Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1827
Peer-review started: December 1, 2020
First decision: December 24, 2020
Revised: January 6, 2021
Accepted: January 25, 2021
Article in press: January 25, 2021
Published online: March 16, 2021
Processing time: 94 Days and 2.6 Hours
Patent ductus arteriosus (PDA) is a common congenital heart abnormality in preterm neonates with a high incidence in neonates with very low birth weights. When PDA persists, interstitial lung water content increases, which could lead to abnormal circulation hemodynamics and pulmonary edema. It is important to perform early and reliable assessment of lung water content in very low-weight preterm neonates with persistent PDA.
To evaluate the role of bedside cardiopulmonary ultrasonography in the lung water content assessment in very low-weight preterm neonates with persistent PDA.
From January 2018 to March 2020, 69 very low-weight preterm neonates with echocardiography-confirmed PDA were selected as the PDA group. At the same time, 89 very low-weight preterm neonates without PDA were randomly selected as the control group. All neonates underwent echocardiography and 6-segment lung ultrasonography on the fourth day after birth. The clinical characteristics and main ultrasonography results were compared between the two groups. Pearson’s analysis was used to analyze the correlation between lung ultrasonography score (LUS) and other related clinical and ultrasonography results in all neonates. In the PDA group, PDA diameters were recorded, and the correlation with LUS and left atrium to aortic (LA/AO) dimension ratio were also analyzed. LA/AO ratio is one of the ultrasonic diagnostic criteria for hemodynamically significant PDA. When the ratio is ≥ 1.5, it suggests the possibility of hemodynamic changes in persistent PDA. A receiver operating characteristic curve was established using the sensitivity of LUS to predict the hemodynamic changes in neonates with PDA as the ordinate and 1-specificity as the abscissa.
A total of 158 neonates were enrolled in this study, including 69 in the PDA group and 89 in the control group. There were no statistical differences in sex, gestational age, birth weight, ventilator dependence, hospitalization length and left ventricular ejection fraction between the two groups (P > 0.05). The LUS and LA/AO ratio in the PDA group were higher than those in the control group (P < 0.05), but there was no difference of LUS in neonates with or without use of the ventilator (t = 0.58, P = 0.16). In all cases, LUS was negatively correlated with gestational age (r = -0.28, P < 0.01) and birth weight (r = -0.36, P < 0.01), while positively correlated with the LA/AO ratio (r = 0.27, P < 0.01). In the PDA group, PDA diameter was positively correlated with the LA/AO ratio (r = 0.39, P < 0.01) and LUS (r = 0.31, P < 0.01). Receiver operating characteristic results showed that LUS had the moderate accuracy for predicting hemodynamic changes in PDA (area under the curve = 0.741; sensitivity = 93.75%; specificity = 50.94%).
Bedside cardiopulmonary ultrasonography can evaluate lung content in neonates with PDA and predict the possibility of hemodynamic changes in persistent PDA.
Core Tip: Patent ductus arteriosus (PDA) has a high incidence in very low-weight preterm neonates. If PDA persists, then interstitial lung water content increases, which could cause hemodynamic changes and pulmonary edema. Thus, early and reliable assessment of lung water content is important in these cases. In this study, we performed cardiopulmonary ultrasonography on the fourth day after birth to investigate whether there are differences between neonates with or without PDA. The results indicated that neonates with PDA had higher lung water content. Lung ultrasonography score had moderate accuracy for predicting the possibility of hemodynamic changes in persistent PDA.
- Citation: Yu LF, Xu CK, Zhao M, Niu L, Huang XM, Zhang ZQ. Bedside cardiopulmonary ultrasonography evaluates lung water content in very low-weight preterm neonates with patent ductus arteriosus. World J Clin Cases 2021; 9(8): 1827-1834
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1827.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1827
Patent ductus arteriosus (PDA) is a common congenital heart abnormality in preterm neonates. Neonates with prematurity and low birth weights, especially those with a birth weight less than 1500 g have poorly developed organs and thus a higher incidence of PDA[1]. The direct communication between the aorta and pulmonary artery can result in abnormal pulmonary circulation, left heart overload and poor systemic perfusion[2], which may present with pulmonary edema, pulmonary hemorrhage, left ventricular dysfunction and bronchopulmonary dysplasia[3,4]. Therefore, it is important to perform an early and reliable lung water content and hemodynamic assessment in very low-weight preterm neonates with persistent PDA.
With recent advancements in lung ultrasonography, the detection of a variety of lung diseases in neonates has improved markedly. Studies have reported that changes in the ratio of fluid in the interstitial or pleural spaces can produce artifacts, making it possible to diagnose pulmonary diseases[5,6]. The B line is an artifact caused by excessive water outside of pulmonary blood vessels, and the number of B lines is related to the severity of pulmonary edema[7]. The lung ultrasonography score (LUS) is a quantitative assessment of B line. In very low birth-weight preterm neonates with PDA, hemodynamic instability mainly manifests as pulmonary edema[8,9].
Also, PDA has a characteristic performance in echocardiography. For typical PDA cases, the diagnostic accuracy of echocardiography can be 100%, and the structure and function of the heart can be simultaneously evaluated. At present, the diagnostic criteria for hemodynamically significant PDA include clinical symptoms and echocardiographic indicators, of which the left atrium to aortic (LA/AO) dimension ratio and PDA diameter are important ultrasonic indicators for hemodynamically significant PDA. A ratio of LA/AO ≥ 1.5 suggests the possibility of hemodynamic changes in persistent PDA[10,11]. Therefore, we performed cardiopulmonary ultrasonography in neonates with or without PDA and compared their ultraso-nography results to explore the differences, evaluate lung water content and predict hemodynamic changes in very low-weight preterm neonates with PDA.
A total of 69 very low-weight preterm neonates with echocardiography-confirmed PDA screened fetal congenital heart defects who were admitted to the neonatal intensive care unit of the Hangzhou First People’s Hospital from January 2018 to March 2020 were enrolled in this study. During the same period, 89 cases without PDA were included as the control group. The inclusion criteria were: Gestational age less than 37 wk; birth weight less than 1500 g; and admission to the neonatal intensive care unit of our hospital within 24 h of birth. The exclusion criteria were: Congenital malformations and other chromosomal abnormalities identified before birth; and lung diseases caused by definite pathogenic factors (including meconium aspiration syndrome, pulmonary hemorrhage, and pneumonia, etc.). The study protocol was approved by the Ethics Committee of the Hangzhou First People’s Hospital in China (approval no. 2018-30-1) and conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the guardians of the neonates prior to inclusion.
Ultrasonic examination was performed with the use of Philips IE33 and Philips CX50 United States instruments (Philips Healthcare, Best, the Netherlands). Neonatal cardiac ultrasound probe (12 MHz), high-frequency shallow probe (12 MHz) and micro-convex probe (8 MHz) were used to evaluate the heart and lung functions.
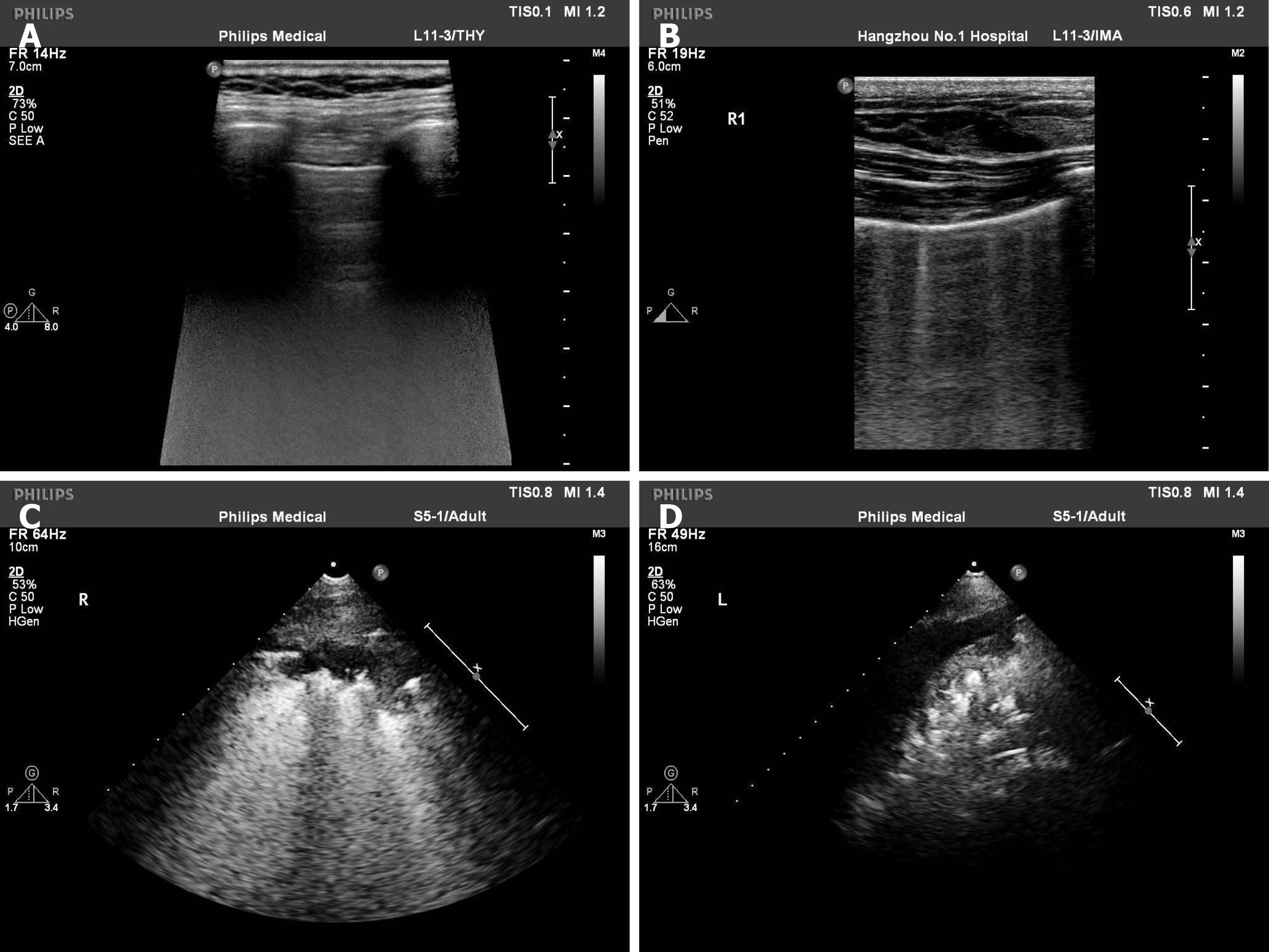
Ultrasonic examinations were completed on the fourth day after birth. Echocardi-ography was performed to measure the left ventricular ejection fraction, LA and AO in M-mode with standard planes. Lung ultrasonography was carried out to evaluate lung function in horizontal and vertical scans. Each lung was divided into three regions, namely anterior superior, anterior inferior and lateral. According to the method of Brat et al[12], we scored the lungs as follows (Figure 1): Lung sliding with line A or slight line B, 0 point; more than three B lines with a gap between B lines and no fusion, 1 point; diffuse B lines and fusion between B lines with moderate subpleural lung consolidation, 2 points; and extensive consolidation, 3 points. Each region with the most severe performance was scored, the final LUS value was the sum of the scores, and the highest score was 18 points. All ultrasound examinations were performed by the same sonographer who was well-experienced in cardiopulmonary ultrasonogra-phy examination. All images were saved and stored in the picture archiving and communication system.
All statistical analyses were conducted using SPSS 17.0 software for Windows (IBM Corp, Armonk, NY, United States). Measured data were presented as means ± standard deviations and categorical variables as percentages. Differences between the two groups were determined by Student’s t-test or Chi-square test. Pearson’s analysis was used to analyze the correlation between the LUS and other data in all cases. In the PDA group, Pearson’s analysis was also used to analyze the correlation between the PDA diameter, LUS and LA/AO ratio. A receiver operating characteristic curve was established using the sensitivity of the LUS to predict the possibility of hemodynamic changes in persistent PDA as the ordinate and 1-specificity as the abscissa. P values less than 0.05 were considered statistically significant.
A total of 158 cases were included in this study. There were 69 neonates in the PDA group (33 males and 36 females; 68 of them were ventilator dependent) with an average gestational age of 29.09 ± 2.15 wk, an average birth weight of 1195.14 ± 235.96 g and an average hospitalization length of 41.61 ± 22.76 d. There were 89 neonates in the control group (52 males and 37 females; 84 of them were ventilator dependent) with an average gestational age of 29.26 ± 1.93 wk, an average birth weight of 1165.11 ± 230.04 g and an average hospitalization length of 48.67 ± 16.43 d. There were no statistical differences in any of the parameters between the two groups (P > 0.05).
Lung ultrasonography results revealed that all cases had different B line characteristics. The LUS of neonates on a ventilator were 8.00 ± 3.66 points, and those not on a ventilator were 5.83 ± 3.12 points. There were no differences between them (t = 0.58, P = 0.16). The ultrasonography results of the two groups are summarized in Table 1. The PDA group had an average LUS of 9.26 ± 3.21 points, with a minimum of 2 points and a maximum of 15 points, whereas the control group had an average LUS of 6.88 ± 3.66 points, with a minimum of 2 points and a maximum of 18 points. The difference in the LUS between the two groups was statistically significant (P < 0.01). There were two deaths in the PDA group with lung scores of 10 points and 16 points, respectively. The average left ventricular ejection fraction value was not statistically different between the two groups (P = 0.22), while the average LA/AO ratio of the PDA group was higher than that of the control group (P = 0.01). Pearson’s analysis showed that the LUS was negatively correlated with gestational age (r = -0.28, P < 0.01) and birth weight (r = -0.36, P < 0.01), while positively correlated with the LA/AO ratio (r = 0.27, P < 0.01).
| Variable | PDA group, n = 69 | Control group, n = 89 | P value |
| LUS | 9.26 ± 3.21 | 6.88 ± 3.66 | < 0.01 |
| LA/AO | 1.28 ± 0.24 | 1.18 ± 0.24 | 0.01 |
| LVEF | 62.70 ± 7.61 | 64.00 ± 5.86 | 0.22 |
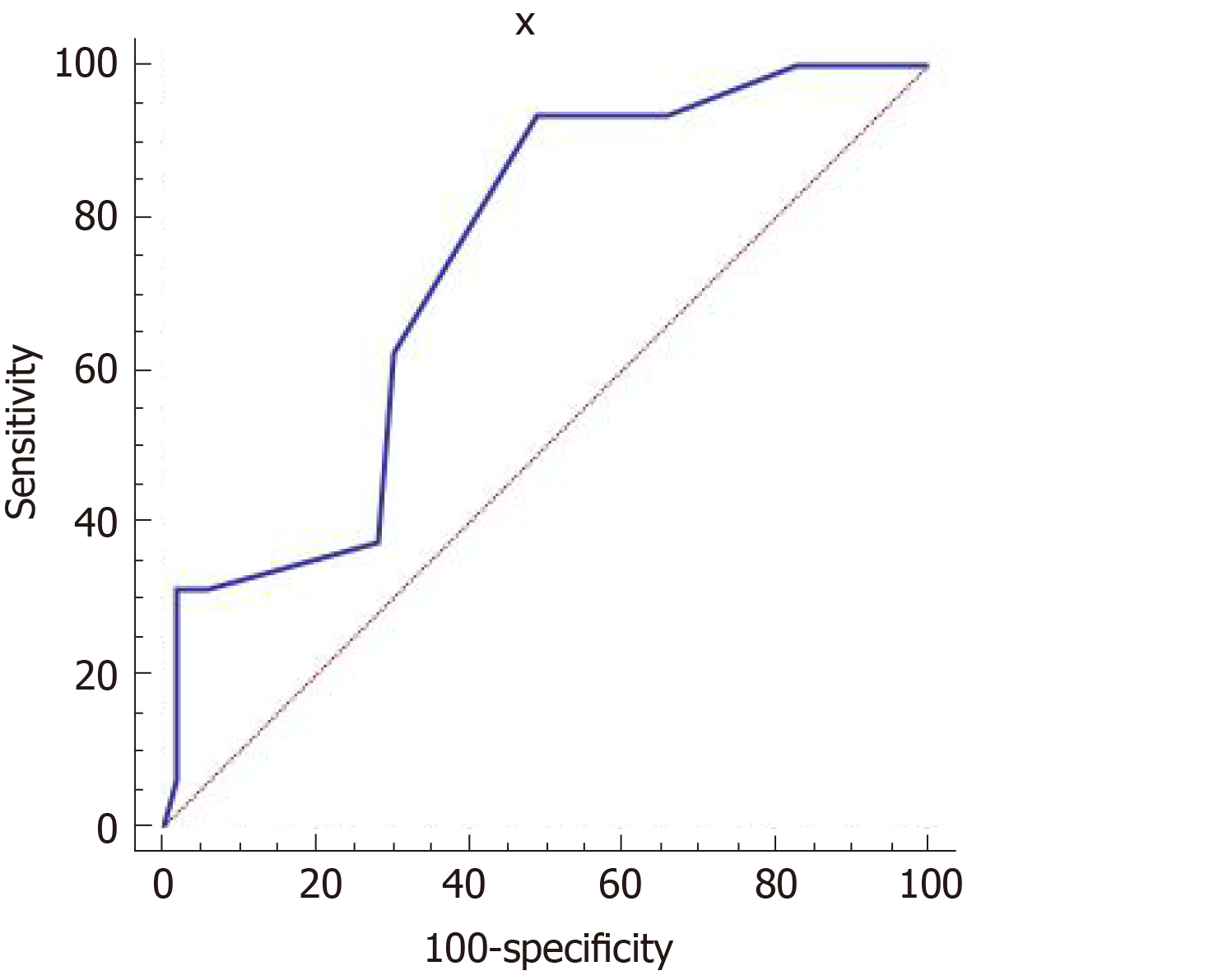
PDA diameter ranged from 0.08 to 0.35 cm, with an average of 0.20 ± 0.06 cm. PDA diameter was positively correlated with the LA/AO ratio (r = 0.39, P < 0.01) and LUS (r = 0.31, P < 0.01). There were 16 cases with an LA/AO ratio ≥ 1.5. The receiver operating characteristic curve results showed that the LUS had moderate accuracy for predicting the possibility of hemodynamic changes in persistent PDA. The area under the curve was 0.741 (95% confidence interval: 0.621-0.839), and sensitivity and specificity were 93.75% and 50.94%, respectively (Figure 2).
The lungs were once considered to be blind spots in ultrasonography. Advancements in lung ultrasonography have made it possible to detect a variety of pulmonary diseases, including respiratory distress syndrome, pneumonia, wet lung and pulmonary hemorrhage in neonates[13,14]. The 2019 Guidelines for Ultrasound Diagnosis of Neonatal Lung Diseases confirmed that lung ultrasonography had higher sensitivity and accuracy compared with traditional chest X-rays in the diagnosis of pneumothorax, pulmonary edema, pulmonary consolidation and pleural effusion[15-17]. Ultrasonography also has many advantages; it produces highly reproducible findings without radiation damage. Scanning can also be performed easily at the bedside, which is especially important for critically ill patients. As such, lung ultrasonography has become increasingly used in the diagnosis of lung diseases in general intensive care and child intensive care units[17-22].
Persistent PDA in very low-weight preterm neonates would cause left ventricular enlargement and increase diastolic blood pressure, resulting in congestion of the left atrium and pulmonary vascular bed causing abnormal circulation hemodynamics and pulmonary edema. It was previously reported that the LUS was related to the presence of lung water content[12,23-25], while it is still controversial whether the LUS can directly reflect the severity of pulmonary diseases[26,27]. The aim of the present study was to evaluate lung water content and predict the possibility of hemodynamic changes in very low-weight preterm neonates with persistent PDA. The results showed that the LUS in the PDA group was statistically higher than in the control group and negatively correlated with gestational age and birth weight, suggesting that neonates with lower gestational age and birth weight would had higher lung water content. This would provide useful information for clinical treatment.
PDA has a characteristic performance in echocardiography, and the LA/AO ratio measured in M-mode is related to the severity of the PDA diameter. The LA/AO ratio and PDA diameter are important ultrasonic indicators for hemodynamically significant PDA, and a LA/AO ratio ≥ 1.5 suggests the possibility of hemodynamic changes in persistent PDA. There were 16 cases with a LA/AO ratio ≥ 1.5 in the PDA group. The receiver operating characteristic curve showed that the LUS had moderate accuracy for diagnosing hemodynamically significant PDA. When the cut-off of the LUS was more than 9 points, its sensitivity and specificity were 93.75% and 50.94%. PDA diameter can be measured and was positively correlated with the LUS and LA/AO ratio. Therefore, it might provide more information for clinicians to determine whether further interventions are needed.
Ultrasonic examinations were completed on the fourth day after birth based on the following reasons: (1) Patent ductus is still present 72 h after birth[28]; (2) Pulmonary blood vessels dilate after birth, and the pulmonary artery pressure decreases rapidly by 72 h[29]; and (3) Examinations at different time points may affect the LUS. Currently, there are several different methods for the LUS calculation. The most widely used are the 6-zone and 12-zone methods[5]. In the present study, the 6-zone method was used. First, as the body surface area of very low-weight preterm neonates was very low, the 6-zone method can cover all inspection areas. Second, some neonates were on ventilators and moving them would increase the risk of tube removal. Last, the dense B line was mainly located in the anterior chest.
This study has several limitations. First, the LUS is a semi-quantitative evaluation of lung function, and there is no established quantitative standard for the number of B lines and the range of lung consolidation. Second, scores are affected by subjective factors of the operator. Different operators may give different scores for the same neonate. It is also difficult to collect large amounts of data from several centers. However, this study involved one sonographer who scored all the cases. Third, very low birth-weight preterm neonates often have other lung problems, indicating that a variety of complex factors may affect the accuracy of the scoring. Fourth, the number of cases in this study is small, and additional studies with a larger sample are needed.
Cardiopulmonary ultrasonography could evaluate lung water content and predict the possibility of hemodynamic changes in very low-weight preterm neonates with PDA. The LUS in lung ultrasonography is higher in PDA neonates and has a moderate accuracy for predicting hemodynamic changes. Echocardiography can detect PDA and indirectly assess left atrial pressure based on LA/AO ratio. The LUS is positively correlated with PDA diameter and LA/AO ratio and comprehensive assessment of the ultrasonic indicators is needed.
Patent ductus arteriosus (PDA) has a high incidence in neonates with very low-birth weights, and abnormal circulation hemodynamics and pulmonary edema may occur in persistent PDA. Lung ultrasonography was reported to be a quantitative method for assessment of lung water content. However, there are few studies reporting its role in neonates with PDA.
With recent advancements in lung ultrasonography, the detection of a variety of lung diseases in neonates has improved markedly. The advantages of bedside, radiation free, relatively cheap and reproducible would make it widely used in clinical work.
To evaluate lung water content and predict the possibility of hemodynamic changes in very low-weight preterm neonates with PDA.
In this study, we performed cardiopulmonary ultrasonography in neonates with or without PDA and compared the ultrasonography results including left ventricular ejection fraction, left atrium to aortic dimension ratio and lung ultrasonography score (LUS) between the two groups.
Results showed that the LUS and left atrium to aortic ratio were higher in neonates with PDA, and the LUS was positively correlated with PDA diameter and left atrium to aortic ratio, indicating that the lung water content was higher in PDA. In addition, the receiver operating characteristic curve showed that the LUS had moderate accuracy for predicting the possibility of hemodynamic changes. The area under the curve was 0.741 (95% confidence interval: 0.621-0.839), and sensitivity and specificity were 93.75% and 50.94%, respectively.
We conclude that bedside cardiopulmonary ultrasonography can evaluate lung water content and predict the possibility of hemodynamic changes in very low-weight preterm neonates with PDA.
The LUS is subjective and operator dependent. A more objective and appropriate evaluation method should be identified. In addition, neonatal lungs are complex, different diseases can have the same ultrasound images, the LUS alone cannot reflect its severity, and comprehensive evaluation is needed.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Stavroulopoulos A S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
| 1. | Sekar KC. Protective strategies to prevent patent ductus arteriosus. Chin Med J (Engl). 2010;123:2914-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zhang L, Shen W, Chen C, Pan JH. Clinical analysis of risk factors and complications of HSPDA in premature infants. Zhonghua Quanke Yixue Zazhi. 2019;8:1299-1301. |
| 3. | Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. 2000;137:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Saldeño YP, Favareto V, Mirpuri J. Prolonged persistent patent ductus arteriosus: potential perdurable anomalies in premature infants. J Perinatol. 2012;32:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Kurepa D, Zaghloul N, Watkins L, Liu J. Neonatal lung ultrasound exam guidelines. J Perinatol. 2018;38:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Lichtenstein DA. Current Misconceptions in Lung Ultrasound: A Short Guide for Experts. Chest. 2019;156:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Corradi F, Via G, Forfori F, Brusasco C, Tavazzi G. Lung ultrasound and B-lines quantification inaccuracy: B sure to have the right solution. Intensive Care Med. 2020;46:1081-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Clyman RI. Patent ductus arteriosus, its treatments, and the risks of pulmonary morbidity. Semin Perinatol. 2018;42:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Letshwiti JB, Semberova J, Pichova K, Dempsey EM, Franklin OM, Miletin J. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum Dev. 2017;104:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Mohlkert LA, Hallberg J, Broberg O, Rydberg A, Halvorsen CP, Liuba P, Fellman V, Domellöf M, Sjöberg G, Norman M. The Preterm Heart in Childhood: Left Ventricular Structure, Geometry, and Function Assessed by Echocardiography in 6-Year-Old Survivors of Periviable Births. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | El-Khuffash A, James AT, Corcoran JD, Dicker P, Franklin O, Elsayed YN, Ting JY, Sehgal A, Malikiwi A, Harabor A, Soraisham AS, McNamara PJ. A Patent Ductus Arteriosus Severity Score Predicts Chronic Lung Disease or Death before Discharge. J Pediatr 2015; 167: 1354-1361. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated With Continuous Positive Airway Pressure. JAMA Pediatr. 2015;169:e151797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 13. | Liu J, Cao HY, Fu W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J Int Med Res. 2016;44:1534-1542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Liu J, Feng X, Hu CB, Qiu RX. Guidelines for ultrasonic diagnosis of lung diseases in neonates. Zhonghua Shiyong Erke Linchuang Zazhi. 2019;33:1057-1064. |
| 15. | Liu J, Copetti R, Sorantin E, Lovrenski J, Rodriguez-Fanjul J, Kurepa D, Feng X, Cattaross L, Zhang H, Hwang M, Yeh TF, Lipener Y, Lodha A, Wang JQ, Cao HY, Hu CB, Lyu GR, Qiu XR, Jia LQ, Wang XM, Ren XL, Guo JY, Gao YQ, Li JJ, Liu Y, Fu W, Wang Y, Lu ZL, Wang HW, Shang LL. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J Vis Exp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 16. | Jones BP, Tay ET, Elikashvili I, Sanders JE, Paul AZ, Nelson BP, Spina LA, Tsung JW. Feasibility and Safety of Substituting Lung Ultrasonography for Chest Radiography When Diagnosing Pneumonia in Children: A Randomized Controlled Trial. Chest. 2016;150:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Chen SW, Fu W, Liu J, Wang Y. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine (Baltimore). 2017;96:e5826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | The Division of Perinatology, Society of Pediatric, Chinese Medical Association, The Division of Neonatal Ultrasound Society, the Chinese Neonatologist Association, Chinese Medical Doctor Association, The Division of Critical Ultrasound Society of Ultrasonics, China Medicine Education Association, Chinese College of Critical Ultrasound, Editorial Board of Chinese Journal of Applied Clinical Pediatrics. Guideline on lung ultrasound to diagnose pulmonary diseases in newborn infants. Zhonghua Shiyong Erke Linchuang Zazhi. 2018;33:1057-1064. |
| 19. | Gravel CA, Bachur RG. Point-of-Care Ultrasound Differentiation of Lung Consolidation and Normal Thymus in Pediatric Patients: An Educational Case Series. J Emerg Med. 2018;55:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Sharma D, Farahbakhsh N. Role of chest ultrasound in neonatal lung disease: a review of current evidences. J Matern Fetal Neonatal Med. 2019;32:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Alonso-Ojembarrena A, Lubián-López SP. Lung ultrasound score as early predictor of bronchopulmonary dysplasia in very low birth weight infants. Pediatr Pulmonol. 2019;54:1404-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Pang H, Zhang B, Shi J, Zang J, Qiu L. Diagnostic value of lung ultrasound in evaluating the severity of neonatal respiratory distress syndrome. Eur J Radiol. 2019;116:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Corradi F, Ball L, Brusasco C, Riccio AM, Baroffio M, Bovio G, Pelosi P, Brusasco V. Assessment of extravascular lung water by quantitative ultrasound and CT in isolated bovine lung. Respir Physiol Neurobiol. 2013;187:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ; Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40:2064-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 26. | Liu J. The Lung Ultrasound Score Cannot Accurately Evaluate the Severity of Neonatal Lung Disease. J Ultrasound Med. 2020;39:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Taveira M, Yousef N, Miatello J, Roy C, Claude C, Boutillier B, Dubois C, Pierre AF, Tissières P, Durand P. [Can a simple lung ultrasound score predict length of ventilation for infants with severe acute viral bronchiolitis? Arch Pediatr. 2018;25:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 29. | Hu Q, Ren WD, Mao J, Li J, Qiao W, Bi WJ, Xiao YJ, Zhan Y, Xu M, Liu CX, Sun L, Tang L, Zhang J. Changes in pulmonary artery pressure during early transitional circulation in healthy full-term newborns. Ultrasonics. 2015;56:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |