugs are associated with a withdrawal and rebound phenomenon[4,5]. Due to the limitations of drug use, novel therapeutic approaches are needed in the treatment of HUA.
The functional status of the intestines and kidneys is critical to the metabolism of UA. The daily production and excretion of UA in the human body is 600-700 mg. Approximately two-thirds of this amount is excreted by the kidneys, and one-third is excreted through the intestines. The intestinal pathway compensates in cases of renal damage and becomes the main pathway for urate elimination[6]. The imbalance between probiotics and pathogenic bacteria affects the expression of intestinal tight junction proteins, which increases the permeability of the intestinal mucosa barrier, leads to gut-derived lipopolysaccharide (LPS) translocation and causes kidney damage via blood circulation. These changes induce renal UA excretion disorder, reduce UA excretion and increase serum UA (SUA) levels[7,8]. The intestinal flora also regulates adenosine triphosphate-binding cassette superfamily G member 2 (ABCG2), solute carrier family 2 member 9 (SLC2A9) and other UA transporters of the intestinal epithelium[9,10].
Faecal microbiota transplantation (FMT) recently emerged as a treatment strategy for HUA. FMT relate to the transplantation of the functional flora of a healthy individual into the gastrointestinal tract of a patient to establish a new intestinal microbiota for the treatment of intestinal and extraintestinal diseases. FMT technology was first used for treating refractory Clostridium difficile infection in 2013[11]. FMT also aids in inducing the remission of ulcerative colitis and improving hepatic encephalopathy and insulin sensitivity[12-14]. Washed microbiota transplantation (WMT), the generation of bacterial solutions via automatic purification systems, is respected in clinic. WMT reduces the adverse events caused by traditional faecal suspension preparations and greatly improves the treatment efficacy[15,16].
Previous studies have found that HUA is closely associated with an intestinal flora imbalance[17,18]. Manipulation of gut dysbiosis with probiotics relieves fructose-induced hyperuricaemia in mice and enhances intestinal barrier function[19]. A pilot study[20] found that all patients (n = 11) exhibited a reduction in SUA levels on day 28 post-FMT (P < 0.05). Our study expanded the sample size to retrospectively examine the short-term and mid-term effects and safety of WMT on SUA levels in patients with HUA and normal UA (NUA) levels.
A total of 144 patients who received WMT treatment from July 2016 to April 2020 in the First Affiliated Hospital of Guangdong Pharmaceutical University and had SUA data before treatment were selected. All Patients in this study provided informed consent. Patients who received WMT treatment were divided into an HUA group and an NUA group based on their SUA levels before treatment. The inclusion criterion for the HUA group was an SUA > 416 μM. The inclusion criterion for the NUA group was an SUA ≥ 202 μM to ≤ 416 μM.
Patients included in the study met the following criteria: (1) Aged 18-85 years; (2) received WMT treatment; and (3) had SUA data before and after treatment (Figure 1). The following exclusion criteria were used: (1) Gastrointestinal infection, cardiopulmonary failure or serious liver and kidney diseases; (2) pregnancy; (3) malignant tumours; (4) rejection to transendoscopic enteral tubing; (5) rejection or failure to complete the follow-ups; and (6) a high-purine diet.
All the patients were treated with WMT. Mixed multi-donor faeces were used as the source of the bacterial suspension for WMT. Healthy young men aged 18-25 years was one of requirements of the donors. The donors underwent health examinations to exclude metabolic diseases, genetic diseases, infectious diseases, digestive tract diseases, malignant tumours, and other associated diseases. The donors did not take antibiotics or drugs that affected alimentary canal dynamics and/or caused gut microecological disorders in the previous 3 mo. Utilizing an automatic purification system (GenFMTer; FMT Medical, Nanjing, Jiangsu Province, China), bacterial solution (200 mL) was isolated and injected into the sick’s gut through the lower or middle alimentary canal within 30 min. Two transplantation routes were available for use. One route was the middle alimentary canal route in which transendoscopic enteral tubing was placed in the jejunum under gastroscopy, and proton pump inhibitors were administered intravenously 1 h before the injection. This procedure was performed to lower the inactivation of bacteria when moving through the stomach. Metoclopramide hydrochloride (10 mg) was injected intramuscularly to decrease side effects, like abdominal distension or vomiting, resulted from stimulus of the digestive tract by the fresh faecal liquid. The sick was placed in a sitting position during injection of the fresh faecal liquid. The injection procedure was mild and slow, and needed at least 30 min for 200 mL of the fresh faecal liquid. After the injection, the patient sustained standing or seated not less than 2 h. The other method was the lower alimentary canal route in which transendoscopic enteral tubing was placed into the caecum by means of enteroscopy. During injection of the fresh faecal liquid, the sick was placed in the right sided position, and this process lasted for 30 min. Completed this procedure, the sick rested in the right sided position for at least 2 h. One course was administered once daily for 3 d. Four courses were administered at one course per month in the first month, second month, third month, and sixth month.
The main outcome was the changes in SUA levels before and after WMT treatment. The SUA measured in the last test within 3 mo after the first WMT represented the short-term effect, and the SUA measured in the last test within 3-6 mo after the first WMT was the mid-term effect. The secondary outcomes included the WMT course and occurrence of adverse events (AEs) after WMT. In accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0, We graded the AEs and classified the relationship between AEs and WMT as unrelated and possibly, probably and definitely related.
Statistical product and service solutions version 26.0 were used for data analyses. Categorical data are expressed as the frequency and percentage, and numerical data are expressed as the mean ± SD deviation. If a normal distribution was observed, Student’s t test was used for comparison between groups. Otherwise, the Wilcoxon signed rank test was used. Differences were defined as statistically significant when P < 0.05.
Most of the patients were not treated with WMT due to HUA (Table 1). The patients received different short- and mid-term courses of WMT in the HUA group and the NUA group (Table 2).
| Main diagnosis | n (%) |
| Irritable bowel syndrome | 32 (22.22) |
| Functional constipation | 22 (15.28) |
| Ulcerative colitis | 14 (9.72) |
| Gastroesophageal reflux disease | 14 (9.72) |
| Nonalcoholic fatty liver disease | 11 (7.64) |
| Childhood autism | 9 (6.25) |
| Functional enteropathy | 5 (3.47) |
| Functional diarrhea | 5 (3.47) |
| Gout | 5 (3.47) |
| Cirrhosis after hepatitis | 3 (2.08) |
| Functional abdominal pain syndrome | 3 (2.08) |
| Radiation colitis | 2 (1.39) |
| Chronic viral hepatitis B | 2 (1.39) |
| Other | 16 (11.11) |
| Time | Group | n | 1 course | 2 courses | 3 courses | 4 courses |
| Short-term | ||||||
| HUA group | 32 | 1 | 21 | 10 | - | |
| NUA group | 74 | 1 | 51 | 22 | - | |
| Mid-term | ||||||
| HUA group | 9 | - | 1 | 6 | 2 | |
| NUA group | 20 | - | - | 15 | 5 |
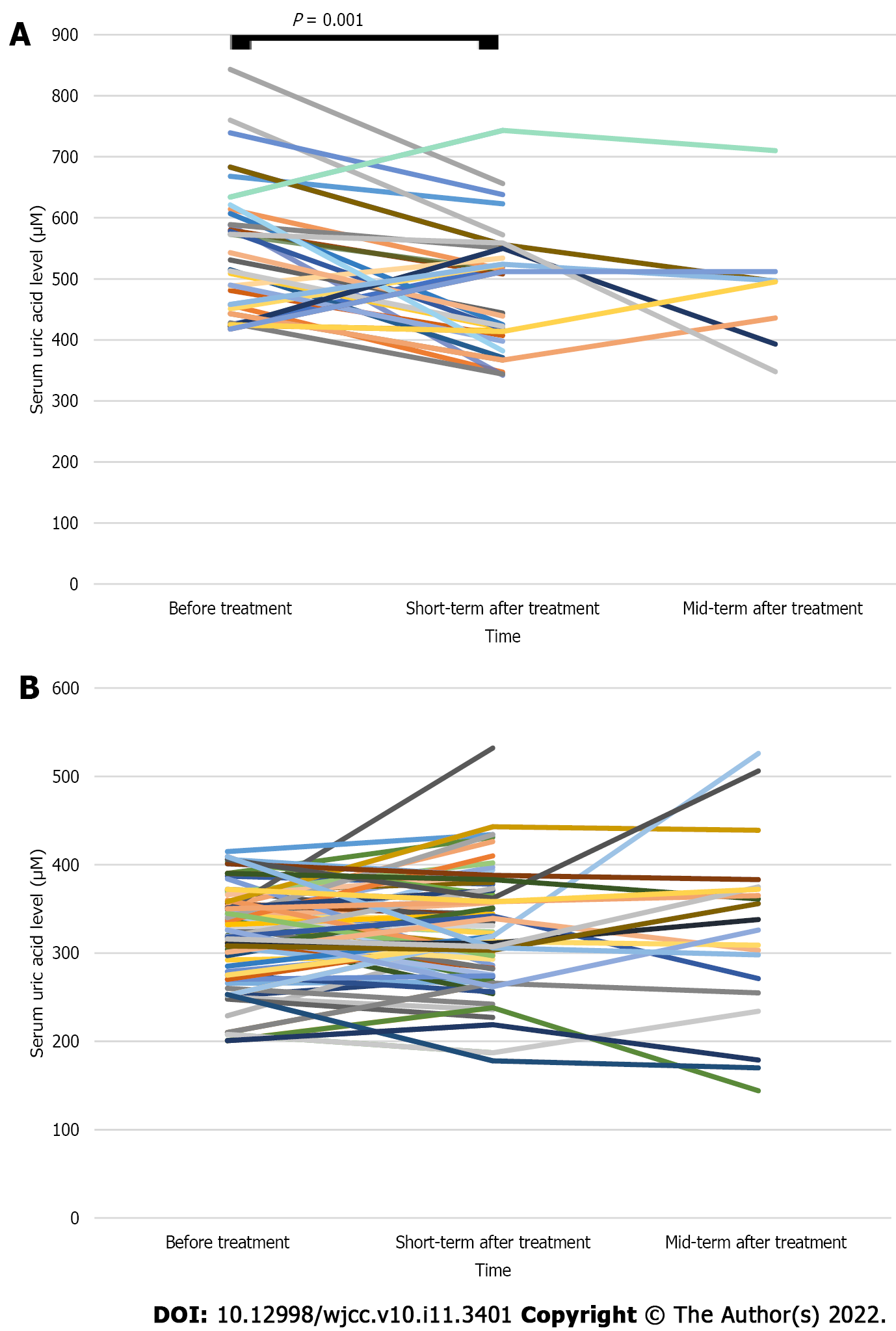
The short-term average SUA level after WMT treatment in the HUA group was lower than that before treatment (481.00 ± 99.85 vs 546.81 ± 109.64 μM, n = 32, P < 0.05; Table 3 and Figure 2A). The mid-term average SUA level after WMT treatment was lower than that before treatment, but the difference was not statistically significant (483.00 ± 101.21 vs 504.00 ± 100.30 μM, n = 9, P > 0.05; Table 3 and Figure 2A). The mid-term average SUA level after WMT treatment decreased compared with the short-term SUA level after treatment, but the difference was not statistically significant (485.88 ± 107.80 vs 528.12 ± 111.89 μM, n = 8, P > 0.05; Table 3 and Figure 2A). The average short-term and mid-term SUA levels after treatment in the NUA group were similar to the levels before treatment (P > 0.05; Table 3 and Figure 2B).
| Group | SUA levels (μM) | P value | SUA levels (μM) | P value | SUA levels (μM) | P value | |||
| Before treatment | Short-term effect after treatment | Before treatment | Mid-term effect after treatment | Short-term effect after treatment | Mid-term effect after treatment | ||||
| HUA group (n = 44) | 546.81 ± 109.64 (n = 32) | 481.00 ± 99.85 (n = 32) | 0.001 | 504.0 ± 100.3 (n = 9) | 483.00 ± 101.21 (n = 9) | 0.596 | 528.12 ± 111.89 (n = 8) | 485.88 ± 107.80 (n = 8) | 0.276 |
| NUA group (n = 100) | 322.8 ± 52.0 (n = 74) | 326.62 ± 63.30 (n = 74) | 0.500 | 308.30 ± 70.28 (n = 20) | 325.50 ± 101.03 (n = 20) | 0.350 | 309.10 ± 68.32 (n = 20) | 325.50 ± 101.03 (n = 20) | 0.301 |
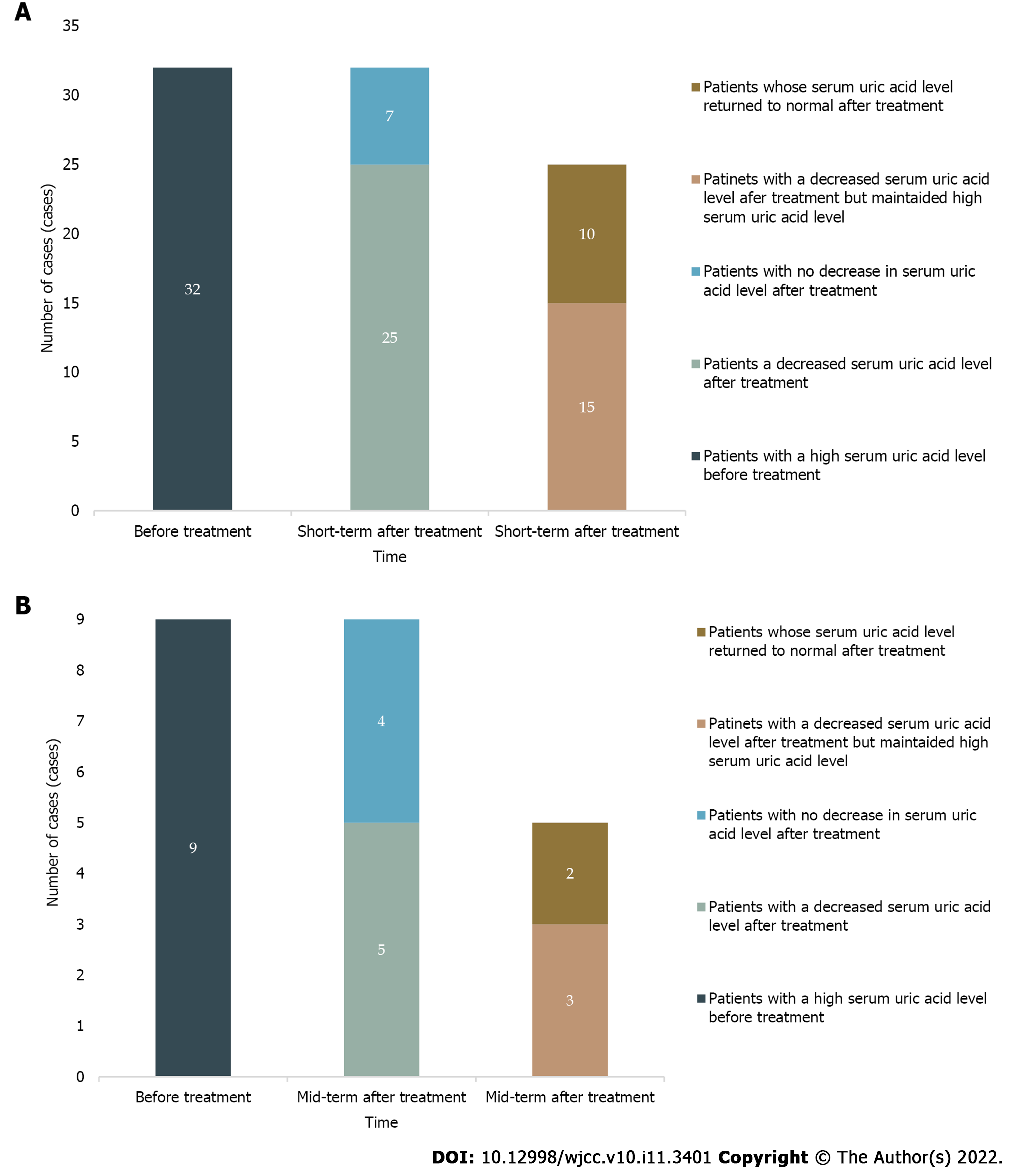
SUA levels decreased in the short term after treatment in 25 patients in the HUA group (78.12%), and SUA levels returned to normal in 10 patients (31.25%). A total of 55.56% (5/9) of patients had a decrease in SUA levels in the mid-term after treatment, and the SUA levels of 22.22% (2/9) of these patients returned to normal (Figure 3).
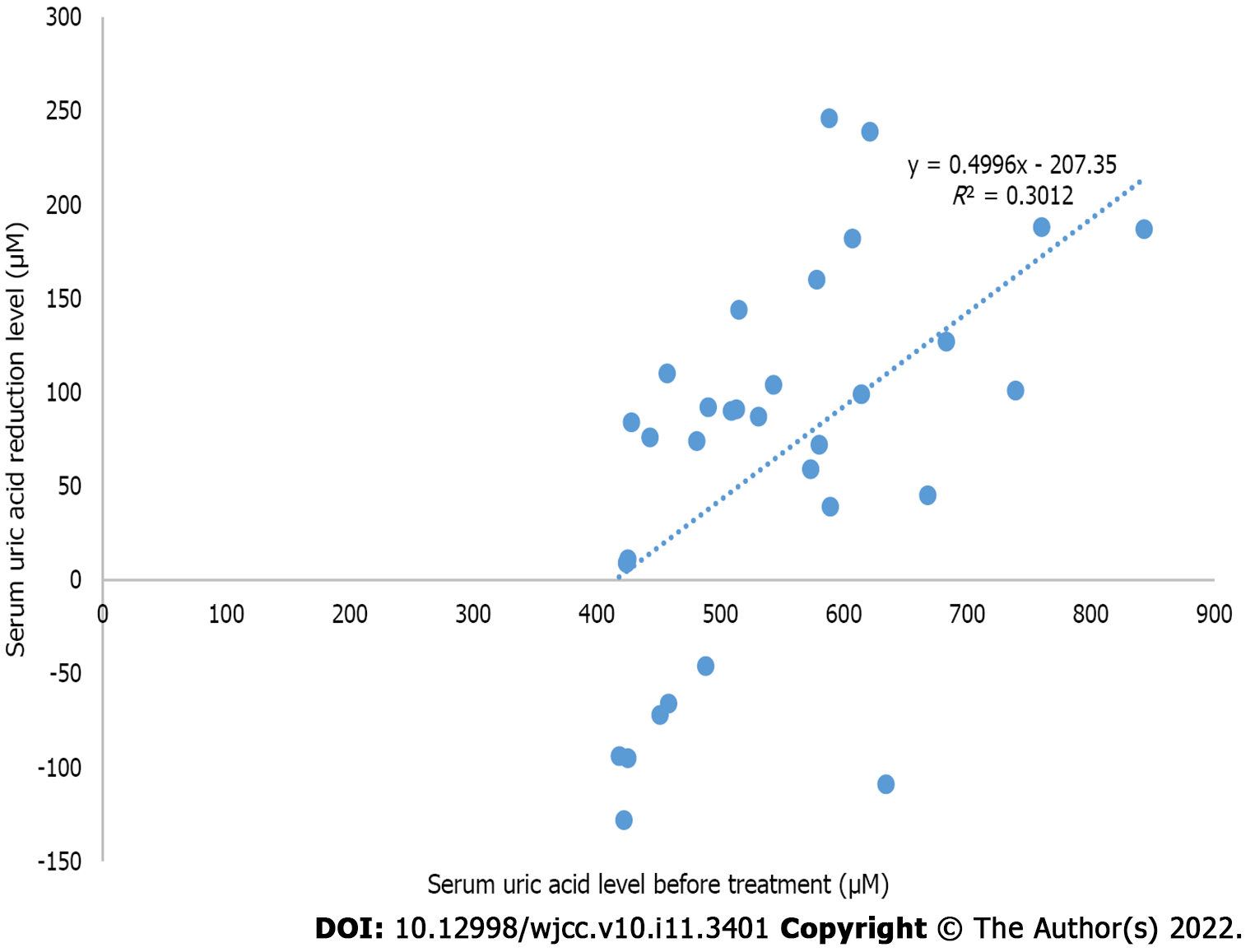
The relationship between the short-term SUA reduction level after WMT treatment and the SUA level before treatment in the HUA group showed r = 0.549 and R² = 0.300 (P < 0.05), which suggested that the short-term SUA reduction level after WMT treatment and the SUA level before treatment were moderately correlated (Figure 4).
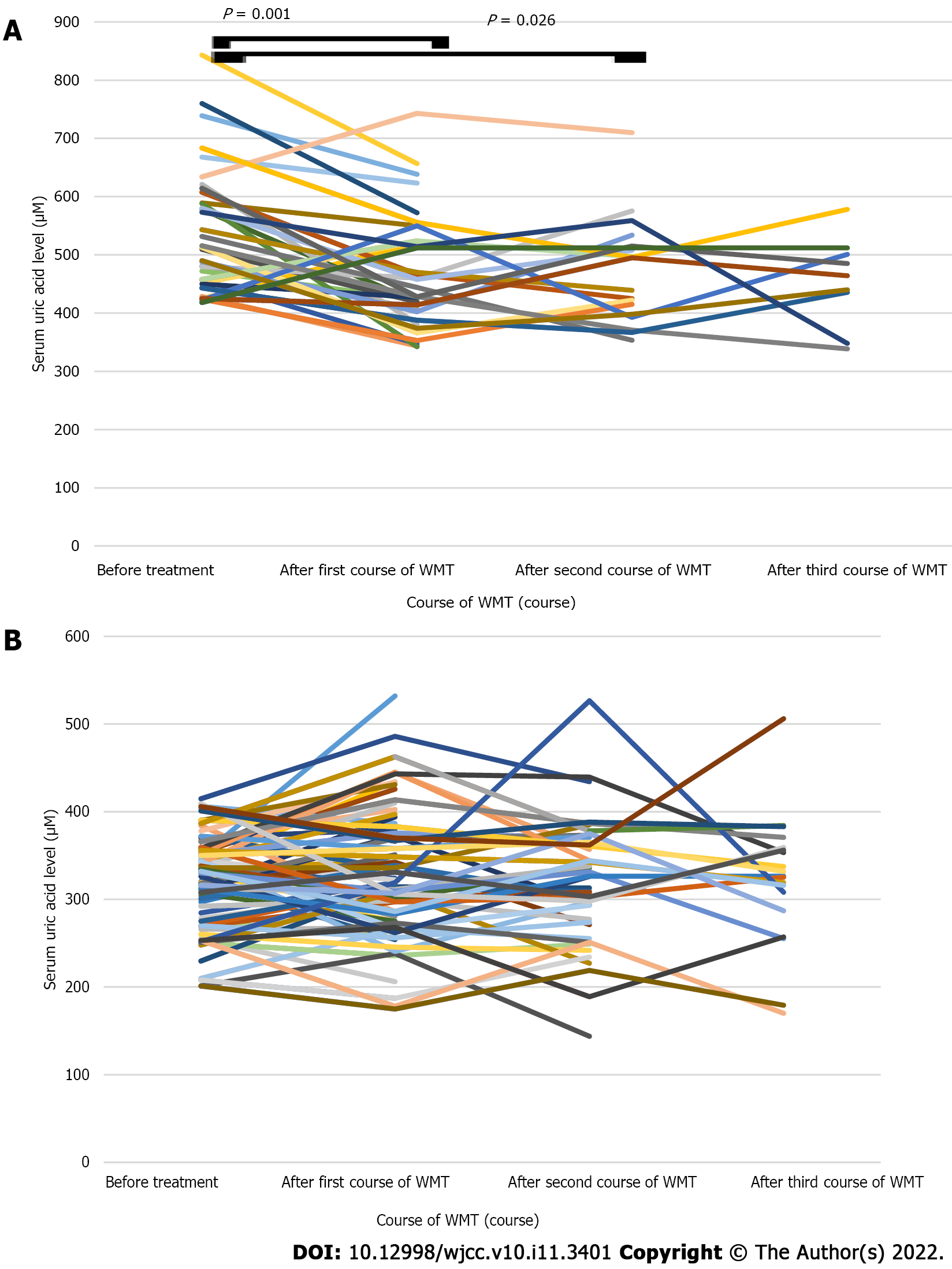
The average SUA level of the patients in the HUA group decreased after the first course of WMT compared with that before treatment (469.74 ± 97.68 vs 540.00 ± 107.16 μM, n = 35, P < 0.05; Table 4 and Figure 5A). The average SUA level of the patients in the HUA group was lower after the second course of WMT than before treatment (465.57 ± 88.88 vs 513.19 ± 78.14 μM, n = 21, P < 0.05; Table 4 and Figure 5A). The average SUA level of the patients in the HUA group after the third course of WMT was decreased compared with that before treatment, but the difference was not statistically significant (417.36 ± 92.84 vs 526.73 ± 111.30 μM, n = 11, P > 0.05; Table 4 and Figure 5A). The average SUA level of the patients in the NUA group (n = 100) after receiving different courses of WMT was similar to that before treatment (P > 0.05; Table 4 and Figure 5B).
| Group | SUA levels (μM) | P value | SUA levels (μM) | P value | SUA levels (μM) | P value | |||
| Before treatment | After first course of WMT | Before treatment | After second course of WMT | Before treatment | After third course of WMT | ||||
| HUA group (n = 44) | 540.00 ± 107.16 (n = 35) | 469.74 ± 97.68 (n = 35) | 0.001 | 513.19 ± 78.14 (n = 21) | 465.57 ± 88.88 (n = 21) | 0.026 | 526.73 ± 111.30 (n = 11) | 417.36 ± 92.84 (n = 11) | 0.101 |
| NUA group (n = 100) | 320.55 ± 52.73 (n = 77) | 328.86 ± 71.91 (n = 77) | 0.184 | 317.29 ± 57.44 (n = 51) | 323.18 ± 68.06 (n = 51) | 0.442 | 333.00 ± 55.49 (n = 27) | 328.59 ± 73.52 (n=27) | 0.628 |
The average short-term and mid-term serum creatinine levels after treatment were similar to those before treatment in both groups (P > 0.05; Table 5).
| Group | SCR levels (μM) | P value | SCR levels (μM) | P value | ||
| Before treatment | Short-term effect after treatment | Before treatment | Mid-term effect after treatment | |||
| HUA group (n = 44) | 73.00 ± 20.17 (n = 29) | 72.07 ± 17.27 (n = 29) | 0.575 | 68.44 ± 14.67 (n = 9) | 67.78 ± 13.03 (n = 9) | 0.798 |
| NUA group (n = 100) | 64.85 ± 13.74 (n = 73) | 64.67 ± 13.22 (n = 73) | 0.791 | 66.3 ± 14.6 (n = 20) | 66.10 ± 14.02 (n = 20) | 0.890 |
No serious adverse reactions occurred during WMT treatment in the 144 treated patients. Only one patient developed mild diarrhea during the second WMT treatment, which gradually returned to normal within 3 d. This AE was classified as possibly related to WMT.
HUA is closely related to intestinal flora imbalance. A bacterial disorder was observed in patients with HUA that manifested as an increase in the total count of aerobes, Escherichia coli and Bacteroides and a decrease in the number of Lactobacillus and Bifidobacteria[17]. The composition of the intestinal flora also changed in animal models of HUA caused by a high-fat diet, a high-glucose diet, a high-fructose diet, and a high-oxalic-acid diet[18,21,22]. Liu et al[23] transplanted the faecal flora of HUA rats into recipient rats. After 3 wk, the diversity and richness of the intestinal flora of recipient rats changed, and UA levels of recipient rats also increased.
Previous studies have shown that probiotics effectively treat HUA[24,25]. However, one study demonstrated that symbiotic and probiotic interventions had no effect on SUA levels after 12 wk of intervention[26]. A meta-analysis showed that UA levels were significantly increased in the intervention group compared to a control group[27].
These studies have clarified the differences in the intestinal flora between patients with HUA and healthy individuals, but controversy over the role of probiotics in reducing SUA remains. WMT significantly improves intestinal bacterial disorders and is currently recognized as the most effective method to restore the intestinal microecological balance. A pilot study[20] found that all patients (n = 11) had a reduction in SUA levels on day 28 post-FMT (P < 0.05). However, this study did not examine the short- and mid-term effects of WMT on patients with NUA levels or the mid-term effect of WMT on patients with HUA and had a relatively small sample size.
The average SUA level in the HUA group decreased within 3 mo after WMT in our study (P < 0.05). Short-term SUA levels decreased in 25 patients (78.12%) after treatment, and SUA levels returned to normal in 10 patients (31.25%). The mechanism of WMT treatment in the reduction of UA may involve two pathways, promotion of UA decomposition and excretion. The intestinal flora degrade UA into allantoin[28]. The intestinal flora also affect the metabolism of UA by regulating ABCG2, SLC2A9 and other UA transporters of the intestinal epithelium[9,10]. Some scholars[7,8] have hypothesised a “metabolic endotoxemia”, wherein changes in the structure of the intestinal flora can increase the permeability of the intestinal tract and cause microbial metabolites, such as endotoxins or LPS, to increase in the host circulatory system. LPS forms an immune complex with its receptor CD14, which is recognized by Toll-like receptor 4 on the surface of immune cells and causes kidney damage through blood circulation. These effects subsequently lead to renal UA excretion disorders and reduced UA excretion, which increase SUA levels. Wang et al[19] treated mice with fructose-induced HUA with isolated Lactobacillus brevis DM9218. The results showed that DM9218 decreased SUA levels, hepatic xanthine oxidase activity and liver LPS in fructose-fed mice. Diamine oxidase and endotoxin levels decreased after FMT in a clinical study (P < 0.05)[20]. Because our study was a retrospective study, further studies are needed to explore the mechanism of WMT reduction of SUA. The SUA levels of some patients in the HUA group did not decrease. The high SUA levels in these patients may not be the result of an intestinal flora imbalance, and WMT had no obvious effect, or it interfered with other factors, such as a high-purine diet. Therefore, we should clarify that the role of WMT in HUA results from different causes in further studies.
After WMT in the HUA group, SUA levels at the mid-term observation point were reduced compared with those before treatment and at the short-term observation point. However, there was no significant difference (P > 0.05). It may be associated with the small sample included in the mid-term observation. Alternatively, WMT may have no effect on mid-term SUA levels in HUA. Follow-up studies should further expand the sample size and extend follow-up time to clarify the mid-term and long-term effects of WMT treatment.
There was a difference in the number of courses of WMT in the evaluation of short-term and long-term effects (Table 2). Therefore, we further analysed the effect of WMT on SUA in different populations based on the number of treatment courses. After one and two courses of WMT in the HUA group, the average SUA level decreased (P < 0.05), which indicated that the first and second courses of WMT played a significant impact on reducing SUA levels in patients with HUA. After three courses, the average SUA level decreased compared with that before treatment, but the difference was not statistically significant (P > 0.05). This finding may be related to the small sample size or may suggest that the third course of WMT cannot reduce SUA levels. Follow-up researches with a larger sample capacity are needed to elucidate the effect on SUA level of the third course of treatment. Because of the limited number of patients in this study with complete UA data before treatment and after each treatment, it was difficult to further analyse the relationship between the number of courses of treatment and the effect of WMT. Future research should investigate the optimal course of treatment for HUA.
The average SUA level in the NUA group was similar to that before treatment, and the difference was not statistically significant. This finding suggests that WMT treatment does not interfere with normal UA metabolism. These results also provide evidence to support the safety of WMT treatment from the perspective of UA metabolism.
The safety of WMT is noteworthy. Only one patient developed mild diarrhea during the second WMT treatment, which gradually returned to normal within 3 d. A systematic review analysed the FMT-related AEs reported in 129 studies worldwide from 2000 to 2020, and the results showed that the total incidence of FMT-related AEs was 19%[29]. The low number of adverse reactions in the current study may be related to the use of WMT instead of FMT or the small sample size. Due to the short follow-up time, the current study could not clarify the long-term safety of WMT for the treatment of HUA.
The current study also has the following limitations: (1) No analysis of the effects of WMT on the improvement of gout flares in people with HUA; (2) no placebo control group or UA-lowering drug group; and (3) a single-centre design, which may lead to regional and genetic background bias.
WMT reduces SUA levels of patients with HUA in the short term with mild side effects but has no obvious effect on the SUA level of patients with NUA.
Hyperuricaemia (HUA) pathogenesis is closely associated with intestinal bacteria.
Current treatments for HUA have failed to obtained satisfactory clinical results.
To investigate the effect and safety of washed microbiota transplantation (WMT) on serum uric acid (SUA) levels in different populations.
A total of 144 patients who received WMT from July 2016 to April 2020 in the First Affiliated Hospital of Guangdong Pharmaceutical University and had SUA data before treatment were selected. The changes in SUA levels before and after treatment were retrospectively reviewed. According to the pretreatment SUA level, the patients were divided into a hyperuricaemia group (HUA group: SUA > 416 μM) and a normal uric acid group (NUA group: SUA ≥ 202 μM to ≤ 416 μM). Statistical product and service solutions 26.0 was used to analyse the data.
The average short-term SUA levels in the HUA group decreased after WMT (481.00 ± 99.85 vs 546.81 ± 109.64 μM, n = 32, P < 0.05). The levels decreased in 25/32 patients and returned to normal in 10/32 patients. The short-term level of SUA reduction after treatment moderately correlated with the SUA levels before treatment (r = 0.549, R² = 0.300, P < 0.05). The average SUA levels decreased after the first and second courses of WMT (469.74 ± 97.68 vs 540.00 ± 107.16 μM, n = 35, 465.57 ± 88.88 vs 513.19 ± 78.14 μM, n = 21, P < 0.05). Short-term and mid-term SUA levels in the NUA group after WMT and SUA levels after the first, second and third courses of WMT were similar to those before WMT (P > 0.05). Only 1/144 patients developed mild diarrhoea after WMT.
WMT can lower the SUA level in patients with HUA in the short term with mild side effects, but WMT has no obvious effect on the SUA level of patients with NUA.
WMT may be a novel treatment for HUA.
We thank the information department and medical records room of the First Affiliated Hospital of Guangdong Pharmaceutical University for their help on data collection and patients follow-up from the bottom of our heart. We honestly acknowledge Zheng YM at the First Affiliated Hospital of Guangdong Pharmaceutical University for her guidance during the submission process. We truly thank Fu SL, Wang XH, Zhu JW and Guo JD at the Inner Mongolia Ewenki Autonomous Banner People’s Hospital for their help on patients follow-up. We also sincerely thank the patients for their enthusiastic participation in our study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Innocenti T, Italy; Patel VJ, India; Rothschild B, United States S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Wu J, Qiu L, Cheng XQ, Xu T, Wu W, Zeng XJ, Ye YC, Guo XZ, Cheng Q, Liu Q, Liu L, Xu CL, Zhu GJ. Hyperuricemia and clustering of cardiovascular risk factors in the Chinese adult population. Sci Rep. 2017;7:5456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Joosten LAB, Crişan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol. 2020;16:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 3. | Yamanaka H; Japanese Society of Gout and Nucleic Acid Metabolism. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30:1018-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Pisaniello HL, Fisher MC, Farquhar H, Vargas-Santos AB, Hill CL, Stamp LK, Gaffo AL. Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review. Arthritis Res Ther. 2021;23:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Strilchuk L, Fogacci F, Cicero AF. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin Drug Saf. 2019;18:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 6. | Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, Stamp LK. Gout. Nat Rev Dis Primers. 2019;5:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 7. | Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 8. | Xu D, Lv Q, Wang X, Cui X, Zhao P, Yang X, Liu X, Yang W, Yang G, Wang G, Wang P, Wang Z, Li Z, Xing S. Hyperuricemia is associated with impaired intestinal permeability in mice. Am J Physiol Gastrointest Liver Physiol. 2019;317:G484-G492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Hosomi A, Nakanishi T, Fujita T, Tamai I. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS One. 2012;7:e30456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | DeBosch BJ, Kluth O, Fujiwara H, Schürmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-98; quiz 499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1185] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 12. | Lima SF, Gogokhia L, Viladomiu M, Chou L, Putzel G, Jin WB, Pires S, Guo CJ, Gerardin Y, Crawford CV, Jacob V, Scherl E, Brown SE, Hambor J, Longman RS. Transferable Immunoglobulin A-Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology. 2022;162:166-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 13. | Bloom PP, Tapper EB, Young VB, Lok AS. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021;75:1452-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S, Birch DW, Samarasinghe KK, Walter J, Madsen KL. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 15. | Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, Chen Y, Yin H, Wang H, Marcella C, Cui B, Cheng L, Ji G, Zhang F. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11:251-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 16. | . Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J (Engl). 2020;133:2330-2332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Ren KY, Yong CM, JIN YC, Cao B, Wei LZ. Analysis of intestinal flora in patients with hyperuricemia in Qindao District. Zhongguo Yishi Zazhi. 2014;16:1649-1651; 1656. |
| 18. | Yu Y, Liu Q, Li H, Wen C, He Z. Alterations of the Gut Microbiome Associated With the Treatment of Hyperuricaemia in Male Rats. Front Microbiol. 2018;9:2233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Wang H, Mei L, Deng Y, Liu Y, Wei X, Liu M, Zhou J, Ma H, Zheng P, Yuan J, Li M. Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition. 2019;62:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Xie WR, Yang XY, Deng ZH, Zheng YM, Zhang R, Wu LH, Cai JY, Kong LP, Xia HH, He XX. Effects of washed microbiota transplantation on serum uric acid levels, symptoms and intestinal barrier function in patients with acute and recurrent gout: a pilot study. Dig Dis. 2021; In press. |
| 21. | Silva JCP, Mota M, Martins FO, Nogueira C, Gonçalves T, Carneiro T, Pinto J, Duarte D, Barros AS, Jones JG, Gil AM. Intestinal Microbial and Metabolic Profiling of Mice Fed with High-Glucose and High-Fructose Diets. J Proteome Res. 2018;17:2880-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | García-Arroyo FE, Gonzaga G, Muñoz-Jiménez I, Blas-Marron MG, Silverio O, Tapia E, Soto V, Ranganathan N, Ranganathan P, Vyas U, Irvin A, Ir D, Robertson CE, Frank DN, Johnson RJ, Sánchez-Lozada LG. Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS One. 2018;13:e0202901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Liu X, Lv Q, Ren H, Gao L, Zhao P, Yang X, Yang G, Xu D, Wang G, Yang W, Wang P, Wang Z, Xing S. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ. 2020;8:e8664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Yamanaka H, Taniguchi A, Tsuboi H, Kano H, Asami Y. Hypouricaemic effects of yoghurt containing Lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomised, double-blind, placebo-controlled study. Mod Rheumatol. 2019;29:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Szulińska M, Łoniewski I, van Hemert S, Sobieska M, Bogdański P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 26. | Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effect of Synbiotic and Probiotic Supplementation on Serum Levels of Endothelial Cell Adhesion Molecules in Hemodialysis Patients: a Randomized Control Study. Probiotics Antimicrob Proteins. 2019;11:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Firouzi S, Haghighatdoost F. The effects of prebiotic, probiotic, and synbiotic supplementation on blood parameters of renal function: A systematic review and meta-analysis of clinical trials. Nutrition. 2018;51-52:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Sorensen LB. Degradation of uric acid in man. Metabolism. 1959;8:687-703. [PubMed] |
| 29. | Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |