Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.801
Peer-review started: November 3, 2020
First decision: November 20, 2020
Revised: November 23, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: February 6, 2021
Processing time: 83 Days and 9.7 Hours
Malignant obstructive jaundice is mainly caused by cholangiocarcinoma. Only a few patients are indicated for surgical resection, and the 3-year survival rate is < 50%. For patients who are not eligible for surgery, biliary stent placement can relieve biliary obstruction and improve liver function and quality of life. However, restenosis after biliary stents has a poor prognosis and is a clinical challenge. Biliary stent combined with iodine-125 (125I) seed implantation can prolong stent patency and improve survival.
To evaluate the safety and efficacy of biliary stent combined with 125I seed strand implantation in malignant obstructive jaundice.
We enrolled 67 patients between January 2016 and June 2018 with malignant obstructive jaundice and randomized them into a biliary stent combined with 125I seed strand treatment (combined) group (n = 32) and biliary stent (control) group (n = 35). All patients underwent enhanced computed tomography and magnetic resonance imaging and were tested for biochemical and cancer markers. Twelve patients underwent pathological examination before surgery. All patients were followed up by telephone or clinical visit. Postoperative liver function improvement, postoperative complications, stent patency time, and survival time were compared between the two groups. Prognostic risk factors were evaluated.
Technical success was achieved in all patients in both groups. Postoperative liver function improved significantly in all patients (total bilirubin, direct bilirubin, alanine aminotransferase, and aspartate aminotransferase decreased significantly in all patients, the P values were less than 0.05). There was no significant difference in preoperative or postoperative indexes between the two groups for changes in total bilirubin (P = 0.147), direct bilirubin (P = 0.448), alanine aminotransferase (P = 0.120), and aspartate aminotransferase (P = 0.387) between the two groups. The median stent patency time of the combined group was 9.0 ± 1.4 mo [95% confidence interval (CI): 6.3-11.8 mo], which was significantly longer than the that of the control group (6.0 ± 0.3 mo, 95%CI: 5.5-6.5 mo, P = 0.000). The median survival time of the combined group was 11.0 ± 1.4 mo (95%CI: 8.2-13.7 mo), which was significantly longer than that of the control group (7.0 ± 0.3 mo, 95%CI: 6.4-7.6 mo, P = 0.000). Location of obstruction and number of stents were independent risk factors affecting prognosis.
Biliary stent combined with 125I seed strand implantation is safe and effective in malignant obstructive jaundice and improves stent patency time and median survival time.
Core Tip: For patients who are unable to undergo surgery, biliary stent placement can relieve biliary obstruction and prolong survival. However, previous studies relieved stenosis caused by an existing tumor lesion by placing an iodine-125 seed in the middle of the stent while ignoring both ends. Both ends of the stent can be reobstructed. We observed that extending the iodine-125 seed strand along the length of the stent produced a safe and effective result in malignant obstructive jaundice.
- Citation: Wang HW, Li XJ, Li SJ, Lu JR, He DF. Biliary stent combined with iodine-125 seed strand implantation in malignant obstructive jaundice. World J Clin Cases 2021; 9(4): 801-811
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/801.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.801
Malignant obstructive jaundice is caused by cholangiocarcinoma or peribiliary malignant tumor invasion and compression; onset of symptoms is generally insidious with only 10%-20% of patients indicated for surgical resection. The 3-year survival rate is < 50%[1,2]. For patients who are not indicated for or unwilling to undergo surgery, biliary stent placement can relieve biliary obstruction, improve liver function and quality of life, and prolong survival[3-5]. However, restenosis after a biliary stent may significantly worsen prognosis and remains a clinical challenge. The main causes of restenosis include tumor growth, cholestatic accumulation, and granulation tissue hyperplasia. In recent years, reports of biliary stents combined with iodine-125 (125I) seed implantation seemed to prolong stent patency time and improved patient survival[6-8]. However, most attempts to relieve stenosis caused by tumors utilized a technique of placing the 125I seed in the middle of the stent while ignoring both ends. Because of overgrowth of the tumor and fibrous granulation tissue hyperplasia caused by frictional stimulation between the two ends of the stent and the bile duct wall, both ends of the stent can be reobstructed[9,10]. Therefore, measures to prevent reobstruction may provide a greater benefit to patients. In this study, we evaluated extending the 125I seed strand across the entire length of the stent.
This study was approved by the Medical Ethics Review Committee of Harbin Medical University Cancer Hospital and registered for the Chinese clinical trial (Registration Number: ChiCTR2000030878).
This was a single center, randomized, controlled study. The inclusion criteria were as follows: (1) Age 18-80 years; (2) Eastern Cooperative Oncology Group score ≤ 2 points; (3) Obstructive jaundice caused by pathologically or clinically diagnosed malignant tumor, and patient not indicated or unwilling to undergo surgery; (4) No severe cardiovascular and cerebrovascular diseases; (5) No severe coagulation abnormalities; and (6) Willing to participate in this study and give signed informed consent. The exclusion criteria were as follows: (1) Suspicion of benign biliary stricture; and (2) Patients with reobstruction after biliary stenting or drainage.
Based on previous reports, the median survival time after traditional biliary stent implantation is 3-8 mo[6,11-13]. We assumed median survival time of 3 mo in the control group and 8 mo in the combined group. The α value was 0.05 (unilateral test), the power was 0.9, and we needed 30 cases in each group for the sample size.
Between January 2016 and June 2018, 67 patients with malignant obstructive jaundice were treated in our center. All patients underwent enhanced computed tomography and magnetic resonance imaging and were tested for biochemical and cancer markers. Twelve patients underwent pathological examination before surgery. All patients were diagnosed with malignant obstructive jaundice. Envelopes were randomly assigned to the simple stent (control) group and the biliary stent combined with 125I seed strand treatment (combined) group according to a 1:1 ratio. There were 35 patients in the control group and 32 in the combined group (one patient in the combined group was transferred to the control group for economic reasons). There were 35 patients with pancreatic cancer (21 in the control group and 14 in the combined group); 19 patients with gallbladder cancer and cholangiocarcinoma (8 in the control group and 11 in the combined group); and 13 patients with lymph node metastasis from digestive malignant tumors (6 in the control group and 7 in the combined group). The statistician was blinded to group assignment. The general clinical characteristics of the two groups of patients are shown in Table 1. The differences between the two groups were not significant.
| Combined | Control | χ2/t | P value | |
| Age in yr | 63.25 ± 9.92 | 63.46 ± 10.43 | -0.0831 | 0.934 |
| Sex | ||||
| Male | 16 | 15 | 0.343 | 0.558 |
| Female | 16 | 20 | ||
| ECOG score | ||||
| < 2 | 20 | 25 | 0.604 | 0.437 |
| 2 | 12 | 10 | ||
| Child grade | ||||
| B | 25 | 26 | 0.136 | 0.713 |
| C | 7 | 9 | ||
| Type of obstruction1 | ||||
| High | 16 | 16 | 0.123 | 0.726 |
| Low | 16 | 19 | ||
| Obstruction length (cm) | 3.22 ± 1.04 | 3.26 ± 0.98 | -0.1562 | 0.877 |
| Number of stents | ||||
| Unilateral | 30 | 32 | 1.0003 | |
| Bilateral | 2 | 3 | ||
| Tumor type | ||||
| Pancreatic cancer | 14 | 21 | 1.820 | 0.403 |
| Bile duct and gallbladder cancer | 11 | 8 | ||
| Gastrointestinal cancer metastasis | 7 | 6 | ||
| Combined infusion chemotherapy | ||||
| Yes | 13 | 14 | 0.003 | 0.958 |
| No | 19 | 21 |
125I seeds were produced by Beijing Science and Technology Biotechnology Co. Ltd. (Beijing, China). Dimensions: length = 4.5 mm, diameter = 0.8 mm, effective radiation radius of 15-20 mm, half-life of 59.43 d, energy of 27.4 keV for X rays and 35.5 keV for R rays. The stent was a biliary stent produced by Nanjing Minitron Co. Ltd. (Nanjing, China) with a diameter of 10 mm and length of 40-80 mm.
Under the guidance of ultrasound and digital subtraction angiography, an 18G needle was used to puncture the intrahepatic dilated bile duct. A guide wire was introduced in place of the puncture needle core, and the puncture needle sheath was pulled out. An 8F long arterial sheath was introduced through the guide wire, and two guide wires were introduced through the long arterial sheath after withdrawing the core of the arterial sheath. After removing the arterial sheath, the stent conveyor was placed along one of the guide wires. A customized 125I seed strand was placed along an 8F long arterial sheath that was introduced along the other guide wire (Figure 1A-F). The 125I seeds were placed in a 4F plastic tube via a seed gun, and both ends were heated and then clamped shut (Figure 1G). The number of 125I seeds was calculated by: N = L/4.5 + 2 (L = stent length). Only simple stents were placed in the control group. After successful stenting and 125I seed strand implantation, an external drainage tube was placed through the guide wire. After 2 mo, imaging examination was performed to confirm that the stent was unobstructed before the drainage tube was removed.
All patients were followed up by telephone or clinical visit. Telephone follow-up was performed every 2 mo to document the patient’s status. If a patient had new jaundice or a significant increase in total bilirubin, abdominal enhanced computed tomography or magnetic resonance imaging was recommended to determine stent obstruction. Follow-up continued until a patient died or the end of the study. The overall survival period was calculated from the time the stent was placed to when the patient died or the end of the study. The stent patency time was from stent placement to stent blockage or the end of the study.
The c2 test was used to compare the numerical data. Continuous variables were expressed as mean ± standard deviation, and changes in the laboratory tests in the same group were evaluated by paired t test. Comparison of changes in preoperative and postoperative indexes in both groups was performed by independent t test. Survival was calculated using the Kaplan-Meier method, and factors affecting prognosis were analyzed using a Cox regression model. P < 0.05 indicated that the difference was significant. Statistical analysis was performed using SPSS version 17.0.
Both groups of patients were successfully implanted with biliary stents or 125I seed strand, and the technical success rate was 100%. Liver function was significantly improved in both groups 5 d after surgery (total bilirubin, direct bilirubin, alanine aminotransferase, and aspartate aminotransferase decreased significantly in all patients; the P values were less than 0.05). There was no significant difference in preoperative or postoperative indexes between the two groups for changes in total bilirubin (P = 0.147), direct bilirubin (P = 0.448), alanine aminotransferase (P = 0.120), and aspartate aminotransferase (P = 0.387) (Table 2). No serious complications occurred in either group.
| Combined | Control | P value | |
| Total bilirubin, μmoL/L | 0.147 | ||
| Before | 358.98 ± 134.05 | 347.86 ± 131.73 | |
| After | 180.08 ± 110.94 | 199.43 ± 113.88 | |
| P value | 0.000 | 0.000 | |
| Direct bilirubin, μmoL/L | 0.448 | ||
| Before | 196.02 ± 74.90 | 194.76 ± 73.52 | |
| After | 97.89 ± 67.33 | 108.97 ± 66.91 | |
| P value | 0.000 | 0.000 | |
| ALT, U/L | 0.120 | ||
| Before | 209.82 ± 148.62 | 209.65 ± 152.53 | |
| After | 80.73 ± 54.18 | 95.41 ± 68.73 | |
| P value | 0.000 | 0.000 | |
| AST, U/L | 0.387 | ||
| Before | 195.46 ± 154.29 | 193.51 ± 136.76 | |
| After | 61.97 ± 47.25 | 80.71 ± 67.98 | |
| P value | 0.000 | 0.000 |
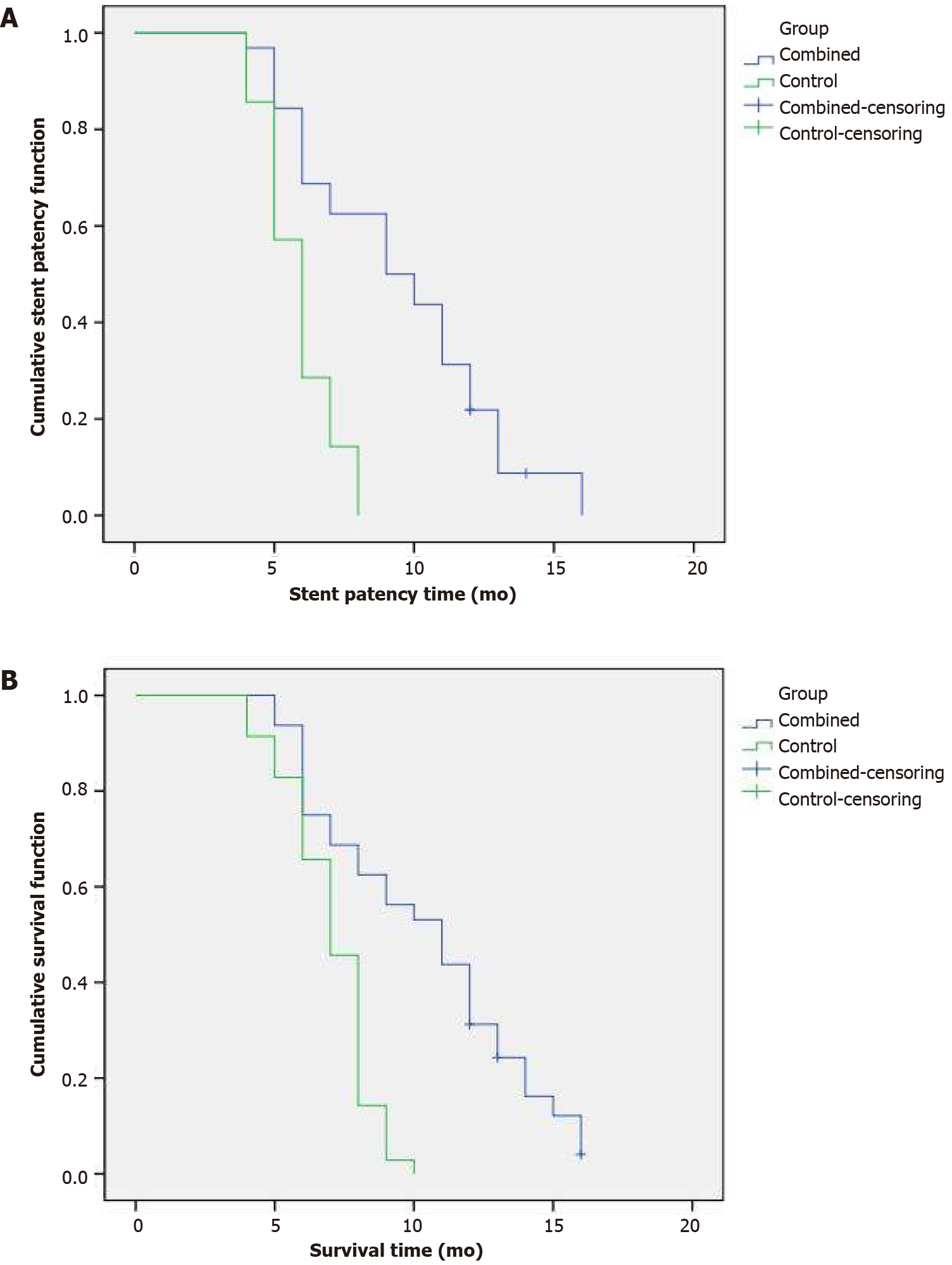
The mean and median stent patency times for the combined group were 9.5 ± 0.6 mo [95% confidence interval (CI): 8.3-10.7 mo] and 9.0 ± 1.4 mo (95%CI: 6.3-11.8 mo), respectively. For the control group, they were 5.8 ± 0.2 mo (95%CI: 5.4-6.3 mo) and 6.0 ± 0.3 mo (95%CI: 5.5-6.5 mo), respectively. There was a significant difference between the two groups (P = 0.000) (Figure 2A). By the end of the study, 18 patients in the combined group had stent occlusion, of whom four had percutaneous transhepatic cholangial drainage, and 19 patients in the control group had stent occlusion, of whom seven had percutaneous transhepatic cholangial drainage.
The median survival time for the combined group was 11.0 ± 1.4 mo (95%CI: 8.3-13.8 mo). For the control group, it was 7.0 ± 0.3 mo (95%CI: 6.4-7.6 mo). There was a significant difference between the groups (P = 0.000) (Figure 2B).
Univariate and multivariate analysis showed that the type of obstruction and the number of stents were independent risk factors that affected the prognosis for survival. Patients with low biliary obstruction and a single stent had a better prognosis (P values were 0.015 and 0.035, respectively; hazard ratios were 2.060 and 3.489, respectively) (Table 3).
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| ECOG score, < 2/2 | 1.569 | (0.931-2.646) | 0.091 | |||
| Child rating, B/C | 0.901 | (0.504-1.610) | 0.724 | |||
| Type of obstruction, low/high | 2.395 | (1.412-4.062) | 0.001 | 2.060 | (1.153-3.678) | 0.015 |
| Obstruction length in cm | 1.188 | (0.904-1.560) | 0.216 | |||
| Number of stents, unilateral/bilateral | 6.129 | (2.310-16.262) | 0.000 | 3.489 | (1.094-11.126) | 0.035 |
| Tumor type1, pancreatic cancer/biliary cancer/gastrointestinal metastatic cancer | 1.192 | (0.665-2.135) | 0.555 | |||
| 0.532 | (0.269-1.053) | 0.070 | ||||
| Combined perfusion, yes/no | 0.766 | (0.465-1.264) | 0.297 | |||
To treat unresectable malignant obstructive jaundice, the first task is to remove biliary blockage, improve liver function, and relieve symptoms to prepare for further radiotherapy, chemotherapy, or other treatments. Biliary stent placement has become the preferred treatment method[3-5,14]. However, after stent placement due to tumor overgrowth, tissue-reactive hyperplasia, and biliary formation, stent restenosis significantly affects the survival of patients[15-18]. Therefore, prevention of restenosis after stenting has been a hot topic for clinical research and investigation in recent years and has included the study of covered and drug-eluting stents. Covered stents are prone to displacement, and stent displacement tends to increase the incidence of cholecystitis and pancreatitis. These two approaches have not achieved satisfactory clinical outcomes[19-21].
125I seeds are new micro-low-dose-rate particle sources developed in recent years with an effective irradiation radius of 15-20 mm, half-life of 59.43 d, and energy of 27.4 keV for X rays and 35.5keV for R rays. Low dose rate and continuous irradiation lead to high dose within the target, resulting in a strong targeted killing effect on tumor cells by destroying DNA double strands of tumor cells and reducing their ability to proliferate without significantly affecting surrounding normal tissues. Because of these advantages, 125I seed implantation has achieved good results in the treatment of various solid malignancies in recent years.
Chen et al[22] first implanted 125I seeds into porcine bile duct. They demonstrated that 125I seeds were safe and did not cause serious damage to the bile duct. There are also reports that biliary stents combined with 125I seed strands for patients with malignant obstructive jaundice. Due to the killing effect of 125I seeds on tumor tissues, they can increase stent patency time and prolong survival[6,8,11,12,23]. Some researchers placed the 125I seed strands into biliary stenosis through an external drainage tube and then removed the drainage tube and 125I seed strands 2 mo later. This approach also improved the stent patency time and survival of patients compared with traditional stents[6,8,11,12,23], but the drainage tube was unstable due to respiratory movement and other daily activities and affected quality of life. Because the drainage tube and 125I seed strands were removed after 2 mo, the 125I seed strands had a shorter duration of action, which inevitably affected treatment efficacy.
Subsequently, Zhu et al[6] developed a biliary stent carrying 125I seeds and placed the biliary stent into 12 patients with malignant obstructive jaundice. They showed that radioactive particle stents were superior to traditional bare stents. However, because the stent was divided into a jacket and an inner stent carrying particles, the inner diameter of the stent for drainage was reduced, and the narrowing of the stent inevitably affected bile drainage and stent patency time[15,24]. Additionally, other researchers inserted the 125I seed strands between the stent and bile duct wall, and the stent patency time and patient survival also improved[25]. The 125I seed strands are fixed and stable, and the quality of life can be significantly improved without carrying an external drainage tube for a long time. We also used this approach with a satisfactory outcome and quality of life was significantly improved. However, all of the aforementioned studies focused on the stenosis caused by existing tumors and placed 125I seeds in the middle of the stent, ignoring both ends of the stent.
Tumor growth will occur along the outside of the stent, causing obstruction at both ends of the stent as reported previously[9]. Guo et al[26,27] found that after esophageal stent implantation, both ends were prone to reocclusion in animal experiments and clinical practice. Pathological examination revealed that the obstruction was mainly caused by overgrown tumor tissue and hyperplastic granulation tissue. After biliary stenting, granulation tissue hyperplasia may also occur with local frictional stimulation between both ends of the stent and the biliary tract[10]. These factors can cause reobstruction at both ends of the stent. 125I seeds destroy double-stranded DNA of tumor cells and stop tumor proliferation. Additionally, it has been found that radioactive stents increase the activity of the caspase-3 gene through γ rays, promoting canine bile duct smooth muscle cell apoptosis, and therefore inhibiting canine bile duct stenosis in animal experiments[28].
Based on the inhibitory effect of 125I seeds on tumor and granulation tissues, we extended the coverage of 125I seed strands to both ends of the biliary stent to prevent reobstruction at either end. Our results showed that the survival time of the combined group reached 11.0 ± 1.4 mo, which is longer than that from the Zhu et al[6] study (median survival time 7.4 mo). The stent patency time reached 9.0 ± 1.4 mo, which was significantly better than that reported by Zhu et al[6] (stent patency time and median survival time of 194 d)[13]. A few factors may have contributed to the improved outcomes in our study. Firstly, the extended 125I seed strand played a role in preventing obstruction at both ends of the stent. Secondly, the diameter of our stent was 10 mm. Some studies have shown that the larger the diameter of the stent, the longer the restenosis time appears to be[15]. Thirdly, if there was biliary tract infection, we performed conventional percutaneous transhepatic cholangial drainage before biliary stent placement to reduce biliary edema and control infection. When the biliary stent was implanted, the catheter was first used to drain the accumulated viscous bile, and normal saline was used to wash out the remaining bile.
The stent was placed after bile drainage, which seemed to prolong stent patency time, presumably because thick bile can rapidly cause reobstruction of the stent[15,24]. Our study also found that the type of obstruction and number of stents were independent risk factors that affected prognosis. The higher the location of the obstruction, the more and thinner the bile duct branches would be. Because left and right hepatic ducts are disconnected, bilateral stents are required. One stent can only resolve one obstruction. If there are more than two biliary obstructions, then the stents cannot resolve all the obstructions. Therefore, multibranch biliary obstruction has a generally poor bilirubin declining effect, which directly affects liver function and prognosis.
There were some limitations to our study. First, the sample size was small, and a multicenter study with a large sample size is needed. Second, due to the lack of advanced measuring instruments, accurate metrological data could not be obtained.
In conclusion, biliary stent combined with 125I seed strand implantation is safe and effective for treatment of malignant obstructive jaundice. The present study suggests that it could improve stent patency and median survival.
Biliary stent placement can relieve biliary obstruction and improve liver function and quality of life. However, restenosis after biliary stents has a poor prognosis and is a clinical challenge.
In order to solve the problem of biliary stent restenosis, we should seek a better method for clinical treatment.
To evaluate the safety and efficacy of biliary stent combined with iodine-125 seed strand implantation in malignant obstructive jaundice.
Sixty-seven patients were randomly divided into the biliary stent with iodine-125 group (combined group) and the biliary stent group (control group). The stent patency time and total survival time were compared between the two groups.
The present study suggests that biliary stent combined with iodine-125 seed strands could improve stent patency and median survival.
Biliary stent combined with 125I seed strand implantation is safe and effective for the treatment of malignant obstructive jaundice.
A multicenter study with a large sample size is needed. Accurate metrological data is needed.
CONSORT 2010 Statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Faloppi L, Otto G, Raisch K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Skipworth JR, Olde Damink SW, Imber C, Bridgewater J, Pereira SP, Malagó M. Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol Ther. 2011;34:1063-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8103] [Article Influence: 506.4] [Reference Citation Analysis (2)] |
| 3. | Crosara Teixeira M, Mak MP, Marques DF, Capareli F, Carnevale FC, Moreira AM, Ribeiro U Jr, Cecconello I, Hoff PM. Percutaneous transhepatic biliary drainage in patients with advanced solid malignancies: prognostic factors and clinical outcomes. J Gastrointest Cancer. 2013;44:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Tsuyuguchi T, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Kondo S, Furuse J, Saito H, Suyama M, Kimura F, Yoshitomi H, Nozawa S, Yoshida M, Wada K, Amano H, Miura F; Japanese Association of Biliary Surgery; Japanese Society of Hepato-Biliary-Pancreatic Surgery; Japan Society of Clinical Oncology. Stenting and interventional radiology for obstructive jaundice in patients with unresectable biliary tract carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Meller MT, Arts GR, Dean JR. Outcomes in percutaneous stenting of non-hepato-biliary/pancreatic malignant jaundice. Eur J Cancer Care (Engl). 2010;19:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Zhu HD, Guo JH, Zhu GY, He SC, Fang W, Deng G, Qin YL, Li GZ, Coldwell DM, Teng GJ. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol. 2012;56:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Li S, He X, Dang L, Xu F, Fang J, Li F, Wang W. Efficacy of 125I Versus Non-125I Combined with Transcatheter Arterial Chemoembolization for the Treatment of Unresectable Hepatocellular Carcinoma with Obstructive Jaundice. Dig Dis Sci. 2018;63:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Chen W, Fang XM, Wang X, Sudarshan SKP, Hu XY, Chen HW. Preliminary clinical application of integrated 125I seeds stents in the therapy of malignant lower biliary tract obstruction. J Xray Sci Technol. 2018;26:865-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Lee SJ, Kim MD, Lee MS, Kim IJ, Park SI, Won JY, Lee DY. Comparison of the efficacy of covered versus uncovered metallic stents in treating inoperable malignant common bile duct obstruction: a randomized trial. J Vasc Interv Radiol. 2014;25:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Hausegger KA, Kleinert R, Lammer J, Klein GE, Flückiger F. Malignant biliary obstruction: histologic findings after treatment with self-expandable stents. Radiology. 1992;185:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Yang M, Yan Z, Luo J, Liu Q, Zhang W, Ma J, Zhang Z, Yu T, Zhao Q, Liu L. A pilot study of intraluminal brachytherapy using 125I seed strand for locally advanced pancreatic ductal adenocarcinoma with obstructive jaundice. Brachytherapy. 2016;15:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Jiao D, Wu G, Ren J, Han X. Study of self-expandable metallic stent placement intraluminal 125I seed strands brachytherapy of malignant biliary obstruction. Surg Endosc. 2017;31:4996-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Zhou WZ, Fu YM, Yang ZQ, Shi HB, Liu S, Xia JG, Zhou CG. Study of Percutaneous Stent Placement with Iodine-125 Seed Strand for Malignant Biliary Obstruction. Cardiovasc Intervent Radiol. 2019;42:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | van Delden OM, Laméris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur Radiol. 2008;18:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 15. | Guo Y, Liu Y, Lu Z, Shi X, Zou D, Wang D, Liu F, Jin Z, Li Z. Obstructive component analysis of radioactive stents and common plastic stents in the bile duct. Eur J Gastroenterol Hepatol. 2014;26:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kida M, Miyazawa S, Iwai T, Ikeda H, Takezawa M, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Recent advances of biliary stent management. Korean J Radiol. 2012;13 Suppl 1:S62-S66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Kim HS, Lee DK, Kim HG, Park JJ, Park SH, Kim JH, Yoo BM, Roe IH, Moon YS, Myung SJ. Features of malignant biliary obstruction affecting the patency of metallic stents: a multicenter study. Gastrointest Endosc. 2002;55:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H, Shiratori Y, Kawabe T, Omata M. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 459] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 20. | Song TJ, Lee SS, Yun SC, Park DH, Seo DW, Lee SK, Kim MH. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: a prospective comparative pilot study. Gastrointest Endosc. 2011;73:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Yoon WJ, Lee JK, Lee KH, Lee WJ, Ryu JK, Kim YT, Yoon YB. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Chen Y, Wang XL, Yan ZP, Wang JH, Cheng JM, Gong GQ, Li GP. Damage to pig bile duct caused by intraluminal brachytherapy using a (125)I ribbon. Acta Radiol. 2013;54:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Hasimu A, Gu JP, Ji WZ, Zhang HX, Zhu DW, Ren WX. Comparative Study of Percutaneous Transhepatic Biliary Stent Placement with or without Iodine-125 Seeds for Treating Patients with Malignant Biliary Obstruction. J Vasc Interv Radiol. 2017;28:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Rey JF, Maupetit P, Greff M. Experimental study of biliary endoprosthesis efficiency. Endoscopy. 1985;17:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Ma J, Luo J, Gu J, Liu Q, Liu L, Zhang W, Zhang Z, Yan Z. Malignant obstructive jaundice treated with intraluminal placement of Iodine-125 seed strands and metal stents: An analysis of long-term outcomes and prognostic features. Brachytherapy. 2018;17:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Guo JH, Teng GJ, Zhu GY, He SC, Deng G, He J. Self-expandable stent loaded with 125I seeds: feasibility and safety in a rabbit model. Eur J Radiol. 2007;61:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Guo JH, Teng GJ, Zhu GY, He SC, Fang W, Deng G, Li GZ. Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer. Radiology. 2008;247:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | He GJ, Yu FQ, Wu R, Gao QY, Xu SH, Gao H, Jiang WG, Jiang T, Dai XW. 103Pd-induced apoptosis of proliferative smooth muscle cells in bile ducts of dogs: significance and effects on related genes. Hepatobiliary Pancreat Dis Int. 2007;6:521-526. [PubMed] |