Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1793
Peer-review started: September 19, 2020
First decision: December 13, 2020
Revised: December 21, 2020
Accepted: December 27, 2020
Article in press: December 27, 2020
Published online: March 16, 2021
Processing time: 167 Days and 6.7 Hours
Post-hepatectomy liver failure (PHLF) is a serious complication and a leading cause of death after hepatectomy, an accurate prediction of PHLF is important for improvement of prognosis after hepatectomy.
To retrospectively analyze the risk factors for postoperative liver failure in patients undergoing hepatectomy for liver tumors.
The clinical data of 80 patients undergoing hepatectomy in our hospital from June 2018 to January 2020 were collected. With laboratory examination as well as pre- and post-operative abdominal three-dimensional reconstructive computed tomography, the demographic data, surgical data, biochemical indicators, coagulation index, routine blood tests, spleen and liver volumes, relative remnant liver volume, and other related indicators were obtained and compared between patients with PHLF and those without PHLF.
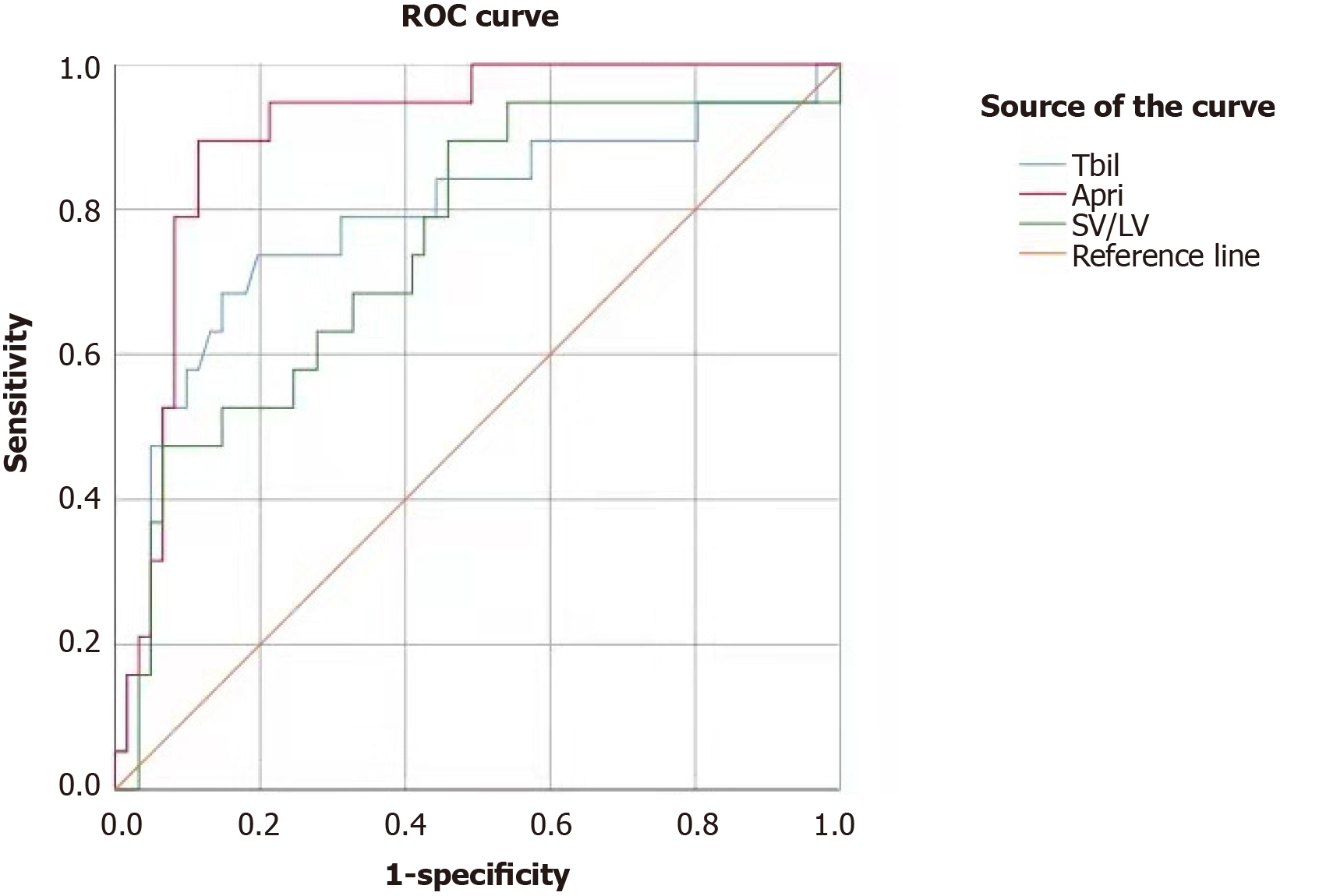
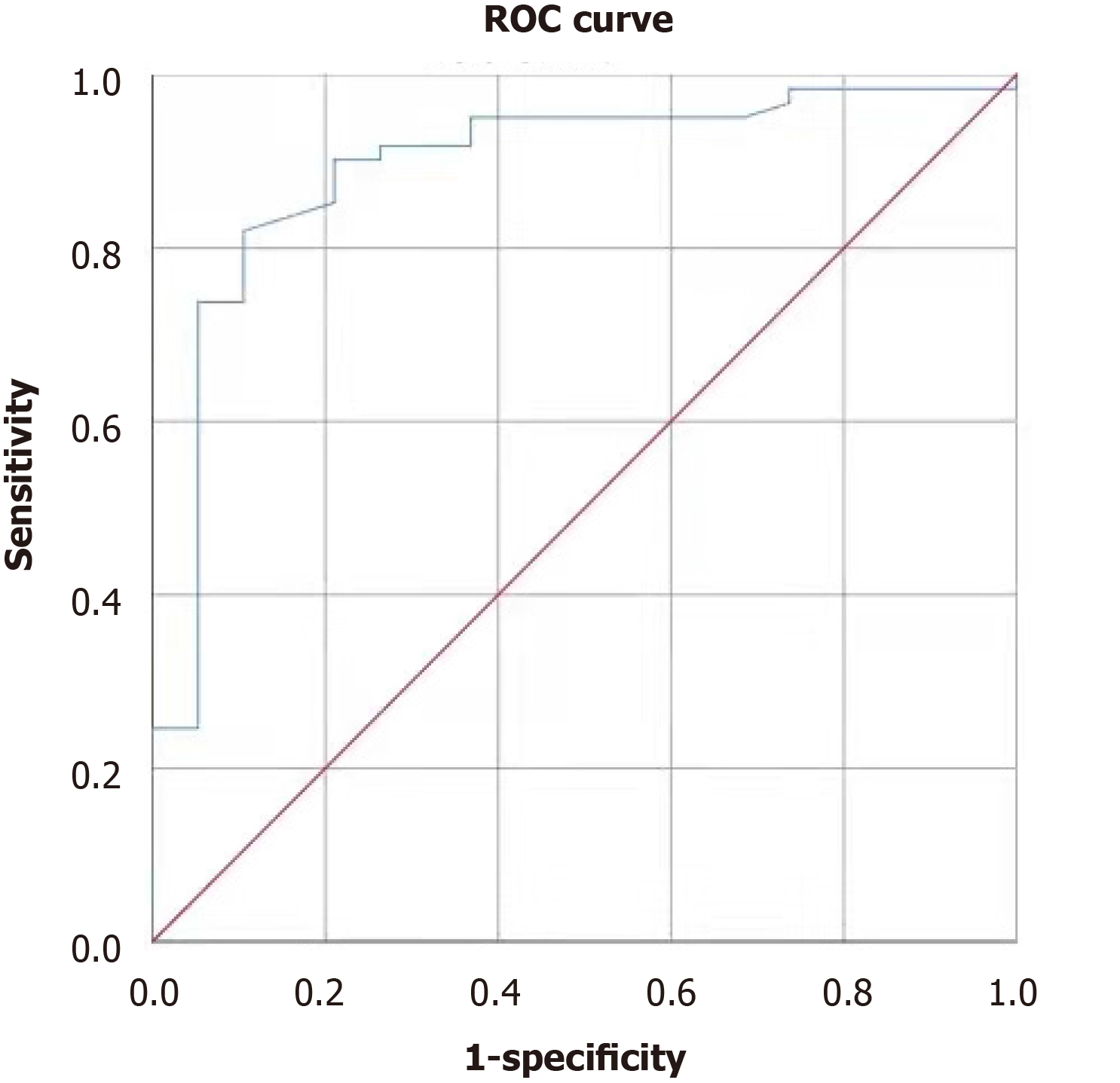
PHLF occurred in 19 (23.75%) patients. Univariate logistic regression analysis showed that gender, history of hepatitis/cirrhosis, and preoperative bilirubin, albumin, coagulation function, albumin-bilirubin ratio, aspartate amino-transferase-to-platelet ratio index (APRI), Model for End-Stage Liver Disease score, spleen volume (SV), spleen volume/liver volume ratio (SV/LV), and relative remnant liver volume were statistically associated with the occurrence of PHLF (all P < 0.05). Multivariate regression analysis showed that preoperative total bilirubin, platelets (PLT), APRI, and SV/LV were independent risk factors for PHLF (all P < 0.05). The area under the curve and cut-off values were 0.787 and 18.6 mmol/L for total bilirubin, 0.893 and 146 × 1012/L for PLT, 0.907 and 0.416 for APRI, and 0.752 and 20.84% for SV/LV, respectively.
For patients undergoing liver resection, preoperative total bilirubin, PLT, APRI, and SV/LV are independent risk factors for PHLF. These findings may provide guidance to safely perform liver surgery in such patients.
Core Tip: The etiology of post-hepatectomy liver failure (PHLF) is unclear. The volume of liver resection and the liver functional reserve are mainly used to evaluate the risk of PHLF. In this study we explored the risk factors for PHLF in patients with liver tumors. We found that preoperative total bilirubin, platelets, aspartate amino-transferase-to-platelet ratio index, and spleen volume/liver volume ratio are independent risk factors for PHLF. Both biochemical results and spleen volume on imaging should be considered before establishing a surgical plan to minimize the risk of PHLF. These findings may provide guidance to safely perform liver surgery in such patients.
- Citation: Xing Y, Liu ZR, Yu W, Zhang HY, Song MM. Risk factors for post-hepatectomy liver failure in 80 patients. World J Clin Cases 2021; 9(8): 1793-1802
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1793.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1793
Cancer is the leading cause of death worldwide in the 21st century, and its incidence is increasing annually. Liver cancer is the 7th most common malignancy worldwide, there were 841080 new cases and 781685 deaths in 2018[1]. Nearly one-half of new liver cancer cases occur in China, and approximately 70% of patients are complicated with hepatitis B[2].
So far, surgery remains the mainstay treatment for patients with liver cancer. In recent years, the prognosis of liver cancer patients has been markedly improved[3]. The surgical approaches vary with tumor location and involvement. For patients requiring extensive liver resection, post-hepatectomy liver failure (PHLF) is the leading cause of postoperative death[4]. The incidence of PHLF has ranged from 1.2% to 32%[5-8]. However, the perioperative mortality rate due to PHLF has been as high as 40%-60% in the past 15 years[9-11]. As the etiology of PHLF is still unclear, there is no recognized method for predicting the occurrence of PHLF before surgery. The risk of PHLF is mainly evaluated in terms of the volume of liver resection and the liver functional reserve. Recent studies have shown that spleen stiffness, spleen and liver volume[12] and biochemical indicators such as albumin-bilirubin (ALBI) ratio and aspartate aminotransferase-to-platelet ratio index (APRI)[13] have certain values for predicting PHLF. However, no uniform standard for PHLF prediction is available. In this study we examined the risk factors for PHLF in a Chinese population with liver cancer.
This study retrospectively analyzed patients who underwent partial hepatectomy for liver tumors in our hospital from June 2018 to January 2020. All patients received partial liver resection alone. The exclusion criteria were: (1) Patients who had received liver radiofrequency ablation and/or transarterial chemoembolization before surgery; (2) Patients with severe liver and kidney dysfunction (Child-Pugh class B or higher) before surgery; and/or (3) Patients who had developed distant metastasis before surgery.
All patients underwent open or laparoscopic precision liver resection. According to the different surgical approaches, hepatic portal occlusion was performed (or not) using the Pringle maneuver. The occlusion and reperfusion time was 15 min and 5 min, respectively. After the operation, a drainage tube was placed in the abdominal cavity, and the operating time, intraoperative blood loss, and hepatic hilum occlusion (or not) were recorded. All patients underwent abdominal thin-slice computed tomography (CT) or magnetic resonance imaging (MRI) examination within 1 mo before surgery and within 1 wk after surgery.
PHLF is defined as an increased international normalized ratio (INR) and concomitant hyperbilirubinemia on or after postoperative day 5[5]. The demographic data, past histories, as well as biochemical indicators, routine blood tests, and coagulation index within one week before surgery and on postoperative day 5 were recorded, and the Model for End-Stage Liver Disease (MELD) score, ALBI, APRI, and other indicators were calculated. After the pre- and post-operative CT or MRI images were converted into DICOM format, the IQQA-Liver three-dimensional simulation system was used to calculate the spleen volume (SV), remnant liver volume (RLV), standard remnant liver volume ratio, and volume ratio of liver to spleen (LV/SV ratio). The formulas and definitions involved are as follows: MELD: 3.8 × ln [bilirubin (mg/dL)] + 11.2 × ln (INR) + 9.6 × ln [creatinine (mg/dL)] + 6.4 × ln (etiology, cholestatic/alcoholic 0, others 1); ALBI: 0.66 × log10 [bilirubin (μmol/L) – 0.085 × albumin (g/L)]; APRI: Aspartate aminotransferase/upper limit of normal/platelet count (expressed as platelets × 109/L) × 100; %RLV-: remnant liver volume/total liver volume; RLV-BWR: Remnant liver volume to donor body weight ratio; SRLVR: RLV/SLV (standard liver volume); SLV: 706.2 × body surface area + 2.4; Body surface area: 0.0061 × body height (cm) + 0.0128 × body weight (kg) - 0.1529.
Normally distributed quantitative data are presented as mean ± SD, and P values were calculated using independent samples t test; non-normally distributed quantitative data are presented as median, and P values were calculated using Mann-Whitney U test. Qualitative data are presented as n (%) and compared using the Chi-square test or Fisher's exact test. Univariate and multivariate logistic regression analyses (variables with a P value < 0.05 were included in the multivariate analysis, and forward logistic regression was applied) were performed, and the cut-off values were predicted using the receiver operating characteristic (ROC) curves. Two-sided tests were used for all analyses and a P value less than 0.05 was considered statistically significant. The SPSS software package (version 22.0) was used.
The surgery was uneventful in all patients, and no perioperative deaths were recorded. There were 2 cases of biliary fistula and abdominal hemorrhage, which improved after conservative treatment, and 5 cases of biliary fistula, which improved with retained drainage. No re-operations were required.
PHLF occurred in 19 (23.75%) of 80 patients and improved after symptomatic medical treatment. No patient suffered liver failure. The comparisons between the PHLF group and the non-PHLF group are shown in Table 1.
| PHLF group (n = 19) | Non-PHLF group (n = 61) | |
| Gender | ||
| Male | 16 (84.21%) | 22 (36.07%) |
| Female | 3 (15.79%) | 39 (63.93%) |
| Age (yr) | 60 (50,68) | 57 (51,62) |
| History of hepatitis | 9 (47.37%) | 7 (11.48%) |
| History of diabetes | 4 (21.05%) | 5 (8.2%) |
| Liver cirrhosis | 9 (47.37%) | 4 (6.56%) |
| Operating time (h) | 6.13-2.3 | 5.3-1.78 |
| Bleeding (mL) | 400 (200, 500) | 300 (200, 500) |
| Hepatic hilum occlusion | 9 (47.37%) | 27 (44.26%) |
| ALT | 28 (14.5, 40.1) | 17.2 (10.4, 26.4) |
| AST | 37.6 (25.2, 43.1) | 20.1 (15.4, 25.7) |
| TBIL | 21.4 (17.5, 36.2) | 13.9 (10.6, 18.2) |
| DBIL | 7 (5.8, 10.3) | 5.1 (3.34, 7.7) |
| Creatinine | 53 (41.4, 64.9) | 50.3 (43.8, 61.1) |
| eGFR | 120.66 (99.73, 129.65) | 113.59 (105.84, 120.44) |
| ALB | 35.67-6 | 39.45-3.66 |
| WBC | 4.8 (3.19, 6.73) | 6.14 (4.98, 7.35) |
| LY | 1.29 (0.86, 1.6) | 1.39 (1.12, 1.85) |
| PLT | 119.11-43.9 | 214.11-67.27 |
| PT | 11.5 (10.8, 13.3) | 11.1 (10.6, 11.9) |
| INR | 1.07 (1.03, 1.21) | 1.02 (0.98, 1.1) |
| MELD | 10 (8, 11) | 7 (7, 8) |
| ALBI | -2.1-0.62 | -2.59-0.33 |
| APRI | 0.78 (0.64, 1.04) | 0.24 (0.17, 0.4) |
| Total liver volume | 1385.26 (1239.85, 1487.93) | 1294.16 (1174.19, 1385.63) |
| Remnant liver volume | 854.12 (767.42, 1085.13) | 984.13 (874.59, 1095.52) |
| Spleen volume | 206.19 (176.83, 439.16) | 162.79 (153.86, 175.23) |
| SV/LV | 0.15 (0.13, 0.34) | 0.12 (0.11, 0.14) |
| %RLV | 0.65-0.14 | 0.76-0.08 |
| BMI | 23.11-2.4 | 23.6-2.48 |
| Body surface area | 1.73-0.14 | 1.68-0.12 |
| SLV | 1223.28-97.52 | 1188.79-86.27 |
| SRLVR | 0.7 (0.58, 0.85) | 0.83 (0.75, 0.94) |
Nineteen (23.75%) patients developed PHLF after surgery. Univariate logistic regression analysis showed that gender, history of hepatitis/cirrhosis, and preoperative bilirubin, albumin, coagulation function, ALBI, APRI, MELD score, SV, SV/LV, and RLV were significantly associated with the occurrence of PHLF (all P < 0.05). Multivariate regression analysis showed that preoperative total bilirubin, platelets (PLT), APRI, and SV/LV were independent risk factors for PHLF (all P < 0.05) (Table 2).
| Univariate analysis | Multivariate analysis | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Gender (reference = female) | 9.455 | (2.477-36.081) | 0.001 | |||
| Age | 1.047 | (0.987-1.111) | 0.13 | |||
| History of hepatitis | 6.943 | (2.099-22.963) | 0.001 | |||
| History of diabetes | 2.987 | (0.713-12.517) | 0.135 | |||
| Liver cirrhosis | 12.825 | (3.305-49.769) | < 0.001 | |||
| Operating time | 1.250 | (0.953-1.639) | 0.107 | |||
| Bleeding | 1.000 | (0.999-1.001) | 0.521 | |||
| Hepatic hilum occlusion | 1.133 | (0.404-3.183) | 0.812 | |||
| ALT | 1.007 | (0.997-1.017) | 0.188 | |||
| AST | 1.019 | (0.995-1.043) | 0.119 | |||
| TBIL | 1.031 | (1.005-1.058) | 0.018 | 1.177 | (1.017, 1.362) | 0.029 |
| DBIL | 1.026 | (1.001-1.051) | 0.038 | |||
| Creatinine | 1.006 | (0.992-1.021) | 0.398 | |||
| eGFR | 0.995 | (0.971-1.019) | 0.661 | |||
| ALB | 0.832 | (0.733-0.944) | 0.004 | |||
| WBC | 0.820 | (0.624-1.078) | 0.155 | |||
| LY | 0.927 | (0.425-2.018) | 0.848 | |||
| PLT | 0.968 | (0.952-0.983) | < 0.001 | 0.949 | (0.908, 0.992) | 0.021 |
| PT | 1.512 | (1.028-2.223) | 0.036 | |||
| INR | 97.870 | (1.336-7169.878) | 0.036 | |||
| MELD | 1.375 | (1.111-1.703) | 0.003 | |||
| ALBI | 11.662 | (2.96-45.95) | < 0.001 | |||
| APRI | 62.012 | (8.376-459.096) | < 0.001 | 2.954 | (1.021, 8.544) | 0.046 |
| Total liver volume | 1.001 | (1-1.002) | 0.205 | |||
| Remnant liver volume | 0.998 | (0.996-1.001) | 0.222 | |||
| Spleen volume | 1.006 | (1.002-1.009) | 0.005 | |||
| SV/LV | 123.308 | (2.076-7324.329) | 0.021 | < 0.001 | (0,0.043) | 0.036 |
| %RLV | 0.000 | (0-0.008) | < 0.001 | |||
| BMI | 0.922 | (0.749-1.136) | 0.446 | |||
| Body surface area | 25.376 | (0.325-1984.13) | 0.146 | |||
| SLV | 1.005 | (0.998-1.011) | 0.146 | |||
| SRLVR | 0.068 | (0.002-1.906) | 0.114 | |||
Analysis of the ROC curves showed that the area under the curve (AUC) for total bilirubin (TBIL) in predicting PHLF was 0.787 (95%CI: 0.653-0.920, P < 0.001); the cut-off value of 18.6 mmol/L was associated with a sensitivity of 0.737 and a specificity of 0.803 (Figure 1). The AUC for PLT in predicting PHLF was 0.893 (95%CI: 0.806-0.981, P < 0.001); the cut-off value of 146 × 1012/L was associated with a sensitivity of 0.902 and a specificity of 0.789 (Figure 2). The AUC for APRI in predicting PHLF was 0.907 (95%CI: 0.836-0.977, P < 0.001); the cut-off value of 0.416 was associated with a sensitivity of 0.947 and a specificity of 0.787 (Figure 1). The AUC for SV/LV in predicting PHLF was 0.752 (95%CI: 0.623-0.880, P < 0.001); the cut-off value of 20.84% was associated with a sensitivity of 0.474 and a specificity of 0.934 (Figure 1).
As a leading cause of death following major liver resection, PHLF was first defined by the International Study Group of Liver Surgery in 2011 as a “postoperative acquired deterioration” in the ability of the liver to maintain its synthetic, excretory and detoxifying functions, which are characterized by biochemical and clinical changes without other causes and an increased INR and concomitant hyperbilirubinemia on or after postoperative day 5[5]. The incidence of PHLF is reported to be 1.2%-32%[5-8]. In the present study, the incidence of PHLF was 23.75%, which was basically consistent with that reported in the literature. The risk factors for PHLF remain controversial, and mainly include liver reserve dysfunction and small postoperative RLV. In addition, intraoperative and postoperative management may also be associated with the development of PHLF[8]. The incidence of liver cancer is high in China, and most patients have hepatitis B and cirrhosis before they ultimately develop liver cancer; as a result, most patients have different degrees of cirrhosis when they undergo surgery for liver cancer, and thus the incidence of PHLF is particularly high due to poor liver reserve function. Therefore, accurate liver function evaluation before surgery is essential. Child-Pugh classification is a commonly used tool for assessing liver function, but its accuracy has been questioned in recent years[14]. ICG-15R can accurately assess hepatic function but has not been widely applied due to limited facilities in some hospitals.
In 1993, Makuuchi et al[15] proposed the use of bilirubin as one of the main parameters for evaluating liver cancer surgery. For patients with normal serum bilirubin, the safe limit of hepatectomy can be determined based on ICG-15R. In the present study, patients with elevated TBIL (higher than 18.6 mmol/L) before surgery were at significantly higher risk of PHLF (P < 0.001). Also, the upper limit of normal for TBIL was set at 19 mmol/L in our study, and a TBIL level of higher than 19 mmol/L was regarded as hepatic insufficiency; accordingly, the extent of surgical resection should be minimized, which is consistent with the standard proposed by Makuuchi et al[15].
Most patients with accompanying hepatitis and/or cirrhosis have thrombocyto-penia, which are significantly associated with the risk and outcome of hepatectomy. In a meta-analysis of 5260 patients[16], thrombocytopenia before surgery was considered an independent risk factor for PHLF. In our study, PLT was found to be an independent risk factor for PHLF, and its cut-off value was set at 146 × 109/L, which yielded a sensitivity of 0.902 and a specificity of 0.789. This cut-off value was in line with the lower limit (150 × 109/L) of normal PLT in our hospital, suggesting that low PLT is an independent risk factor for PHLF.
In addition to a single laboratory index, liver function-related indicators such as ALBI, APRI, and MELD score have increasingly been used to assess the risk of PHLF. MELD score was initially used for predicting the prognosis of patients after transjugular intrahepatic portosystem stent-shunt[17] and was later adopted for organ allocation in liver transplantation[18]. It is particularly useful in the prognostic evaluation of patients with end-stage liver disease. In recent years, it has been reported that MELD score might be an independent risk factor for PHLF[19]. In the present study, MELD score was significantly different between the PHLF group and non-PHLF group; however, MELD score was not an independent risk factor for PHLF in the multivariate analysis.
In 2015, Johnson for the first time proposed the concept of ALBI, which refers to the assessment of liver function in liver cancer patients using the albumin-bilirubin ratio[20]; however, its role in assessing the severity of liver cirrhosis has been controversial[13]. The reasons for this may be that as patients with severe hypoprotei-nemia are often considered unsuitable for surgery, ALBI has a low assessment value in patients undergoing liver surgery. Wai et al[21] in 2003[21], proposed that APRI was believed to be able to effectively assess the degree of liver cirrhosis, and its accuracy was similar to that of liver biopsy. Ichikawa et al[22] confirmed that APRI could be used as an effective independent predictor of PHLF[22]. Similarly, in our study, patients with a preoperative APRI > 0.416 had a significantly higher incidence of PHLF than those with a preoperative APRI ≤ 0.416; when APRI was 0.416, the sensitivity was 0.947 and the specificity was 0.787, indicating that APRI could effectively predict the occurrence of PHLF.
Furthermore, with the advances in three-dimensional visualization technology in surgery, it is now possible to calculate the LV and SV before surgery and to plan the resection range through virtual surgery. A comparison of the liver volume before and after surgery makes it possible to determine the impact of resection range on the occurrence of PHLF. Both RLV and SV have been proven to be effective in predicting the occurrence of PHLF[23]. Generally, a %RLV larger than 25% is safe for a normal liver; however, the %RLV should not exceed 40% in patients with liver cirrhosis[24]. Therefore, preoperative liver function should be considered when estimating the postoperative RLV. Fewer studies have investigated the role of SV. Cirrhotic patients often have hypersplenism, and SV can effectively reflect the status of liver cirrhosis and portal hypertension. The combination of SV and LV may be more effective in predicting the outcomes of liver resection.
According to Peng et al[12], the spleen-RLV ratio could effectively predict the occurrence of PHLF. In our study, the preoperative SV/LV ratio was again confirmed to be an independent risk factor for PHLF. The AUC was 0.752 and the cut-off value was 20.84%. The %RLV showed no significant difference between the PHLF group and non-PHLF group. This might be because the %RLV alone cannot reflect the liver reserve function and thus has a poor predictive value; in contrast, the combination of SV with %RLV reflects both the volume and function of the liver and thus can be more accurate in predicting prognosis.
In addition, studies have reported that the age and BMI of patients were also independent risk factors for PHLF[10,25]. However, in the present study, age and BMI did not significantly affect the occurrence of PHLF, which may be due to the narrow ranges of various indicators in our study.
In this retrospective analysis of 80 patients undergoing liver resection, serum total bilirubin, PLT, APRI, and SV/LV were independent risk factors for PHLF. In particular, serum bilirubin and PLT higher or lower than normal may indicate the possibility of PHLF occurrence. These findings were consistent with previous reports, which suggest that both biochemical results and SV on imaging should be considered before establishing a surgical plan, so as to minimize the risk of PHLF. Our study was limited by its small sample size. When a larger sample is obtained, we will further validate our findings by establishing prediction models.
Post-hepatectomy liver failure (PHLF) is the main cause of death after hepatectomy, which was first defined by the International Study Group of Liver Surgery in 2011. The incidence of PHLF ranges between 1.2%-32%.
Earlier studies showed that PHLF is related to many preoperative factors, the analysis of these factors can be helpful in the prevention of PHLF.
To analyze possible risk factors for PHLF in Chinese patients undergoing hepatectomy.
Eighty patients who underwent partial hepatectomy for liver tumors from June 2018 to January 2020 were enrolled, they were divided into two groups according to whether PHLF occurred. Laboratory examination, Model for End-Stage Liver Disease score, albumin-bilirubin ratio, aspartate aminotransferase-to-platelet ratio index (APRI), spleen volume (SV), remnant liver volume, standard remnant liver volume ratio, and volume ratio of liver to spleen were compared and discussed.
Of 80 patients, 19 (23.75%) developed PHLF. Gender, history of hepatitis/cirrhosis, and preoperative bilirubin, albumin, coagulation function, albumin-bilirubin ratio, APRI, Model for End-Stage Liver Disease score, SV, spleen volume/liver volume ratio (SV/LV), and % remnant liver volume were statistically associated with the occurrence of PHLF according to univariate logistic regression analysis (all P < 0.05). Preoperative total bilirubin (TBIL), platelets (PLT), APRI, and SV/LV were independent risk factors for PHLF in multivariate regression analysis (all P < 0.05). The area under curve and cut-off values were 0.787 and 18.6 mmol/L for TBIL, 0.893 and 146 × 1012/L for PLT, the two cut-off values are consistent with the upper and lower limit of TBIL and PLT in our hospital; furthermore, area under curve and cut-off values were 0.907 and 0.416 for APRI, and 0.752 and 20.84% for SV/LV, respectively.
Elevated preoperative total bilirubin, decreased PLT and APRI higher than 0.416, SV/LV higher than 20.85% are independent risk factors for PHLF in patients undergoing liver resection.
The etiology of PHLF is unclear, and there is no standard method for predicting the occurrence of PHLF before surgery. More patients should be analyzed to obtain more precise data on the prediction of PHLF.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabozzi M S-Editor: Zhang L L-Editor: Webster JR P-Editor: Xing YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55843] [Article Influence: 7977.6] [Reference Citation Analysis (132)] |
| 2. | Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 3. | Farges O, Goutte N, Bendersky N, Falissard B; ACHBT-French Hepatectomy Study Group. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697-704; discussion 704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | van Mierlo KM, Schaap FG, Dejong CH, Olde Damink SW. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J Hepatol. 2016;65:1217-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1729] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 6. | Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, Belghiti J. Prospective validation of the "fifty-fifty" criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-62; discussion 862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 10. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 11. | Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Büchler MW, Weitz J. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol. 2011;18:3640-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Peng W, Zhang XY, Li C, Wen TF, Yan LN, Yang JY. Spleen stiffness and volume help to predict posthepatectomy liver failure in patients with hepatocellular carcinoma. Medicine (Baltimore). 2019;98:e15458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Mai RY, Wang YY, Bai T, Chen J, Xiang BD, Wu GB, Wu FX, Li LQ, Ye JZ. Combination Of ALBI And APRI To Predict Post-Hepatectomy Liver Failure After Liver Resection For HBV-Related HCC Patients. Cancer Manag Res. 2019;11:8799-8806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Surveillance group. ; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2018;117:381-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 597] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | Mehrabi A, Golriz M, Khajeh E, Ghamarnejad O, Probst P, Fonouni H, Mohammadi S, Weiss KH, Büchler MW. Meta-analysis of the prognostic role of perioperative platelet count in posthepatectomy liver failure and mortality. Br J Surg. 2018;105:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 18. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 19. | Kong FH, Miao XY, Zou H, Xiong L, Wen Y, Chen B, Liu X, Zhou JJ. End-stage liver disease score and future liver remnant volume predict post-hepatectomy liver failure in hepatocellular carcinoma. World J Clin Cases. 2019;7:3734-3741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2013] [Article Influence: 201.3] [Reference Citation Analysis (0)] |
| 21. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 22. | Ichikawa T, Uenishi T, Takemura S, Oba K, Ogawa M, Kodai S, Shinkawa H, Tanaka H, Yamamoto T, Tanaka S, Yamamoto S, Hai S, Shuto T, Hirohashi K, Kubo S. A simple, noninvasively determined index predicting hepatic failure following liver resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Gruttadauria S, Pagano D, Liotta R, Tropea A, Tuzzolino F, Marrone G, Mamone G, Marsh JW, Miraglia R, Luca A, Vizzini G, Gridelli BG. Liver Volume Restoration and Hepatic Microarchitecture in Small-for-Size Syndrome. Ann Transplant. 2015;20:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |