Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1996
Peer-review started: November 23, 2020
First decision: December 13, 2020
Revised: December 14, 2020
Accepted: January 15, 2021
Article in press: January 15, 2021
Published online: March 16, 2021
Processing time: 101 Days and 19.6 Hours
The incidence of infection with Mycobacterium abscessus (M. abscessus) has increased in recent years. This increase is partly associated with invasive cosmetic procedures.
The purpose of this case summary is to increase clinicians' awareness of M. abscessus infection and reduce mycobacterial infection caused by cosmetic procedures. We report the case of a 45-year-old woman who received acetyl hexapeptide-8 (argireline) injections in the forehead and temples, and erythema, nodules, and abscesses appeared at the injection sites after one week. The pus specimens were examined by microbiological culture and confirmed to be positive for M. abscessus. Clarithromycin 500 mg twice daily and moxifloxacin 400 mg once daily were administered for 5 mo and the lesions gradually subsided.
We report here for the first time a case of infection with M. abscessus after argireline injection. This condition is easily misdiagnosed as a common bacterial infection. Microbiological examinations are helpful for diagnosis and standardized cosmetic procedures can prevent infection with M. abscessus.
Core Tip: Mycobacterium abscessus (M. abscessus) is a rapidly growing nontubercul-ous mycobacterium that can lead to infections of the lung, lymph node, skin, and soft tissue. The incidence of infection with M. abscessus has increased in recent years. This increase is partly associated with invasive cosmetic procedures. We report here for the first time a case of infection with M. abscessus after argireline injection. The purpose of this case summary is to increase clinicians' awareness of M. abscessus infection and reduce mycobacterial infection caused by cosmetic procedures.
- Citation: Chen CF, Liu J, Wang SS, Yao YF, Yu B, Hu XP. Mycobacterium abscessus infection after facial injection of argireline: A case report. World J Clin Cases 2021; 9(8): 1996-2000
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1996.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1996
Mycobacterium abscessus (M. abscessus) is a rapidly growing nontuberculous mycobacterium (NTM) that can lead to infections of the lung, lymph node, skin, and soft tissue[1]. Compared with Mycobacterium tuberculosis, NTM has weaker virulence and pathogenicity. In humans, M. abscessus is usually an opportunistic pathogen, with poor pathogenicity, and it is more likely to infect patients with chronic diseases or immunocompromised patients. With the recent popularization of cosmetic procedures, the number of healthy patients infected with NTM is further increasing[2]. We report here for the first time a case of M. abscessus infection in a middle-aged woman 1 wk following argireline injection in the forehead and temples.
Erythema, nodules, and abscesses appeared at the face injection sites for 9 wk.
Ten weeks prior to presentation, to improve the appearance of wrinkles in the forehead and temples, a 45-year-old female patient went to a private cosmetic clinic and received an injection of a bottle of argireline (concentration unknown). Injection sites should not come into contact with water for 2 d. Erythema, nodules, and abscesses began to appear at the injection sites after 1 wk, with mild pain but no fever. Six weeks prior to presentation, the patient went to a private clinic and received injections of cephalosporin antibiotics for 10 d. The skin lesions slightly improved but then relapsed after treatment was stopped. Four weeks prior to presentation, she went to the district hospital for an abscess incision and drainage. The pus specimens were cultured and found to be positive for mycobacteria. Three weeks prior to presentation, she came to the outpatient clinic of our hospital.
The patient was healthy.
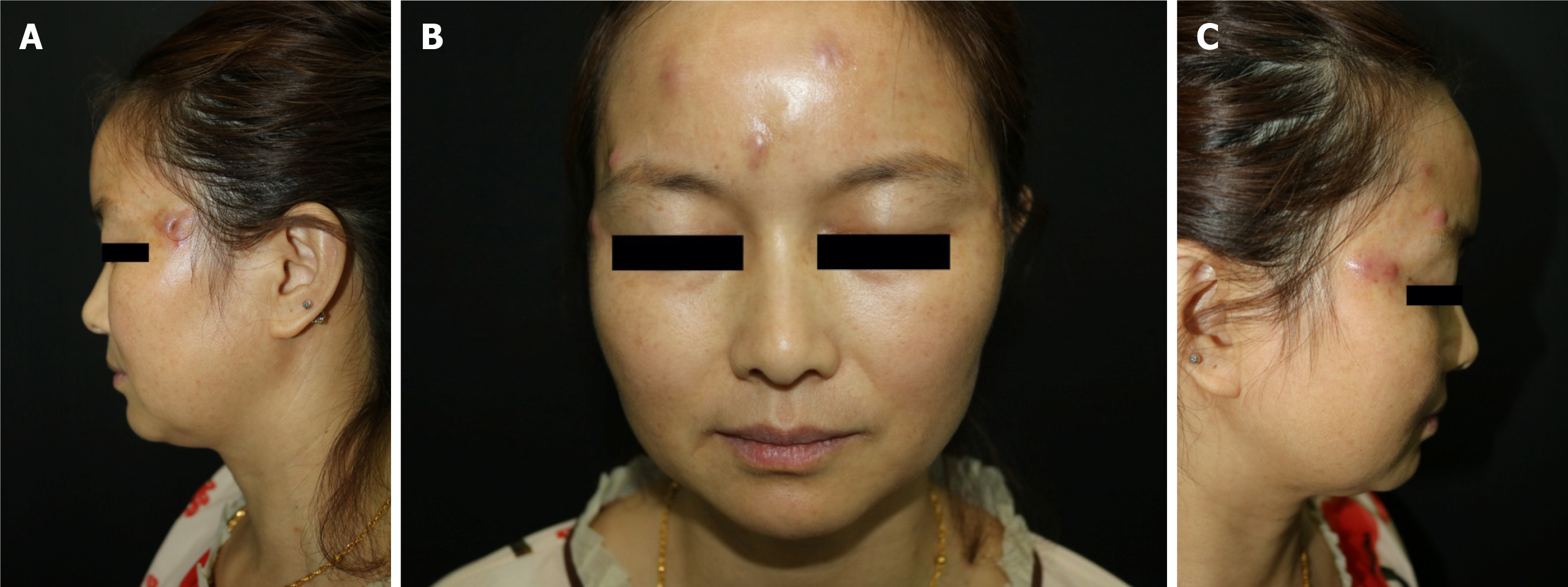
Physical examination showed that there were eight red nodules and abscesses on the forehead and temples. The diameter was approximately 0.5-1.0 cm (Figure 1).
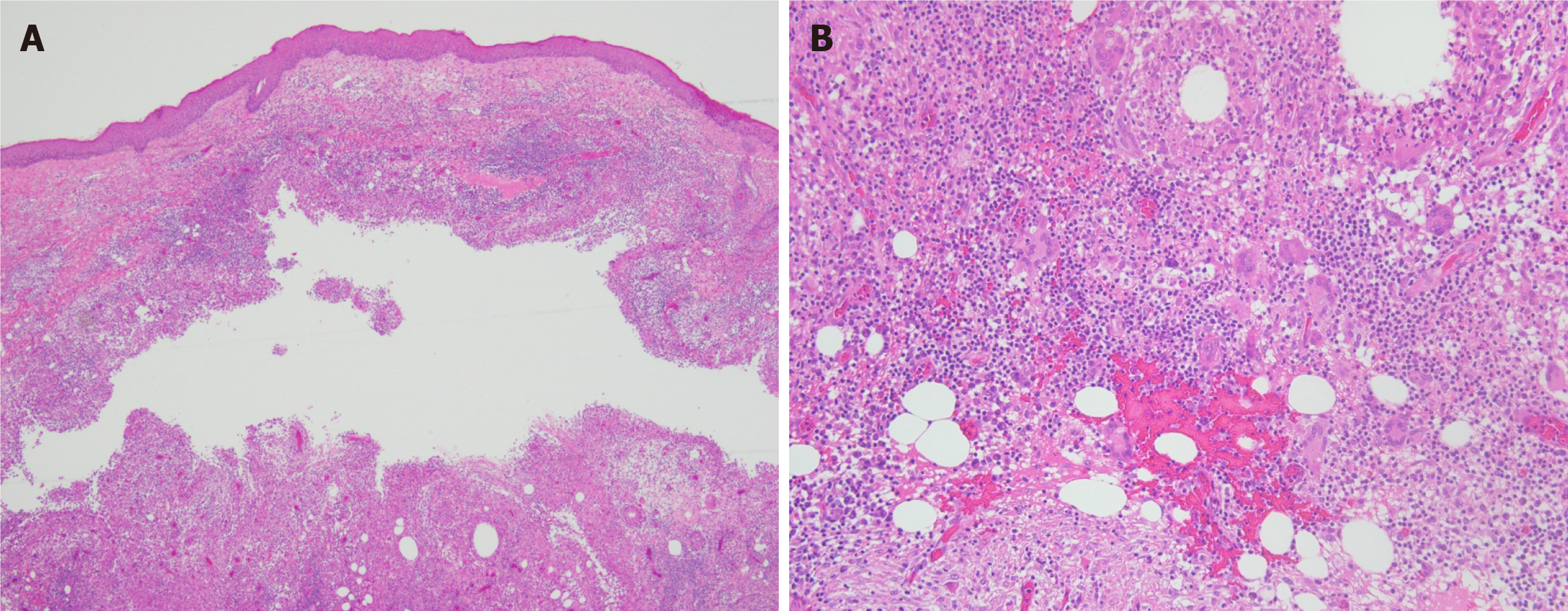
Biopsy was performed. Hematoxylin-eosin staining revealed a cyst in the dermis that was surrounded by granulomatous structures formed by histiocytes, scattered multinucleate giant cells, and lymphocyte, plasma cell, and neutrophil infiltration (Figure 2). Periodic acid-Schiff staining was negative. Pus was taken for mycobacterial culture. Colonies appeared after 4 d of mycobacterial culture. M. abscessus was identified with a Vitek MS MALDI-TOF mass spectrometry system (bioMerieux, France).
Facial M. abscessus infection.
The patient was given oral clarithromycin 500 mg twice daily combined with oral minocycline 100 mg twice daily. After a week of treatment, the patient developed significant gastrointestinal side effects. Then, we adjusted the oral clarithromycin treatment to 500 mg twice daily and moxifloxacin 400 mg once daily for 5 mo.
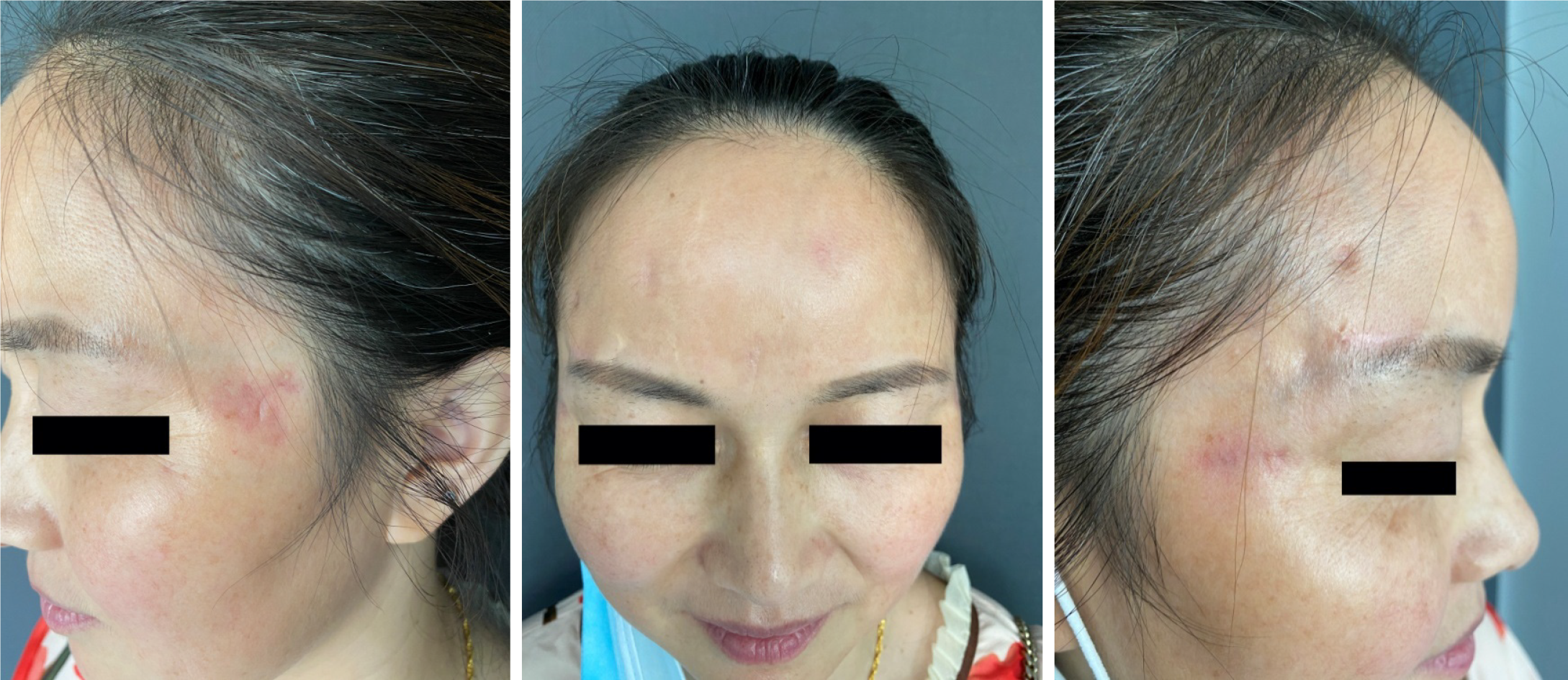
The lesions resolved, with scars and pigmentation (Figure 3) still observed at follow-up.
Most mycobacteria are found in the native environment, for example, in soil, water, or aerosols, and are usually not pathogenic[3]. NTM can cause infections in the lungs, lymph nodes, and skin and subcutaneous soft tissues. Being dependent on the growth rate, NTM can be divided into rapidly growing mycobacteria (RGM) or slowly growing mycobacteria (SGM)[4]. Common RGM include M. abscessus, Mycobacterium fortuitum, and Mycobacterium chelonae. Lesions can be characterized by erythema, nodules, abscesses, and mild pain[5]. Biopsy and microbiological examination should be carried out in patients in whom this disease is highly suspected. In this case, the microbiological examination provided evidence for the diagnosis. However, the low positive rate of bacterial culture is the main factor that makes the disease difficult to diagnose. In this patient, microbial culture of a pus specimen was performed, mycobacteria were observed 4 d later, and M. abscessus was identified with a Vitek MS MALDI-TOF mass spectrometry system (bioMerieux, France). The Vitek MS MALDI-TOF mass spectrometry system was approved by the United States Food and Drug Administration on July 31, 2017 for the identification of mycobacteria. M. abscessus is naturally resistant to first-line anti-tuberculosis drugs. The results of the in vitro susceptibility test for M. abscessus are inconsistent. The relatively consistent results show that M. abscessus is sensitive (greater than 90%) to clarithromycin, amikacin, and cefoxitin; moderately sensitive (40%-60%) to linezolid, imipenem, moxifloxacin, and tetracycline antibiotics; and poorly sensitive (approximately 10%) to ciprofloxacin and the sulfamethoxazole tobramycin[6]. There is no standardized treatment for cutaneous M. abscessus infection, and many NTM strains show drug resistance with limited drug selection. Clarithromycin is the cornerstone of all therapeutic drugs. The treatment effect of a combination of clarithromycin as the preferred drug with other antibacterial agents lasts for at least 4 mo; however, surgical treatment is sometimes required[7]. In this case, we chose clarithromycin combined with minocycline for oral treatment, but after 1 wk of taking the drug, the patient developed a significant gastrointestinal reaction, so we adjusted the treatment plan to clarithromycin combined with moxifloxacin. The lesions improved, although with hyperpigmentation and scarring.
There are two major factors contributing to cutaneous NTM infection. The first is trauma, surgery, or direct exposure to the environment, and the other is systemic infection with cutaneous or subcutaneous soft tissue complications[5]. In recent years, cases of M. abscessus infection have been reported, mainly related to mesodermal therapy or cosmetic fillings[7]. It has been declared in the literature that M. abscessus infection has occurred after tattooing, cosmetic injection, acupuncture, and even hair transplantation[8-11]. Infection with M. abscessus after facial injection of argireline has not previously been reported.
In this case, infection occurred at all injection sites, and we suspect that the drug, drug solvent, or syringe was contaminated with the bacteria. Erythema, mound pustules, nodules, or abscesses appear within 1 wk to several weeks at the injection site. We should suspect infection with nontuberculous mycobacteria or fungi. Cosmetic procedures have become popular worldwide. Many clinics perform tattooing and injection operations. The environment must be strictly disinfected when we are performing treatment, and the surgical instruments should be sterilized. In addition, water and solvent may also cause infection with M. abscessus during surgery, and sterile injection water should be utilized to dissolve the drug. When choosing locations to undergo cosmetic procedures, cosmetic patients must not mistakenly believe in false advertisements and must choose a formal institution.
Argireline is a novel anti-ageing product that is mostly used in cosmetics and is formulated into ointments and creams to prevent skin wrinkles. The anti-ageing mechanism of argireline is similar to that of botulinum toxin, which inhibits the release of neurotransmitters at neuromuscular synapses. Argireline is rarely used for subcutaneous or intramuscular injection[12]. We report here for the first time a case of M. abscessus infection following facial injection with argireline. When undergoing cosmetic injection, attention should be paid to the side effects and risks of treatment. If there is a nodule or abscess at the treatment site, the possibility of NTM infection should be investigated.
We report here for the first time a case of infection with M. abscessus after argireline injection. This condition is easily misdiagnosed as a common bacterial infection. Microbiological examinations are helpful for diagnosis and standardized cosmetic procedures can prevent infection with M. abscessus.
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fateh A, Munerato MC S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li JH
| 1. | Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3867] [Cited by in RCA: 4195] [Article Influence: 233.1] [Reference Citation Analysis (0)] |
| 2. | Lee WJ, Kang SM, Sung H, Won CH, Chang SE, Lee MW, Kim MN, Choi JH, Moon KC. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Bottai D, Stinear TP, Supply P, Brosch R. Mycobacterial Pathogenomics and Evolution. Microbiol Spectr. 2014;2:MGM2-0025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15:716-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 621] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 5. | Kothavade RJ, Dhurat RS, Mishra SN, Kothavade UR. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 2013;32:161-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Huang YC, Liu MF, Shen GH, Lin CF, Kao CC, Liu PY, Shi ZY. Clinical outcome of Mycobacterium abscessus infection and antimicrobial susceptibility testing. J Microbiol Immunol Infect. 2010;43:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus Complex Infections in Humans. Emerg Infect Dis. 2015;21:1638-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 8. | Bechara C, Macheras E, Heym B, Pages A, Auffret N. Mycobacterium abscessus skin infection after tattooing: first case report and review of the literature. Dermatology. 2010;221:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Fang RY, Sun QN. Mycobacterium abscessus infections following injection of botulinum toxin. J Cosmet Dermatol. 2020;19:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Choi WS, Kim MJ, Park DW, Son SW, Yoon YK, Song T, Bae SM, Sohn JW, Cheong HJ, Kim MJ. Clarithromycin and amikacin vs. clarithromycin and moxifloxacin for the treatment of post-acupuncture cutaneous infections due to Mycobacterium abscessus: a prospective observational study. Clin Microbiol Infect. 2011;17:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Eustace K, Jolliffe V, Sahota A, Gholam K. Cutaneous Mycobacterium abscessus infection following hair transplant. Clin Exp Dermatol. 2016;41:768-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Grosicki M, Latacz G, Szopa A, Cukier A, Kieć-Kononowicz K. The study of cellular cytotoxicity of argireline - an anti-aging peptide. Acta Biochim Pol. 2014;61:29-32. [PubMed] |