Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1976
Peer-review started: November 18, 2020
First decision: December 3, 2020
Revised: December 12, 2020
Accepted: January 8, 2021
Article in press: January 8, 2021
Published online: March 16, 2021
Processing time: 106 Days and 0.2 Hours
Germinoma is a type of germ cell tumor that most frequently arises in the midline axis of the brain. Impaired vision is a clinical manifestation of germinnoma. Although rare, intracranial germinoma seeding to the perioptic arachnoid space is one cause of visual acuity decrease.
An 11yearold girl who presented with polyuria and polydipsia and subsequently developed diminution of vision. Imaging showed bilateral heterogeneous enhancement of the optic nerve sheaths and atrophy of the optic nerve, and transsphenoidal biopsy revealed a germinoma. The patient experienced poor visual recovery following chemotherapy and radiotherapy. Germinomas are rare and they are mostly identified in children and adolescents. The manifestations include diabetes insipidus, pituitary dysfunction, visual complaints, etc. The mechanisms that lead to visual loss include intracranial hypertension, compression of optic chiasma, and tumor invasion. A literature review was performed to summarize the cases with a tumor infiltrating the optic nerve. Most of the reported patients were adolescents and presented with anterior pituitary hormone deficiency. Enhancement of optic nerve sheaths and optic disc pallor could be identified in most of the cases. The purpose of this report is to provide awareness that in cases where a germinoma is associated with visual loss, though rare, perioptic meningeal seeding should be taken into consideration.
The case report suggests that children with diabetes insipidus need a complete differential diagnosis.
Core Tip: Impaired vision is one of the clinical manifestations of a germinoma, and most of the reduction in visual acuity is caused by compression of the optic chiasma. We present a case of impaired vision caused by a germinoma and perioptic meningeal seeding, which is extremely rare. The findings of this case study emphasize that children with diabetes insipidus need a complete differential diagnosis.
- Citation: Yang N, Zhu HJ, Yao Y, He LY, Li YX, You H, Zhang HB. Diabetes insipidus with impaired vision caused by germinoma and perioptic meningeal seeding: A case report. World J Clin Cases 2021; 9(8): 1976-1982
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1976.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1976
Intracranial germ cell tumors (GCTs) affect children and young adults, with a peak incidence in the age range of 10-19 years. According to the 2016 World Health Organization classification for tumors of the central nervous system, GCTs are histologically classified into germinoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, teratoma, teratoma with malignant transformation, and mixed GCT[1]. Germinoma most frequently arises in the midline axis of the brain (especially in the pineal and suprasellar regions)[2]. Extra-axial sites have been reported, mostly in Asian populations, in the thalamic and basal ganglia, corpus callosum, and ventricles[3]. Clinical manifestations as well as associated endocrine abnormalities depend on the location and size of the tumor. Impaired vision is one of these clinical manifestations. Most of the visual acuity decrease is caused by compression of the optic chiasma, whereas it is rarely caused by intracranial germinoma seeding to the perioptic arachnoid space[4].
In this case report, we discuss a girl with germinoma who had visual loss caused by secondary seeding dissemination to the optic nerve instead of compression of the optic chiasma by the tumor.
An 11yearold Chinese girl presented with polyuria, polydipsia, and a sudden eyesight decline in both eyes before she came to our hospital.
The patient presented with polyuria and polydipsia 1 year before the admission to our hospital, and the total 24-h urine output was 3 L. She was diagnosed as enuresis in a local hospital, but the results were not available. Treatment with oral desmopressin was effective. She had a sudden eyesight decline in both eyes 2 mo before the admission. She was diagnosed with central diabetes insipidus (CDI) after the admission.
The patient had a free previous medical history.
The patient had no related family and psycho-social history.
The patient’s height growth had slowed down (stable at 137 cm) in the past year, and her weight had decreased by 3 kg (35 kg to 32 kg) in the past 6 mo. She showed normal intellectual development. The best corrected visual acuity of both sides was 20/200, and the light reflex could not be maintained. Ophthalmic examination indicated pallor of both optic discs.
Anterior pituitary hormonal profile showed low 24-h urine free cortisol (5.9 μg/24 h, reference range: 12.3-103.5), adrenocorticotropic hormone (ACTH) (8.8 pg/mL, reference range: 0-46), serum cortisol concentration (8.3 μg/dL, reference range: 4-22.3), and insulin-like growth factor 1 (IGF-1) (53 ng/mL, reference range: 111-551). All other anterior pituitary function assessments were within the normal range. Serum alpha-fetoprotein (AFP) and beta human chorionic gonadotropin (β-hCG) levels were normal. Cerebrospinal fluid, including AFP and β-hCG, was normal. Tests for serum angiotensin-converting enzyme (ACE), anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, immunoglobulin (Ig) G 4, anti-glial fibrillary acidic protein (anti-GFAP) antibody, anti-flotillin-1/2 antibody, anti-myelin basic protein (anti-MBP) antibody, anti-myelin oligodendrocyte glycoprotein (anti-MOG) antibody, and anti-aquaporin-4 (anti-AQP4) antibody were all negative.
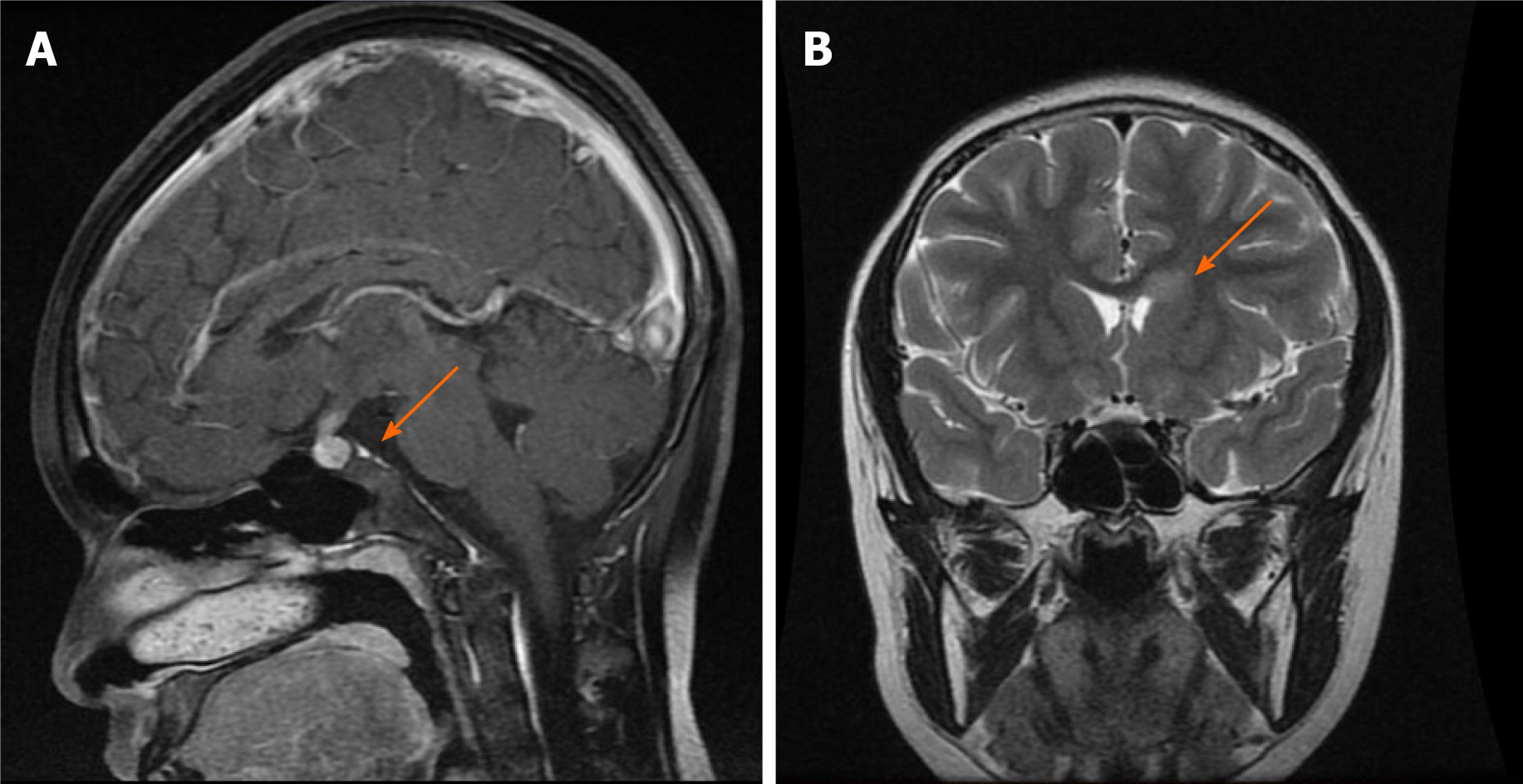
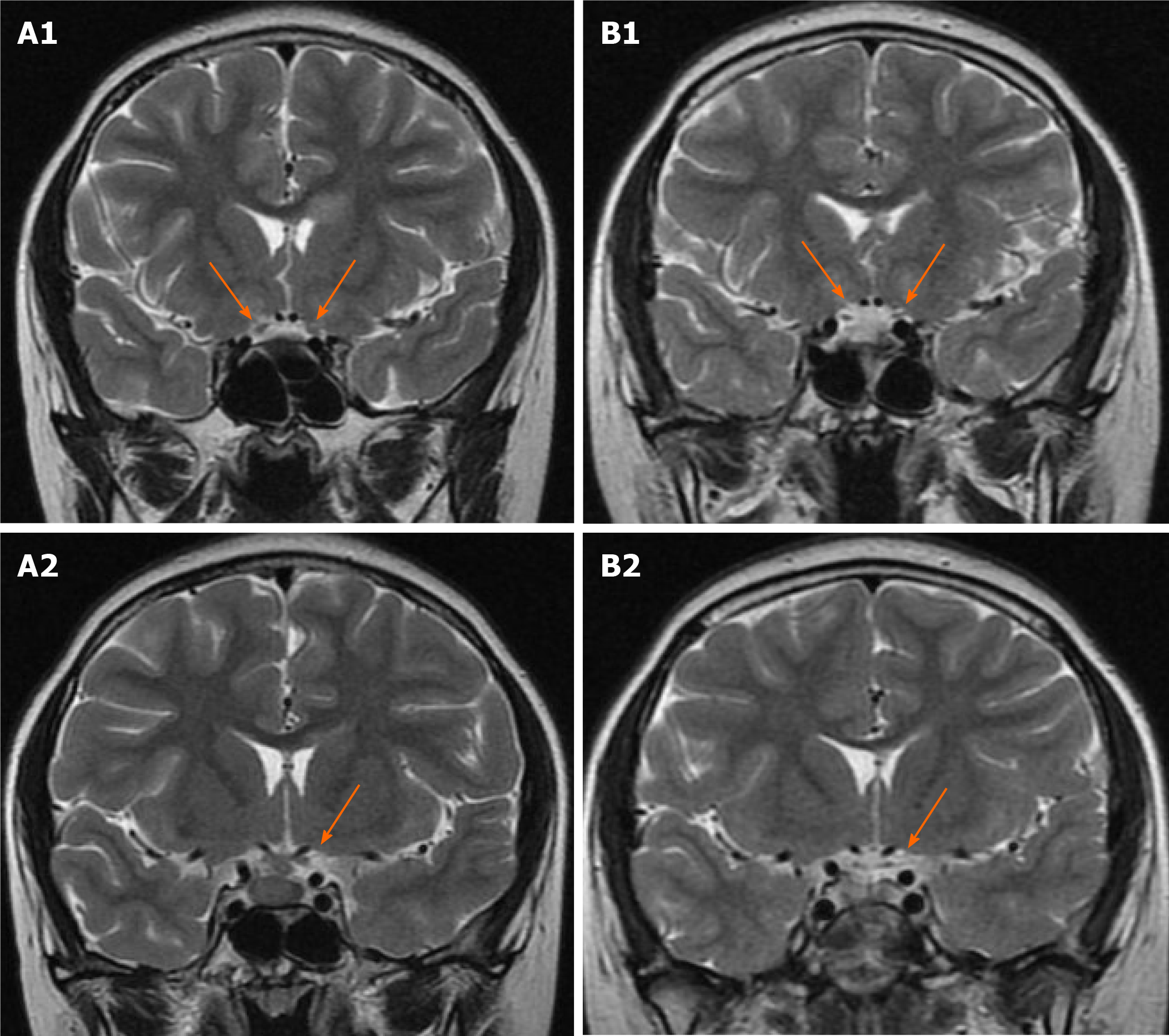
The patient underwent contrast-enhanced magnetic resonance imaging (MRI) of the brain and optic nerve. Imaging revealed a sellar mass with the thickness of the pituitary stalk (Figure 1A), absence of the hyperintensity signal of the posterior pituitary, and abnormal enhancement shadow in the left anterior horn of the ventricle (Figure 1B). Optic nerve MRI showed bilateral enhancement of the optic nerve sheaths and atrophy of the optic nerve (Figure 2A), which was consistent with tumor infiltration. Contrast-enhanced computerized tomography (CT) of the chest, bone scintigraphy, and thyroid ultrasound were all negative.
Transsphenoidal biopsy revealed a germinoma. Immunohistochemistry results were as follows: Etoposide-methotrexate-actinomycin (EMA) (-), AE1/AE3 (partially +), AFP (-), Ki-67 (index 3%), CD30 (Ki-1) (-), CD117 (+), Sox2 (+), OCT3 / 4 (+), and sal-like protein 4 (SALL-4) (+).
In terms of treatment, desmopressin was administered to control the diabetes insipidus (DI), and hydrocortisone was used to treat the adrenal insufficiency. This patient had received chemotherapy and radiotherapy. An etoposide and cisplatin (EP) regimen was selected as the chemotherapy regimen, i.e., cisplatin [28 mg (25 mg/m2), days 1-3] combined with etoposide [115 mg (100 mg/m2), days 1-5]. The irradiation was 19.8 Gy/11F (1.8 Gy/F), 5 F/W for the whole brain and whole spinal cord. The whole ventricle irradiation was boosted to 30.6 Gy/17F (1.8 Gy × 11F + 1.8 Gy × 6F). The main adverse reaction was nausea and vomiting; however, the patient could tolerate it.
After applying the above treatments, the sellar region mass subsided, the optic nerve sheath strengthening was weaker (Figure 2B), and atrophic optic nerve could be seen. Nevertheless, there was no significant improvement in vision.
Germinomas are uncommon. In a retrospective review by Famini et al[5] of 2598 patients undergoing at least one pituitary MRI examination, germinomas were identified in only three patients. Germinomas have the capacity to infiltrate the adjacent brain tissue and commonly disseminate along cerebrospinal fluid pathways[6]; thus, patients with a germinoma may manifest complex clinical findings, such as DI, pituitary dysfunction, etc. In this case, combined with the patient's polydipsia, polyuria, effective desmopressin treatment in the course of the disease, and imaging indicating the absence of the hyperintensity signal of the posterior pituitary, the diagnosis of CDI was clear. She also had anterior hypopituitarism, mainly manifested as poor appetite, growth retardation, laboratory examination revealing hypofunction of the adrenal cortex, low level of growth hormone, and decreased IGF-1. Imaging showed a sellar mass with diffuse ependymal involvement. Optic nerve MRI showed bilateral enhancement of the optic nerve sheath and atrophy of the optic nerve. The patient had no evidence of Langerhans cell histiocytosis, sarcoidosis, or immune system disease. A previous research indicated that only 18.2% of histopathologically diagnosed germinomas were tumor marker positive[7], therefore even though her AFP and β-hCG were negative in the blood and cerebrospinal fluid (CSF), we highly suspected that she had a gernimoma, which was confirmed by postoperative pathology. The limitation of this case report is that an optic nerve biopsy was not performed to protect the optic nerve; hence there was no pathological diagnosis of the optic nerve.
The most common manifestation of germinoma is polyuria, while mass symptoms in the form of headache and visual complaints are observed in approximately half of the cases[6]. The visual complaints always occur after DI, with a median delay of 5 mo (ranging from 1 to 35)[3]. The common mechanisms that lead to visual loss include the following aspects[3]: (1) Intracranial space mass causing intracranial hypertension and papilledema; (2) Compression of the optic chiasma by the sellar mass; and (3) Primary tumor/tumor invasion of the optic nerve, optic radiation, and occipital cortex. However, primary involvement of the optic pathways by germinomas is rare[8].
In this case, the patient's visual acuity decreased 10 mo after DI, and ophthalmic examination revealed optic nerve atrophy and pallor of the optic disc. An MRI examination of the optic nerve showed obvious enhancement of the optic nerve sheaths, with atrophy and thinning of the optic nerve. In previous cases of germinoma with decreased vision, almost all of them involved compression of the optic chiasma. However, the hypophysis of this patient was neither large nor closely related to the optic chiasma; therefore, it was less likely that the visual acuity decrease was due to compression of the optic chiasma. As corroborated by her optic nerve MRI, it was more likely that the tumor infiltrated the optic nerve. A literature review was performed to summarize the cases with a tumor infiltrating the optic nerve in Table 1[4,6,9,10]. Most of the reported patients were adolescents and the age ranged from 12 to 34 years old. Visual complaints always occurred after DI, with a delay ranging from half a year to 12 years. Anterior pituitary hormone deficiency was present in 4/7 of the cases. Enhancement of optic nerve sheaths and optic disc pallor could be identified in most of the cases. After radiotherapy and chemotherapy, 5/7 of the cases showed an improvement in visual acuity.
| Ref. | Age/gender | Duration of CDI | Duration of visual acuity decline | Other clinical presentation | Abnormal hormones | Cranial MRI | Tumor markers (serum) | Tumor markers (CSF) | Funduscopic examination | Treatment | Prognosis |
| Pal et al[6] | 12/F | 6 yr | 3 yr | Headache, vomiting | Low free T4, low cortisol, low ACTH | Suprasellar mass with infundibular stalk thickening, pineal mass | Normal | Normal | Bilateral disk pallor | Chemotherapy | Died of sepsis |
| Huang et al[9] | 21/M | 3 yr | 3 mo | - | - | Suprasellar mass, enhancement of the ventricular surface, bilateral optic nerve and optic chiasm | - | - | Optic disc pallor, a cup to disc ratio (C/D) of 0.4 | Radiotherapy and chemotherapy | Visual acuity of his right eye improved, but left did not |
| Huang et al[9] | 24/M | 2 yr | 1.5 yr | Hypogonadism, visual field defects | - | Suprasellar and pineal region mass | Normal | β-HCG elevated | Optic disc pallor | Radiotherapy | Visual acuity in both eyes improved |
| Huang et al[9] | 13/F | 3.5 yr | 1.5 yr | Nausea, vomiting, fever | - | Suprasellar mass | - | - | Optic disc pallor | Craniotomy for tumor resection | Visual acuity in both eyes was worse |
| Nakajima et al[4] | 31/M | / | 2 yr | Motor weakness, panhypopituitarism | - | Left basal ganglia bilateral mass and the optic nerves enhanced | Normal | Normal | Bilateral optic atrophy | Chemotherapy and Radiotherapy | Visual acuity improved |
| Nakajima et al[4] | 27/M | 5 yr | 8 mo | General fatigue, double vision | - | Pituitary stalk, pineal region, corpus callosum, thalamus and basal ganglia mass, lateral ventricle, optic nerve and the inferior part of the left dentate nucleus enhanced | β-HCG elevated | - | bilateral atrophy of the optic nerves | Chemotherapy and radiotherapy | Visual acuity improved |
| Pereira et al[10] | 34/F | 12 yr | 3 mo | Hypopituitarism, facial paralysis | - | Pituitary stalk mass, optic nerves enhanced | AFP and β-HCG elevated | Normal | Relative afferent pupillary defect and optic disc pallor | Chemotherapy and radiotherapy | Visual acuity improved |
After radiotherapy combined with chemotherapy, although the patient's vision did not improve, enhancement of the optic nerve sheaths weakened. Combined with the characteristics of the above cases, we speculated that the reason for a lack of improvement in the girl's vision might have been the prolonged infiltration of the optic nerve by the tumor, resulting in an irreversible damage of the optic nerve.
In this case report, we present a case with impaired vision caused by germinoma and perioptic meningeal seeding. Although impaired vision is one of the clinical manifestations of germinoma, most of the visual acuity decrease is caused by compression of the optic chiasma, and it is rarely caused by intracranial germinoma seeding to the perioptic arachnoid space. A literature review was performed to summarize the cases with a tumor infiltrating the optic nerve and discovered that most of the reported patients were adolescents presenting with anterior pituitary hormone deficiency. The findings of this case report indicate that children with DI need a complete differential diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mortensen LA S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH
| 1. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10867] [Article Influence: 1207.4] [Reference Citation Analysis (0)] |
| 2. | Reddy MP, Saad AF, Doughty KE, Armstrong D, Melguizo-Gavilanes I, Cheek BS, Opatowsky MJ. Intracranial germinoma. Proc (Bayl Univ Med Cent). 2015;28:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Frappaz D, Pedone C, Thiesse P, Szathmari A, Conter CF, Mottolese C, Carrie C. Visual complaints in intracranial germinomas. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Nakajima T, Kumabe T, Jokura H, Yoshimoto T. Recurrent germinoma in the optic nerve: report of two cases. Neurosurgery. 2001;48:214-7; discussion 217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011;96:1633-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Pal R, Rai A, Vaiphei K, Gangadhar P, Gupta P, Mukherjee KK, Singh P, Ray N, Bhansali A, Dutta P. Intracranial Germinoma Masquerading as Secondary Granulomatous Hypophysitis: A Case Report and Review of Literature. Neuroendocrinology. 2020;110:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Takami H, Fukuoka K, Fukushima S, Nakamura T, Mukasa A, Saito N, Yanagisawa T, Nakamura H, Sugiyama K, Kanamori M, Tominaga T, Maehara T, Nakada M, Kanemura Y, Asai A, Takeshima H, Hirose Y, Iuchi T, Nagane M, Yoshimoto K, Matsumura A, Kurozumi K, Nakase H, Sakai K, Tokuyama T, Shibui S, Nakazato Y, Narita Y, Nishikawa R, Matsutani M, Ichimura K. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019;21:1565-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Iizuka H, Nojima T, Kadoya S. Germinoma of the optic nerve: case report. Noshuyo Byori. 1996;13:95-98. [PubMed] |
| 9. | Huang WB, Zhang XL, Wang W, Dai YL, Qiu HY, Wei SH. Ocular manifestations of intracranial germinomas: three cases report and literature review. Chin Med J (Engl). 2012;125:2790-2793. [PubMed] |
| 10. | Pereira LS, Green AJ, Hwang TN, McCulley TJ. Suprasellar germinoma and late perioptic seeding. Eur J Ophthalmol. 2008;18:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |