Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1318
Peer-review started: September 14, 2020
First decision: November 23, 2020
Revised: December 4, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 26, 2021
Processing time: 144 Days and 22.6 Hours
During surgery for gastric cancer, peritoneal lavage using warm distilled water can cause temporary hemodynamic changes.
To examine the associations between changes in heart rate and single nucleotide polymorphisms (SNPs).
This was a prospective observational study of patients with gastric cancer who underwent gastrectomy and peritoneal hypotonic lavage at the Third Affiliated Hospital of Soochow University from March 2018 to March 2019. Related SNPs were selected, and the verified exons were analyzed. Heart rate and blood pressure (BP) were measured before and after lavage. The patients were grouped as heart rate change ≥ 30% vs < 30%. Comparison and regression analyses of the selected SNPs were performed between the two groups.
According to the inclusion/exclusion criteria, 194 patients were included in the analysis. Of these patients, 138 were male, with a mean age of 65.9 ± 0.8 years, and 56 were female, with a mean age of 65.0 ± 1.3 years. Heart rate dropped by 0%-10% in 65 participants, by 10%-15% in 29, by 15%-20% in 23, by 20%-50% in 39, by 50%-100% in four, six had a cardiac arrest, and 28 had an increase in heart rate. Considering the possible impact of exonic SNPs on the phenotypes, TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) were analyzed. The haplotype analysis suggested that the haplotypes CTT [odds ratio (OR) = 2.018, 95% confidence interval (CI): 1.012-4.025, P = 0.0430] and GCC (OR = 2.293, 95%CI: 1.174-4.477, P = 0.0131) of TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) increased the risk of a drop in heart rate > 30%.
The TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) SNPs were associated with changes in heart rate ≥ 30% during intraperitoneal lavage using distilled water after gastrectomy for gastric cancer.
Core Tip: Haplotype analysis suggested that the haplotypes CTT and GCC of TEP1, TEP1, and RECQL5 increased the risk of a drop in heart rate > 30%. The TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) single nucleotide polymorphisms were associated with changes in heart rate ≥ 30% during intraperitoneal lavage using distilled water after gastrectomy for gastric cancer.
- Citation: Yuan Y, Yao S, Luo GH, Zhang XY. Impact of metabolism-related mutations on the heart rate of gastric cancer patients after peritoneal lavage. World J Clin Cases 2021; 9(6): 1318-1328
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1318.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1318
Gastric adenocarcinomas are tumors of the stomach, including tumors of the noncardia and the subcardia (Siewert type III), with the center starting 2-5 cm below the esophagogastric junction (EGJ)[1,2]. The estimated global incidence of gastric cancer was 1033701 in 2018, with a mortality of 782685[3]. The incidence of gastric cancer is highest in Eastern Asia, Eastern Europe, and South America[3-5]. Men are twice more affected than women[2]. The direct cause of gastric cancer is not clear, but Helicobacter pylori infection and some hereditary cancer predisposition syndromes may play a role[1,2,6,7]. Patients often present with nonspecific symptoms, which may include anorexia, weight loss, abdominal pain, dyspepsia, vomiting, and early satiety[2,7]. The 5-year survival of gastric cancer is 31%; patients with localized disease will have a 5-year survival of 67%, while those with distant disease will have a 5-year survival of 5%[8].
The management of gastric cancer is multidisciplinary and includes surgery, chemotherapy, targeted therapy, and radiation therapy[1]. Gastric cancer spreads regionally to peritoneal lymph nodes, and the Japanese Gastric Cancer Association includes the cytology results of the peritoneal lavage fluid in the staging of gastric cancer[9,10], guiding treatments and chemotherapy[11,12].
Beyond its role for staging, peritoneal lavage can also be used in curative intent. Indeed, peritoneal metastasis is a major cause of relapse after gastrectomy in patients with gastric cancer[1]. Tumor cells can easily disseminate into the abdominal cavity due to the anatomical structures and then serve as seeds for metastases and relapse. Damage to the peritoneum and the exposure of connective tissue during the operation favor tumor cell growth by creating an inflammatory microenvironment[13,14]. Therefore, it is difficult to prevent tumor cells from spreading into the blood and the abdominal cavity[15]. The detection rate of tumor cells after surgery at the operation site or inside the peritoneum can be as high as 93.4%[16]. To address this issue, various methods for peritoneal lavage have been tried to destroy these tumor cells, including high temperature, hypotonic solution, and chemotherapy[17,18]. The value of intraperitoneal chemotherapy is still being debated[19,20]. High-temperature lavage shows some efficacy[21], as well as extensive lavage[18]. Hypotonic lavage emerged in the 1960s. Distilled water with an osmolarity close to 0 is used; since the osmolarity of human tissues is around 280-310 mmol/L, the difference in osmolarity causes the tumor cell to expand and eventually degenerate[22,23].
Nevertheless, observations during the procedure revealed that peritoneal lavage using warm distilled water can cause temporary changes in the cardiovascular system of the patients, such as lowering heart rate and altering blood pressure. In this study, we examined the changes in heart rate in patients with gastric cancer who underwent proximal or total gastrectomy before and after peritoneal lavage using distilled water, and we explored the associations between changes in heart rate and single nucleotide polymorphisms (SNPs) of genes that are involved in metabolism. The results could help identify patients who might be at risk of hemodynamic changes during hypotonic intraperitoneal lavage.
This was a prospective observational study of patients with gastric cancer who underwent gastrectomy at the Third Affiliated Hospital of Soochow University from March 2018 to March 2019. The study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University. All participants signed an informed consent form.
The inclusion criteria were as follows: (1) 30-90 years of age; (2) Gastric cancer stage I-III; and (3) Scheduled to undergo proximal or total gastrectomy. The exclusion criteria were as follows: (1) Scheduled to undergo distal gastrectomy; (2) Arrhythmia or other underlying conditions that may affect the heart rate; or (3) Incomplete data.
Peripheral blood (2 mL) was obtained from the ulnar vein before the operation for subsequent DNA extraction. The surgery was performed by two teams. Both teams had professional experience of over 10 years. Distilled water was used for peritoneal lavage during the operation[24]. After gastrectomy and abdominal lymphadenectomy, warm distilled water at 42°C was used for peritoneal lavage at the xiphoid process within 10 cm from the chest wall. Lavage duration was 3-5 s.
The heart rate and blood pressure were recorded before lavage and around 40-60 s after lavage. The change in heart rate = (the ultimate value of heart rate after lavage - heart rate before lavage)/heart rate before lavage × 100%.
Peripheral blood (2 mL) was obtained from each patient. All the samples were preserved at -80°C until use. Genomic DNA was extracted using the TIANamp blood DNA kit (Tiangen Biotech, Beijing, China) according to the manufacturer's instructions. The concentration and purity of the DNA sample met the polymerase chain reaction (PCR) requirements. The DNA concentration was > 50 ng/μL, and A260/A280 was 1.8-2.0. Primer Premier 5.0 software (Premier Biosoft, Palo Alto, CA, United States) was used to design the primers according to the sequence in NCBI (Table 1). The primers were synthesized and purified by Sangon (Shanghai, China). Genotyping for the gene polymorphisms was examined by real-time PCR on a Light Cycler480® II system (Roche Diagnostics, Basel, Switzerland). The real-time PCR reaction for each gene was performed in a 25-μL volume, containing 0.1 μL of 100 μmol/L each primer, 2 μL of DNA, 2.5 μL of 10 × buffer, 2.5 μL of MgCl2 (25 mmol/L), 0.25 μL of dNTP (10 mmol/L), and 0.5 μL of Taq DNA polymerase. The thermal cycling conditions included the following steps: initial denaturation at 95°C for 3 min, followed by 40 cycles at 95°C for 5 s and 60°C for 15 s.
| Mutation type | Chr | Start | End | Ref | Alt | Location | Gene name | FOC | Primers for sequencing (5’-3’) | |
| Forward | Reverse | |||||||||
| SNP | ||||||||||
| 1 | chr14 | 20757383 | 20757383 | A | G | UTR3 | TTC5 | 0/6 | CCAAAGAAGAGGAAGGACCTAAAG | CTGTCTCTTCCACTGCTCTCTATGTT |
| 2 | chr14 | 20818983 | 20818983 | C | T | intronic | PARP2-920 | 0/6 | TTTATGAGTTGGCATTCAGAAAGTC | GAAAGGTGAAAGGATTTAGGGAAC |
| 3 | chr14 | 20824859 | 20824859 | T | C | intronic | PARP2-156 | 0/6 | ATAAGAGGGAGTCAGAGGAAGGTGT | AGCACTGAGAACATTACTGTATCCCT |
| 4 | chr14 | 20837701 | 20837701 | G | C | exonic | TEP1-886 | 0/6 | GTGTCTTTAGATGTGGTGTTGGCT | TGGCAGAGTCCTCATTTTTGTGT |
| 5 | chr14 | 20841162 | 20841162 | C | T | intronic | TEP1-0948 | 0/6 | CCTCTAACGCCCAGTAAATACACAG | TCTCTTATCCCAGCAGTATCCCAG |
| 6 | chr14 | 20841313 | 20841313 | G | A | intronic | TEP1-0948 | 0/6 | ||
| 7 | chr14 | 20841707 | 20841707 | C | T | exonic | TEP1-449 | 0/6 | TATTCCGCATACCTTCCCCAC | CCCTGCTCCGTCCCTTCTT |
| 8 | chr16 | 1756178 | 1756178 | T | C | upstream | MAPK8IP3 | 0/6 | GGAAGCGACCACTGCCG | CATCTGGATCTCCATCATCGC |
| 9 | chr17 | 8161149 | 8161149 | C | T | exonic | PFAS | 0/6 | GAAAGCCTGTGTAACTCTGATTGC | ATTACTGGCTTCAATGGCAACC |
| 10 | chr17 | 73627539 | 73627539 | T | C | exonic | RECQL5 | 0/6 | GCAATGTCCCTGATGTCCTACC | CTGGAGCAAGACCTGCATCG |
| 11 | chr21 | 35470291 | 35470291 | - | T | UTR3 | SLC5A3 | 0/6 | CGAATGTGCTTGTGTGATACTTGTT | GGCAGACTTGTTGGTCATTTCAG |
| 12 | chr9 | 98691137 | 98691137 | T | C | exonic | ERCC6L2 | 1/6 | CTTGATTACCGACGACTTGATGG | ATGTTGATAAAGGAAATGAGAGAAAAGC |
| Indel | ||||||||||
| 13 | chr17 | 8052526 | 8052532 | AAAAACA | - | intronic | PER1 | 0/6 | TGAAACCCCGTCTCTACTGAAAATAC | TCCCTTAGTGGATTCTTCGCAG |
| 14 | chr12 | 133417982 | 133417982 | - | AA | UTR3 | CHFR | 1/6 | GAAAGGCTTCGTCTCTGCTGC | TGGAGGTGAAGAGAGCGTGTTT |
The PCR products were purified from 1.5% agarose gel using the SanPrep column DNA Agarose Gel Extraction Kit (Sangon Biotech Co., Ltd., Shanghai, China) and were then directly sequenced using PCR primers as sequencing primers by Sangon Biotech Company. For sequence comparison and identification, the chromatogram sequences were inspected using Chromas 2.2 (Technelysium, Queensland, Australia) and searched against the GenBank by the BLAST program provided by the NCBI. Whole exon sequencing (WES) was performed by Shanghai GMINIX Information Technology Co., Ltd., China, according to their standard protocols.
We first screened the genetic mutations (SNP, insertion, or deletion) that occurred in all patients with heart rate changes of over 50% compared with those with changes < 10%, and then performed validation in all the patients included in the study.
We used a 30% change in heart rate as a cutoff, based on clinical experience, to assign the patients into two groups: Patients with changes in heart rate < 30% were considered the control group, while those with changes in heart rate ≥ 30% were considered the observation group. Comparison and regression analyses of the selected SNPs were performed between the two groups.
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, United States). A normality test was performed for all continuous variables using the Kolmogorov-Smirnov test. For the variables that followed normal distribution, the data were displayed as mean ± SD and analyzed using the independent-sample t-test. For those that did not follow normal distribution, the data were displayed as medians (ranges) and analyzed by the Mann-Whitney U-test. The categorical variables were displayed as frequencies and percentages and analyzed by the chi-square or Fisher’s exact test. The Hardy-Weinberg equilibrium test was performed for the SNPs. Logistic regression was performed using a 30% change in the heart rate as the outcome indicator to identify the associated haplotype. Two-sided (except for the chi-square test) P values < 0.05 were considered statistically significant.
According to the inclusion/exclusion criteria, 201 patients were included in the study. Seven patients were subsequently excluded due to incomplete data. Finally, 194 patients were included in the analysis (Table 2). Of these patients, 138 were male, with a mean age of 65.9 ± 0.8 years, and 56 were female, with a mean age of 65.0 ± 1.3 years. The patients’ heart rate changed during peritoneal lavage using warm distilled water. The heart rate dropped by 0%-10% in 65 patients, by 10%-15% in 29 patients, by 15%-20% in 23 patients, by 20%-50% in 39 patients, by 50%-100% in four patients, six patients had a cardiac arrest, and 28 patients had an increase in their heart rate.
| Characteristics | HR decreased < 30% (n = 172) | HR deceased ≥ 30% (n = 22) | P value |
| Age (years), mean ± SD | 65.6 ± 9.1 | 65.8 ± 10.4 | 0.62 |
| Sex, n (%) | |||
| Male | 72.7 | 59.1 | 0.2136 |
| Female | 27.3 | 40.9 | |
| Smoking, n (%) | 14.0 | 4.6 | 0.3190 |
| Drinking, n (%) | 7.0 | 0 | 0.3668 |
| Hypertension, n (%) | 34.3 | 40.9 | 0.6361 |
| Diabetes, n (%) | 8.1 | 13.6 | 0.4171 |
| Hemodynamics before lavage, mean ± SD | |||
| Heart rate (bpm) | 61 ± 10 | 62 ± 13 | 0.511 |
| Diastolic pressure (mmHg) | 66 ± 10 | 65 ± 12 | 0.6207 |
| Systolic pressure (mmHg) | 122 ± 18 | 119 ± 19 | 0.3159 |
| Hemodynamics after lavage, mean ± SD | |||
| Heart rate (bpm) | 56 ± 12 | 27 ± 20 | < 0.0001 |
| Diastolic pressure (mmHg) | 76 ± 14 | 53 ± 23 | < 0.0001 |
| Systolic pressure (mmHg) | 141 ± 24 | 92 ± 48 | < 0.0001 |
| Change in heart rate (%), mean ± SD | 8.3 ± 12.4 | 59.3 ± 27.7 | < 0.0001 |
| Change in diastolic pressure (mmHg), mean ± SD | 10.0 ± 13.1 | -11.8 ± 18.9 | < 0.0001 |
| Change in systolic pressure (mmHg), mean ± SD | 17.5 ± 21.8 | -26.0 ± 41.5 | < 0.0001 |
According to the changes in heart rate, we randomly selected six patients among those with heart rate change > 50%, and those with heart rate change of < 10%, respectively, for WES analysis. Compared to the reference genome, the former patients had 4986 SNPs and 258 insertions or deletions; the latter patients had 22294 SNPs and 1065 insertions/deletions. There were 16192 SNPs and 700 insertions/deletions shared among these 12 patients.
We selected 14 SNPs and insertions/deletions that occurred in all the patients with heart rate change > 50% but did not occur or only occurred once in the patients with heart rate change of < 10% for further validation in a larger population. These SNPs and insertions/deletions included the point mutation in the 3’-untranslated region (UTR) of TTC5 and the AA insertion in CHFR; the exonic point mutations of TEP1-886, TEP1-449, and RECQL5; the intronic mutations in PARAP2-920, PARAP2-156, TEP1-0948, MAPK8IP3, PFA, SLC5A3, ERCC6L2, and PER1; the point mutation in CHFR; and the deletion in PER1. Finally, we successfully validated the point mutations in one of TTC5, TEP1-886, TEP1-449, and two of TEP1-0948, and one of RECQL5, a total of six SNPs.
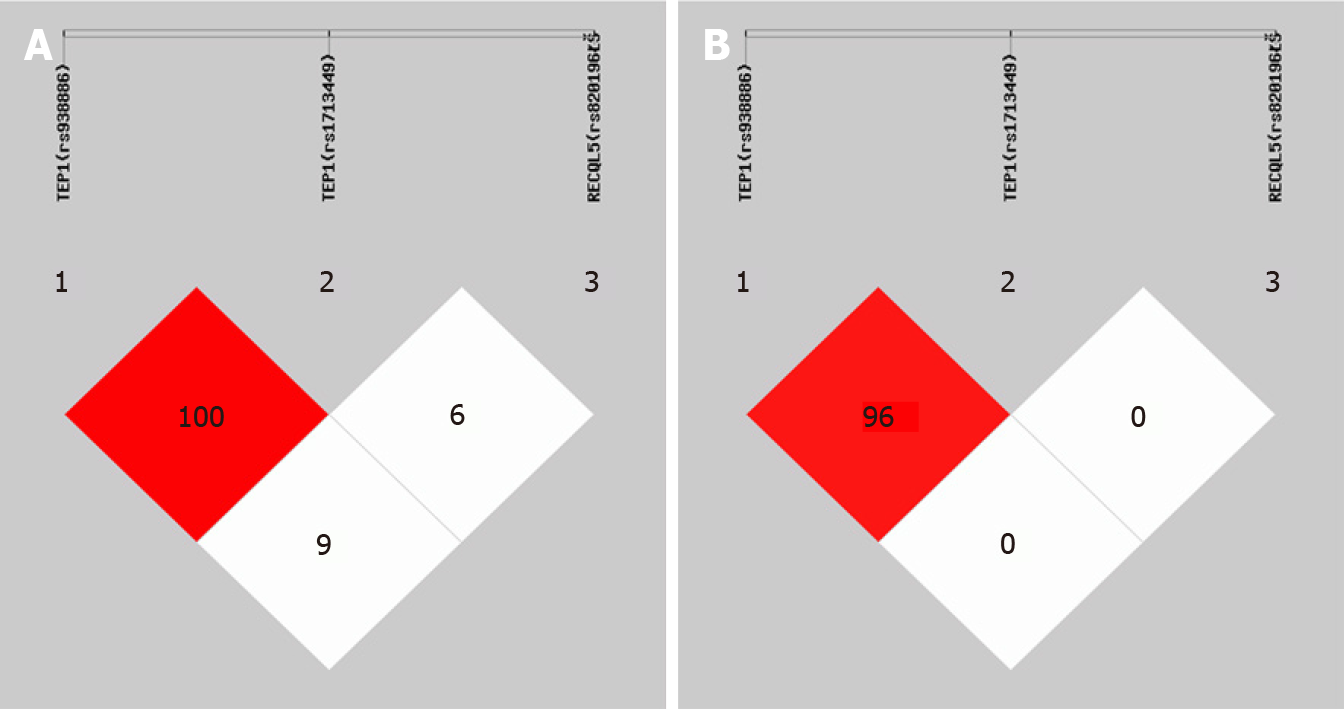
Considering the possible impact of exonic SNPs on the phenotypes, we decided to compare the following SNPs in all patients: TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196). There were no statistically significant differences in the allele frequency and genotype frequency between the two groups (Table 3). The linkage disequilibrium tests suggested a strong disequilibrium between TEP1 (rs938886) and TEP1 (rs1713449) (Figure 1). The haplotype analysis suggested that the haplotypes CTT [odds ratio (OR) = 2.018, 95% confidence interval (CI): 1.012-4.025, P = 0.0430) and GCC (OR = 2.293, 95%CI: 1.174-4.477, P = 0.0131) of TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) increased the risk of a drop in heart rate > 30% (Table 4).
| Characteristics | HR decreased ≥ 30% (n = 22) | HR decreased < 30% (n = 172) | Fisher's P value |
| TEP1 (rs938886), n (%) | |||
| C | 14 (0.32) | 105 (0.31) | 0.8608 |
| G | 30 (0.68) | 239 (0.70) | |
| CC | 1 (0.05) | 16 (0.09) | 0.5022 |
| CG | 12 (0.55) | 73 (0.42) | |
| GG | 9 (0.41) | 83 (0.48) | |
| HWE | 0.2278 | 0.9929 | |
| TEP1 (rs1713449), n (%) | |||
| C | 30 (0.68) | 236 (0.69) | 0.9546 |
| T | 14 (0.32) | 108 (0.31) | |
| CC | 9 (0.41) | 82 (0.48) | 0.4465 |
| CT | 12 (0.55) | 72 (0.42) | |
| TT | 1 (0.05) | 18 (0.11) | |
| HWE | 0.2278 | 0.7110 | |
| RECQL5 (rs820196), n (%) | |||
| C | 16 (0.36) | 109 (0.32) | 0.5319 |
| T | 28 (0.64) | 235 (0.68) | |
| CC | 1 (0.05) | 19 (0.11) | 0.1292 |
| CT | 14 (0.64) | 71 (0.41) | |
| TT | 7 (0.32) | 82 (0.48) | |
| HWE | 0.0787 | 0.5420 |
| Items, n (%) | HR decreased ≥ 30% (n = 22) | HR decreased < 30% (n = 172) | Fisher's P value | Odds ratio (95%CI) |
| CTC | 0.00 (0.00) | 40.98 (0.12) | 0.025601 | 0.000 (0.000-0.008) |
| CTT | 14.00 (0.32) | 64.02 (0.19) | 0.042935 | 2.018 (1.012-4.025) |
| GCC | 16.00 (0.36) | 68.02 (0.20) | 0.013161 | 2.293 (1.174-4.477) |
| GCT | 14.00 (0.32) | 167.98 (0.49) | 0.029281 | 0.481 (0.246-0.939) |
| GTT | 0.00 (0.00) | 3.00 (0.01) | - | - |
Peritoneal lavage can lower the relapse rate caused by peritoneal metastasis[17,18]. During surgery for gastric cancer, peritoneal lavage using warm distilled water can cause temporary hemodynamic changes. This study aimed to examine the changes in heart rate in patients with gastric cancer who underwent proximal or total gastrectomy before and after peritoneal lavage using distilled water and to explore the associations between changes in heart rate and SNPs. The results strongly suggest that the TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) SNPs were associated with changes in heart rate ≥ 30% during intraperitoneal lavage using distilled water after gastrectomy for gastric cancer. Therefore, a genetic test for TEP1 and RECQL5 could be performed before gastrectomy and a different lavage method could be chosen for those at high risk to avoid adverse outcomes such as abnormal heart rate.
Currently, despite the improvement in surgical techniques and multimodal combination therapy, the prognosis of gastric cancer, especially advanced gastric cancer (AGC) complicated by serosal invasion (T3 or T4) is still poor[1]. The overall 5-year survival rate is less than 35%[25]. In 1998, the Japanese Gastric Cancer Association suggested using peritoneal lavage cytology to detect free malignant cells in the abdominal cavity[9]. In 2010, peritoneal lavage cytology was also recommended to stage patients with gastric cancer in the 7th edition of Cancer Tumor Node Metastasis (TNM) staging by the American Joint Cancer Committee (AJCC)[26]. Although it is currently a routine procedure to perform peritoneal lavage using warm water during gastrectomy, very few studies, focus on the mechanisms underlying the changes caused by peritoneal lavage in the internal environment and the circulation system of patients, or focus on the associated genetic mutations. These changes in hemodynamics might represent important challenges for the anesthesiologists, who are responsible for restoring the changes in hemodynamics. Therefore, to address this issue, we carefully examined vital signs during peritoneal lavage using distilled water in 194 patients with gastric cancer. We found that 22 participants had a drop in heart rate of ≥ 30%, and six of them even had a cardiac arrest. A previous study by Ohki et al[23] did not report the changes in hemodynamics. Subsequently, we performed genetic screening and investigated the underlying mechanisms.
In the present study, we found that TEP1 and RECQL5 were associated with a drop in heart rate of ≥ 30% during peritoneal lavage using distilled water. TEP1 (Telomerase-associated protein 1) encodes a hexamer DNA helicase that can unwind the short double-stranded DNA in the 5' -> 3' direction. TEP1, together with the mitochondrial single-stranded DNA binding protein and mtDNA polymerase, is considered to play a crucial role in mtDNA replication. TEP1 exerts its functions by binding to the mitochondrial single-stranded DNA binding protein and plays a crucial role in respiration[27]. TEP1 is located in the mitochondrial matrix and nucleoid. TEP1 mutations can cause infantile-onset spinal cerebellar ataxia (IOSCA) and progressive extraocular paralysis (PEO) and are associated with several mitochondrial disorders; alternative splicing of TEP1 results in several transcript variants, which may lead to changes in heart rate[28,29]. The human RECQL5 (RecQ protein-like 5) gene is located at 17q25.2-25.3 and contains at least 19 exons. It is alternatively spliced into three mRNA variants, RECQL5α, RECQL5β, and RECQL5γ. RECQL5 is one of the five major members of the RECQ helicase family and plays a role in suppressing sister chromatid exchange and DNA repair by homologous recombination as well as preventing double-stranded DNA breakage and the synergistic effect of TOPIIα. RECQL5 may be essential in DNA metabolism and may affect heart rate and be involved in heart diseases[30].
It is believed that emerging evidence will support the association between metabolic genes and the development and progression of heart failure. Therefore, glucose metabolism in the heart or the metabolic pathways relevant to ATP synthesis might be new research directions to prevent heart failure and cardiac arrest during surgery. In this study, some metabolic genes had SNPs that were associated with a drop in heart rate during intraperitoneal lavage using distilled water. If metabolic modulators can be found and proved to be effective in treating patients with heart failure, we could guide treatment decisions and evaluate the patients’ response via metabolic imaging or metabolic biomarkers. In this case, a multidisciplinary method should be proposed by a group of anesthetists, cardio-pathologists, endocrinologists, and geneticists[31-33].
This study has limitations. The sample size was relatively small and from a single center. Also, only a few metabolic parameters were examined. A full array of changes in metabolic parameters should be examined in future studies to better grasp what happens, metabolically, during the heart rate drop as a result of peritoneal lavage. Finally, the SNPs identified here might be specific to an ethnic group, and the results should be confirmed in other groups.
In conclusion, SNPs in TEP1 (rs938886), TEP1 (rs1713449), and RECQL5 (rs820196) were associated with changes in heart rate ≥ 30% during intraperitoneal lavage using distilled water after gastrectomy for gastric cancer. Therefore, a genetic test for TEP1 and RECQL5 could be performed before gastrectomy and a different lavage method could be chosen for those at high risk to avoid adverse outcomes such as abnormal heart rate or even cardiac arrest.
For the first time, we found that severe cardiovascular changes occurred in patients with gastric cancer during intraoperative distilled water lavage, which was also observed in a large cohort.
The internal causes of this phenomenon were studied through detailed clinical case observation and a mechanistic study.
The target population was screened out through gene detection and verification.
We conducted a prospective observational study of the included cases, and selected typical patients for gene screening, and the results were validated in a large cohort.
We successfully validated a site mutation in TEP1-886, TEP1-449 as well as RECQL5, and selected the heart rate change of 30% as the cut-off value for comparison and regression analysis.
SNPs in TEP1 (rs938886), TEP1 (rs1713449) and RECQL5 (rs820196) were associated with changes in heart rate > 30% when peritoneal lavage with distilled water was performed after gastrectomy for gastric cancer patients.
We performed pre-operative detection of TEP1 and RECQL5 genes in patients and selected different lavage methods for high-risk groups to improve peri-operative safety.
We appreciate the great help from all the members of the Department of Gastrointestinal Surgery in the Third Affiliated Hospital of Soochow University.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ekpenyong CE S-Editor: Gao CC L-Editor: Webster JR P-Editor: Xing YX
| 1. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Gastric Cancer. Version 2.2020. Fort Washington: National Comprehensive Cancer Network; 2020. |
| 2. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1462] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55649] [Article Influence: 7949.9] [Reference Citation Analysis (132)] |
| 4. | Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 265] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 5. | Petrillo A, Smyth EC. 27 years of stomach cancer: painting a global picture. Lancet Gastroenterol Hepatol. 2020;5:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1114] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 8. |
American Cancer Society.
Cancer Facts and Figures |
| 9. |
Japanese Gastric Cancer Association.
Japanese Classification of Gastric Carcinoma - 2nd English Edition - |
| 10. | Miyashiro I, Takachi K, Doki Y, Ishikawa O, Ohigashi H, Murata K, Sasaki Y, Imaoka S, Nakaizumi A, Takenaka A, Furukawa H, Hiratsuka M. When is curative gastrectomy justified for gastric cancer with positive peritoneal lavage cytology but negative macroscopic peritoneal implant? World J Surg. 2005;29:1131-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1910] [Article Influence: 238.8] [Reference Citation Analysis (1)] |
| 12. | Kodera Y, Ito S, Mochizuki Y, Ohashi N, Tanaka C, Kobayashi D, Kojima H, Matsui T, Kondo K, Fujiwara M. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer. 2012;15:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4060] [Cited by in RCA: 5718] [Article Influence: 519.8] [Reference Citation Analysis (0)] |
| 14. | Zhang L, Song X, Mohri Y, Qiao L. Role of inflammation and tumor microenvironment in the development of gastrointestinal cancers: what induced pluripotent stem cells can do? Curr Stem Cell Res Ther. 2015;10:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Mahteme H, Påhlman L. Treatment of peritoneal surface malignancy by peritonectomy and intraperitoneal chemotherapy: a novel therapy with curative potential! Minerva Chir. 2005;60:151-157. [PubMed] |
| 16. | Smeenk RM, Verwaal VJ, Zoetmulder FA. Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei--a report of 103 procedures. Eur J Surg Oncol. 2006;32:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Levine EA. Extensive Intraoperative Peritoneal Lavage to Prevent Metastases From Gastric Cancer: The Elegance of Simplicity. JAMA Surg. 2019;154:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Guo J, Xu A, Sun X, Zhao X, Xia Y, Rao H, Zhang Y, Zhang R, Chen L, Zhang T, Li G, Xu H, Xu D. Combined Surgery and Extensive Intraoperative Peritoneal Lavage vs Surgery Alone for Treatment of Locally Advanced Gastric Cancer: The SEIPLUS Randomized Clinical Trial. JAMA Surg. 2019;154:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AA, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 879] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 21. | Beeharry MK, Zhu ZL, Liu WT, Yao XX, Yan M, Zhu ZG. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer. 2019;19:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (2)] |
| 22. | Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia. 2004;20:781-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Ohki A, Abe N, Yoshimoto E, Hashimoto Y, Takeuchi H, Nagao G, Masaki T, Mori T, Ohkura Y, Sugiyama M. Gastric washing by distilled water can reduce free gastric cancer cells exfoliated into the stomach lumen. Gastric Cancer. 2018;21:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, Ward P, Braxton A, Wang M, Buhay C, Veeraraghavan N, Hawes A, Chiang T, Leduc M, Beuten J, Zhang J, He W, Scull J, Willis A, Landsverk M, Craigen WJ, Bekheirnia MR, Stray-Pedersen A, Liu P, Wen S, Alcaraz W, Cui H, Walkiewicz M, Reid J, Bainbridge M, Patel A, Boerwinkle E, Beaudet AL, Lupski JR, Plon SE, Gibbs RA, Eng CM. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1062] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 25. | Fujiwara Y, Doki Y, Taniguchi H, Sohma I, Takiguchi S, Miyata H, Yamasaki M, Monden M. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | La Torre M, Ferri M, Giovagnoli MR, Sforza N, Cosenza G, Giarnieri E, Ziparo V. Peritoneal wash cytology in gastric carcinoma. Prognostic significance and therapeutic consequences. Eur J Surg Oncol. 2010;36:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Liang CP, Tall AR. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J Biol Chem. 2001;276:49066-49076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Kickhoefer VA, Stephen AG, Harrington L, Robinson MO, Rome LH. Vaults and telomerase share a common subunit, TEP1. J Biol Chem. 1999;274:32712-32717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Yajima T, Yagihashi A, Kameshima H, Furuya D, Kobayashi D, Hirata K, Watanabe N. Establishment of quantitative reverse transcription--polymerase chain reaction assays for human telomerase-associated genes. Clin Chim Acta. 2000;290:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Xie X, Zheng YY, Adi D, Yang YN, Ma YT, Li XM, Fu ZY, Ma X, Liu F, Yu ZX, Chen Y, Huang Y. Exome Sequencing in a Family Identifies RECQL5 Mutation Resulting in Early Myocardial Infarction. Medicine (Baltimore). 2016;95:e2737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev. 2002;7:115-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 267] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Hansson A, Hance N, Dufour E, Rantanen A, Hultenby K, Clayton DA, Wibom R, Larsson NG. A switch in metabolism precedes increased mitochondrial biogenesis in respiratory chain-deficient mouse hearts. Proc Natl Acad Sci USA. 2004;101:3136-3141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |