Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.2015
Peer-review started: December 10, 2020
First decision: December 28, 2020
Revised: January 6, 2021
Accepted: January 28, 2021
Article in press: January 28, 2021
Published online: March 16, 2021
Processing time: 84 Days and 2.8 Hours
The roots of Achyranthes japonica Nakai (AJN), called “Useul-puli,” has been traditionally used to control pain and improve dysfunction in osteoarthritis patients in South Korea.
We described 3 patients diagnosed with herbal medicine induced interstitial lung disease after consuming boiled the roots of AJN. They were referred to our hospital because of the modified Medical Research Council grade 4 dyspnea. Chest computed tomography showed bilateral ground-glass opacities with patchy consolidation. After treatment with systemic glucocorticoid therapy and discontinuation of the roots of AJN, their symptoms improved, and almost all ground-glass opacities and patchy consolidations on chest radiography and chest computed tomography resolved.
We present three cases of interstitial lung disease induced by the roots of AJN.
Core Tip: To the best of our knowledge, this is the first case series of interstitial lung disease induced by the roots of Achyranthes japonica Nakai (AJN). Further studies are required to understand the mechanism and evaluate the prevalence of interstitial lung disease induced by the roots of AJN.
- Citation: Moon DS, Yoon SH, Lee SI, Park SG, Na YS. Interstitial lung disease induced by the roots of Achyranthes japonica Nakai: Three case reports. World J Clin Cases 2021; 9(8): 2015-2021
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/2015.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.2015
Many drugs can cause various patterns of interstitial lung disease (ILD). Because drug-induced ILD can be fatal and cause pulmonary interstitial fibrosis, early recognition is essential[1,2]. Herbal medicines are also believed to have the potential to induce pneumonitis. Several case reports on herbal medicine induced ILD have been published in Korea and Japan[3,4].
Case 1: An 83-year-old Korean woman presented with modified Medical Research Council (mMRC) grade 4 dyspnea.
Case 2: A 71-year-old Korean woman presented with mMRC grade 4 dyspnea and febrile sensation.
Case 3: A 72-year-old Korean woman presented with mMRC grade 4 dyspnea.
Case 1: She was referred to our hospital for evaluation of mMRC grade 4 dyspnea for 2 d.
Case 2: She was admitted to our hospital with mMRC grade 4 dyspnea and febrile sensation for 5 d.
Case 3: She was referred to our hospital with mMRC grade 4 dyspnea and nonproductive cough for 2 d.
Case 1: She had been treated for osteoarthritis with medication for 5 years. The patient began consuming the boiled roots of Achyranthes japonica Nakai (AJN) for osteoarthritis (Figure 1).
Case 2: She had been diagnosed with rheumatoid arthritis 5 years before and had undergone bilateral total knee replacement 2 years before. She had consumed the boiled root of AJN to control joint pain a month before.
Case 3: She had primary hypertension. Intermittently, she had been treated with pain control medication for her right knee osteoarthritis for 5 years. Three days before the presentation, she had consumed the boiled roots of AJN to control right knee pain.
Case 1: Examination of the vital signs revealed fever (39.6 °C), tachycardic (heart rate 129 beats/min), normal blood pressure (120/70 mmHg), and tachypnea (26 breaths/min). On chest auscultation, she had inspiratory crackles in bilateral lower lung fields. Upon arrival at the emergency department, arterial saturation was 74.9% on room air.
Case 2: Her vital signs on the first day of admission were: Blood pressure, 130/90 mmHg; Body temperature, 37.3 °C; pulse rate, 115 beats/min; and respiratory rate, 43 breaths/min. On chest auscultation, she had inspiratory crackles in bilateral whole lung fields.
Case 3: At admission, she had tachypnea (30 breaths/min). Her peripheral oxygen saturation (SpO2) was 84% while breathing 4 L/min supplemental oxygen via a nasal cannula.
Case 1: The arterial blood gas analysis performed on room air showed partial pressure of carbon dioxide, the partial pressure of oxygen, SpO2, and pH of 33.3 mmHg, 38.4 mmHg, 74.9%, and 7.438, respectively. Laboratory findings included; white blood cell count was 13750/µL (normal range: 4000-8000/µL), creatinine of 1.06 mg/dL (normal range: 0.5-1.3 mg/dL), C-reactive protein level of 9.94 mg/dL (normal range: 0.0-0.3 mg/dL), and procalcitonin of 0.302 ng/mL (normal range: 0.0-0.5 ng/dL). The serum aspartate aminotransferase (AST) was mildly elevated at 44.7 U/L (normal range: 0-40 U/L), but the serum alanine aminotransferase (ALT) level was normal at 14.1 U/L (normal range: 0-40 U/L). Serology tests for antinuclear antibody, antineutrophil cytoplasmic antibody, and rheumatoid factor were negative. The level of pro-brain natriuretic peptide was 106 pg/mL (normal range < 300 pg/mL). The polymerase change reaction (PCR) of 16 respiratory viruses, including the influenza virus, was negative. And sputum culture was also negative.
Case 2: Arterial blood gas analysis performed on a mask (5 L/min) showed partial pressure of carbon dioxide, the partial pressure of oxygen, SpO2, and pH of 33.8 mmHg, 56.1 mmHg, 88.0%, and 7.379, respectively. On admission, blood tests revealed a white blood cell count of 14350/µL, eosinophil count of 860/mm3 (normal range: 0-450/mm3), creatinine level of 0.54 mg/dL, C-reactive protein level of 30.48 mg/dL, procalcitonin level of 2.8 ng/mL, AST of 54.8 U/L, and ALT of 24.8 U/L. Serology tests for evaluation of connective tissue disease and heart failure were unremarkable. The level of pro-BNP was 34 pg/mL. The PCR for respiratory viruses was negative. And Sputum culture was nothing grown.
Case 3: Laboratory findings showed an elevated C-reactive protein level of 22.13 mg/dL, AST of 60 U/L, ALT of 68.4 U/L, serum creatinine of 1.47 mg/dL, and procalcitonin level of 3.10 ng/mL. The multiplex polymerase chain reaction for atypical pneumonia and serology tests for antinuclear antibody, antineutrophil cytoplasmic antibody, and rheumatoid factor were negative. The level of pro-BNP was 180 pg/mL. The PCR for respiratory viruses was negative. And sputum culture was negative.
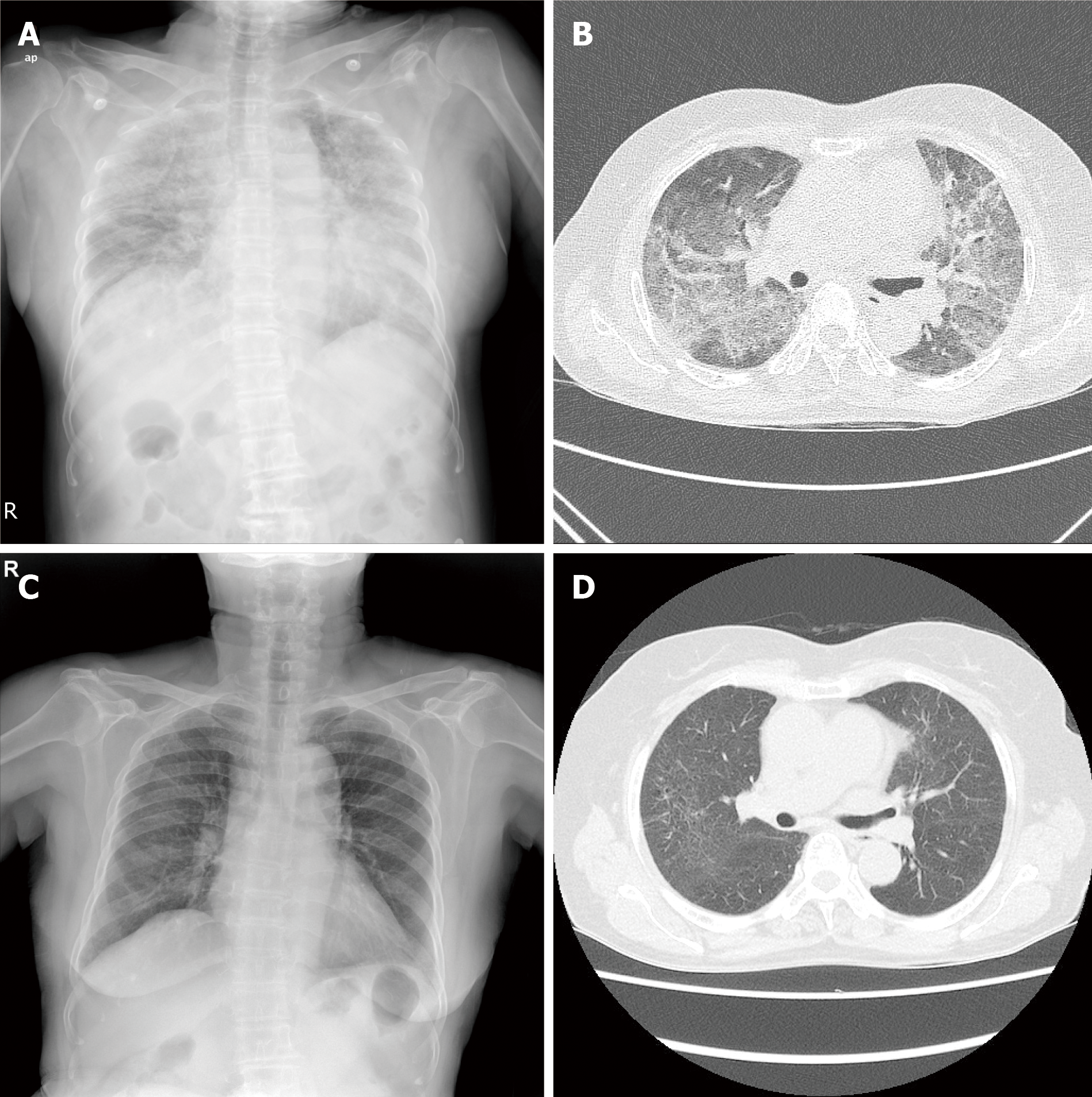
Case 1: Chest radiography showed diffuse bilateral coalescent opacities. Contrast-enhanced chest computed tomography (CT) showed bilateral ground-glass opacities with interlobular interstitial thickening and patchy consolidation (Figure 2A and B). Transthoracic echocardiography revealed normal valvular functions and no regional wall motion abnormalities with an ejection fraction of 58%.
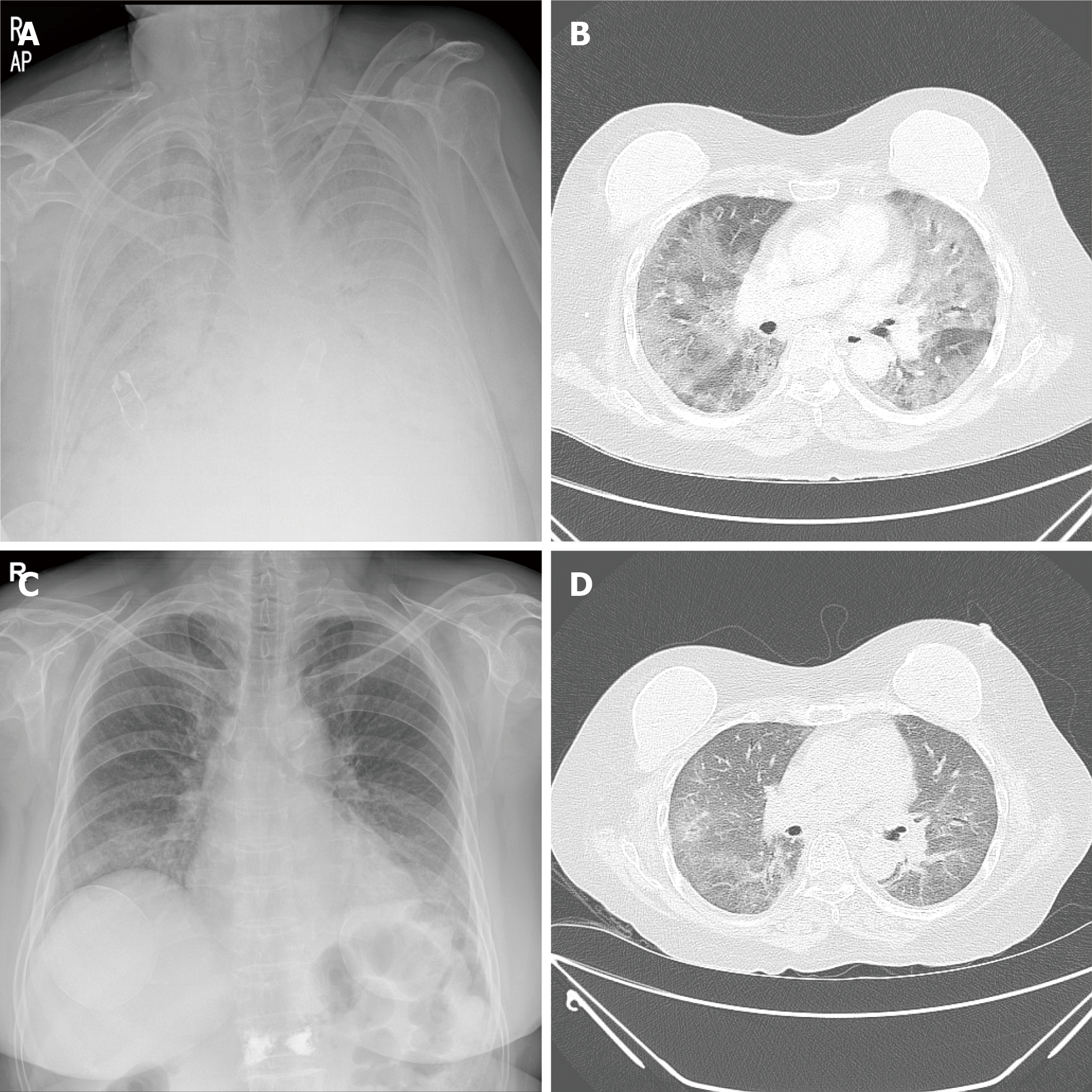
Case 2: Chest X-ray showed bilateral and chest CT showed bilateral areas of ground-glass opacities and patchy consolidations (Figure 3A and B). Transthoracic echocardiography revealed normal valvular functions, without regional wall motion abnormalities with a preserved ejection fraction.
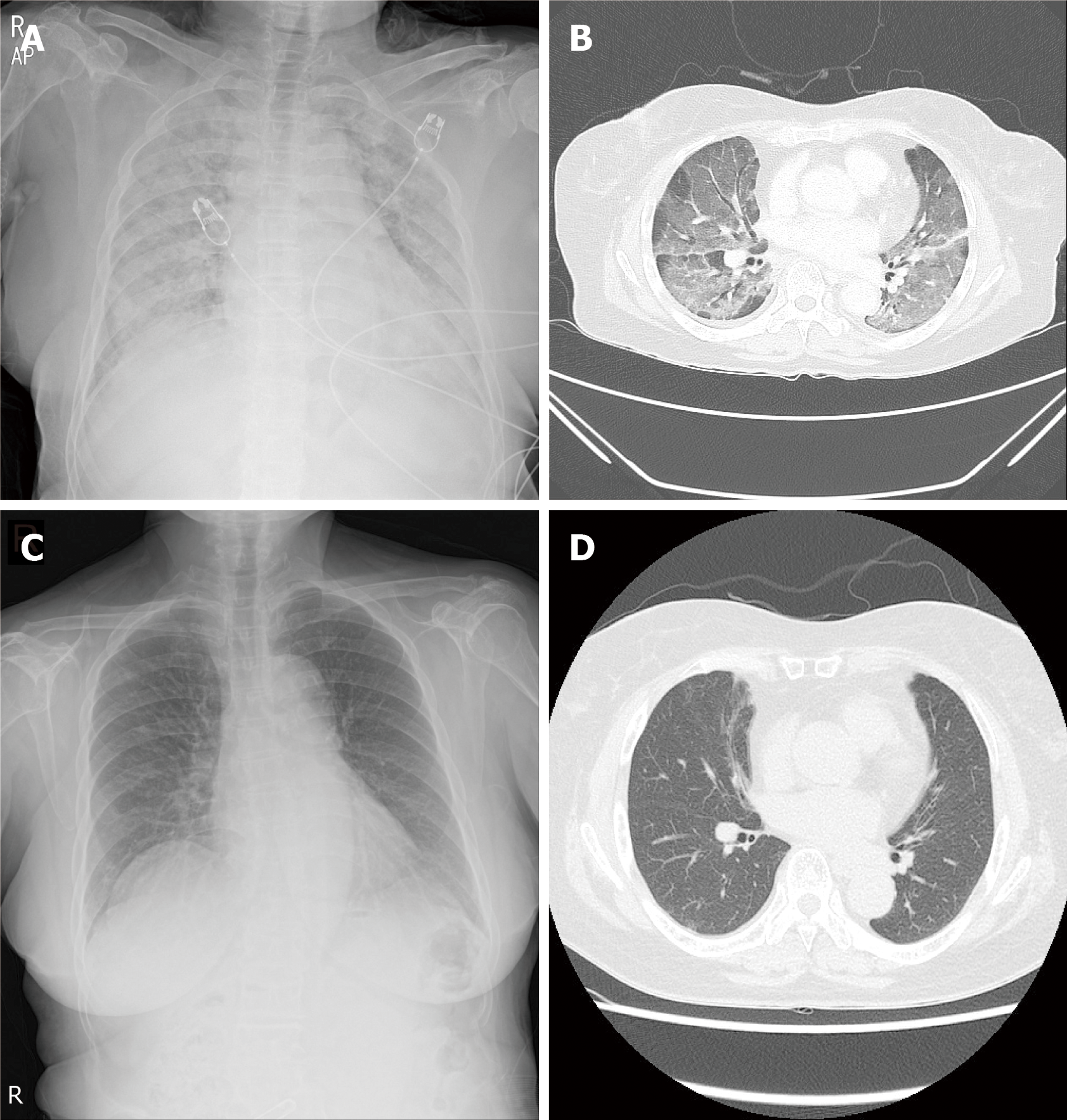
Case 3: The chest radiography showed diffuse lung consolidation. Chest CT showed diffuse bilateral ground-glass opacities with patchy consolidation (Figure 4A and B).
The final diagnosis, in three cases, is herbal medicine induced ILD.
High-flow nasal oxygen cannula was initiated with a fraction of inspired oxygen of 0.6 and a flow of 50 L/min. Treatment glucocorticoids (methylprednisolone 1 mg/kg) and empirical antibiotic therapy with levofloxacin were initiated. The glucocorticoids (methylprednisolone 20 mg) were tapered at the time of hospital discharge (14 d later) and stopped.
She was intubated due to acute respiratory distress. For herb medicine induced ILD, she was treated with glucocorticoid pulse therapy (methylprednisolone 125 mg/6 h) and empirical antibiotics. After 3 d of treatment, hypoxemia resolved, and extubation was performed. Glucocorticoid treatment was maintained for 2 wk and tapered.
Glucocorticoids (methylprednisolone 1 mg/kg) were administered. Seven days later, dyspnea and hypoxemia improved significantly. She was discharged with prednisolone of 40 mg/d.
After 7 d, she showed improvement in symptoms, oxygenation, and chest radiography findings. After 4 mo, chest CT showed near improvement of ground-glass opacities and patchy consolidation (Figure 2C and D).
ILD can be induced by various drugs and biologics, including antibiotics, anti-inflammatory agents, anti-arrhythmic agents, antineoplastic agents, and illicit drugs. Herbal medicine induced ILD has been reported mainly in Korea and Japan. Enomoto et al[3] analyzed 73 cases of Japanese herbal medicine-induced pneumonitis. The most common Japanese herbal medicine was Sho-Saiko-To, followed by Sairei-To. The roots of AJN, called “Useul-ppuli,” has been traditionally used to control pain and improve dysfunction in osteoarthritis patients in Korea. In our case series, we present ILD that occurred after consuming the roots of AJN to relieve joint pain.
The pathogenesis of drug-induced ILD is poorly understood. Cytotoxic lung injuries induced by drugs may include a direct injury to pneumocytes or the alveolar-capillary endothelium with subsequent release of cytokines and recruitment of inflammatory cells and oxidative injury by the generation of reactive oxygen species[5-7]. In addition, immune-mediated lung injury has been reported, including drug hypersensitivity and other immune reactions[8]. However, the mechanism of action of herbal medicine induced ILD is unclear. Further research is required to understand this mechanism.
Drug-induced ILD can be diagnosed when pneumonitis develops shortly after the initiation of drug exposure; improvement of pneumonitis follows the withdrawal of the offending agent, and deterioration occurs upon re-exposure. It is essential to exclude other causes of lung damage, such as infection, cardiogenic pulmonary edema, and other idiopathic causes of ILD[2,7,9]. After consuming the roots of AJN by 3 patients, it took at least 3 d and up to 2 mo until symptoms developed. However, the exact duration and cumulative dose could not be accurately determined in these patients.
The clinical manifestations of drug-induced ILD vary, ranging from a benign course, including cough, dyspnea, low-grade fever, and hypoxemia to life-threatening respiratory failure[9]. The most common radiological patterns on high-resolution CT are bibasilar ground-glass opacities with or without consolidation[2]. Akira et al[10] reported that CT findings in herbal medicine-induced ILD were diffuse ground-glass opacities with patchy consolidation. All patients showed dyspnea, hypoxemia, and bilateral ground-glass opacities with patchy consolidation such as diffuse alveolar damage pattern on chest CT. This CT finding needs to be differentiated from other diseases such as pulmonary edema, eosinophilic pneumonia, and viral pneumonia[11]. We excluded other diseases through PCR of respiratory virus, culture, BNP, and echocardiogram. Bronchoalveolar lavage could be of value in ruling out other problems (e.g., infection, eosinophilic pneumonitis). But in severe hypoxic conditions, bronchoalveolar lavage could be a relative contraindication[12]. Withdrawal of the causative drug, systemic glucocorticoid therapy, and supportive care are the treatment of choice for drug-induced ILD. Empiric glucocorticoid therapy is recommended for patients with rapidly progressive respiratory failure. However, systemic glucocor-ticoid therapy has weak evidence since no randomized control has been conducted[2]. The exact amount and duration of glucocorticoid treatment have not been established. Takatani et al[13] reported that high-resolution CT images with diffuse alveolar damage and organizing pneumonia patterns in drug-induced ILD require a larger cumulative dose of corticosteroids and longer oxygen supply. Three patients showed an improved condition after the withdrawal of the roots of AJN and systemic glucocorticoid therapy. In the treatment of ILD induced by the roots of AJN, if the patient has bilateral ground-glass opacities on high-resolution CT along with rapidly progressive respiratory failure, adjunctive systemic glucocorticoid therapy may be effective.
In summary, to the best of our knowledge, this is the first case series of interstitial lung disease induced by the roots of AJN. Further studies are required to understand the mechanism and evaluate the prevalence of interstitial lung disease induced by the roots of AJN.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen C S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Higenbottam T, Kuwano K, Nemery B, Fujita Y. Understanding the mechanisms of drug-associated interstitial lung disease. Br J Cancer. 2004;91 Suppl 2:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, Giollo A, Wild JM, Waterton JC, Buch M, Linton K, Bruce IN, Leonard C, Bianchi S, Chaudhuri N. Drug-Induced Interstitial Lung Disease: A Systematic Review. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 3. | Enomoto Y Md, Nakamura Y Md PhD, Enomoto N Md PhD, Fujisawa T Md PhD, Inui N Md PhD, Suda T. Japanese herbal medicine-induced pneumonitis: A review of 73 patients. Respir Investig. 2017;55:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Lee MH, Son BW, Kim KM, Jeon SH, Kim YK. A study on korean traditional medicine side effects cases described in domestic western medical journals in the past 10 years. J Int Korean Med. 2018;39:686-698. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ryrfeldt A. Drug-induced inflammatory responses to the lung. Toxicol Lett. 2000;112-113:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Schnyder B, Pichler WJ. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009;84:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 9. | Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Akira M, Ishikawa H, Yamamoto S. Drug-induced pneumonitis: thin-section CT findings in 60 patients. Radiology. 2002;224:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Maansi Parekh, Donuru A, Kapur S. Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era. Radiology. 2020;297:E289-E302. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Delaunois LM. Mechanisms in pulmonary toxicology. Clin Chest Med. 2004;25:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Takatani K, Miyazaki E, Nureki S, Ando M, Ueno T, Okubo T, Takenaka R, Hiroshige S, Kumamoto T. High-resolution computed tomography patterns and immunopathogenetic findings in drug-induced pneumonitis. Respir Med. 2008;102:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |