Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10948
Peer-review started: April 25, 2021
First decision: June 3, 2021
Revised: June 18, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: December 16, 2021
Processing time: 229 Days and 0.9 Hours
Serum gastrin-17 (G-17), pepsinogen I (PGI), and pepsinogen II (PGII) concentrations regulate gastric acid secretion, and hypersecretion of gastric acid increases the risks of peptic ulcer and upper gastrointestinal bleeding. These associations suggest that serum G-17, PGI, and (or) PGII may predict gastrointestinal bleeding risk among peptic ulcer patients.
To evaluate the efficacies of serum G-17, PGI, PGII, and PGI/PGII ratio (PGR) for predicting upper gastrointestinal bleeding among peptic ulcer patients.
A total of 199 patients diagnosed with peptic ulcer confirmed by gastroscopy and positivity for Helicobacter pylori by the 14C-urea breath test were recruited, including 107 patients with simple peptic ulcer and 92 cases complicated by upper gastrointestinal bleeding. Serum PGI, PGII, G-17, and PGR were measured by immune methods and compared between bleeding and non-bleeding groups by univariate analysis. The specificity and sensitivity of PGs and G-17 for evaluating upper gastrointestinal bleeding risk were then assessed by constructing receiver operating characteristic (ROC) curves.
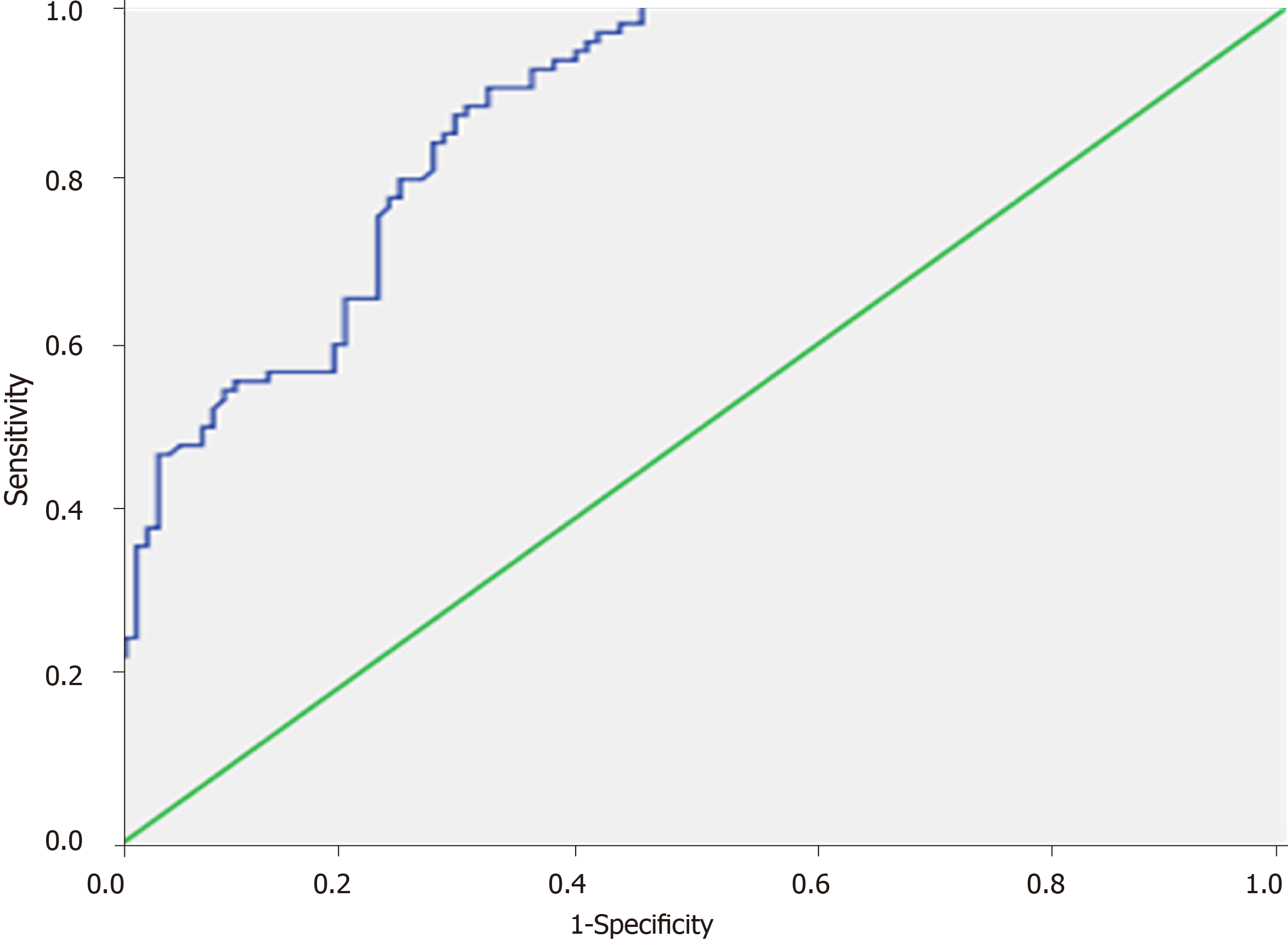
Serum G-17 was significantly higher among peptic ulcer patients with upper gastrointestinal bleeding compared to simple peptic ulcer patients (25.34 ± 14.29 vs 8.84 ± 8.03 pmol/L, t = 9.822, P < 0.01), whereas serum PGI, PGII, and PGR did not differ significantly between bleeding and non-bleeding groups (all P > 0.05). The risk of bleeding was significantly higher among peptic ulcer patients with elevated serum G-17 (> 15 pmol/L) compared to patients with normal or low serum G-17 (73.2% vs 27.4%, χ2 = 40.72, P < 0.01). The area under the ROC curve for serum G-17 was 0.866 ± 0.024, and a cut-off of 9.86 pmol/L yielded 90.2% sensitivity and 68.2% specificity for distinguishing peptic ulcer with and without upper gastrointestinal bleeding.
Serum G-17 is significantly upregulated in peptic ulcer patients and higher levels are predictive of upper gastrointestinal bleeding. Conversely, serum PGI, PGII, and PGR have no predictive value. Further prospective studies are warranted to examine if high G-17 can be used to assess risk of bleeding prior to onset.
Core Tip: Upper gastrointestinal tract bleeding is a severe complication of peptic ulcer that markedly increases mortality. Bleeding risk is elevated by hypersecretion of gastric acid, and secretion rate is associated with serum concentrations of gastrin 17 (G-17), pepsinogen I, and pepsinogen II. Thus, these values may predict bleeding risk among peptic ulcer patients. Indeed, bleeding risk was elevated in patients with G-17 above the normal range (> 15 pmol/L), and serum G-17 > 9.86 pmol/L distinguished bleeding from non-bleeding patients with 90.2% sensitivity and 68.2% specificity. Elevated G-17 may be an effective early predictor of bleeding from peptic ulcer.
- Citation: Wang JX, Cao YP, Su P, He W, Li XP, Zhu YM. Serum gastrin-17 concentration for prediction of upper gastrointestinal tract bleeding risk among peptic ulcer patients. World J Clin Cases 2021; 9(35): 10948-10955
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10948.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10948
Peptic ulcer is among the most common digestive system diseases, and upper gastrointestinal bleeding is the most prevalent complication. Hypersecretion of gastric acid is a critical risk factor for peptic ulcer bleeding, and secretion level is strongly correlated with serum concentration of the gut-derived peptide hormone gastrin-17 (G-17)[1,2]. High serum levels of pepsinogen I (PGI) and PGII are also associated with gastric acid secretion rate by the gastric mucosa and with the incidence of peptic ulcer[3]. Conversely, low serum PGI and PGI/PGII ratio are associated with gastric atrophy. Thus, modulation of G-17, PGI, and PGII secretion levels can be utilized to treat dyspepsia and other gastric diseases[4]. High serum gastrin or PGI/PGII ratio (PGR) will lead to excessive secretion of gastric acid, overcoming the protective capacity of the gastric mucosa and increasing the incidence of peptic ulcer and digestive tract tumors[5]. Indeed, combined detection of serum G-17 and PG is a fundamental screening method for early gastric cancer detection[6]. However, serum PGI, PGII, and G-17 levels among peptic ulcer patients with and without bleeding have not been examined in detail. In the present study, these levels were measured and their predictive values for assessing the risk of peptic ulcer complicated by upper gastrointestinal bleeding tested by univariate and receiver operating characteristic (ROC) curve analyses.
Patients diagnosed with peptic ulcer or peptic ulcer complicated by upper gastr
Approximately 5 mL of fasting (for about 6 h) blood was collected, and the serum was separated for subsequent immunofluorescence measurements of PGI, PGII, and G-17. A serum pepsinogen and G-17 kit was purchased from BIOHIT Healthcare (Hefei) and a microplate reader from Anthos Labtec Instruments (Austria).
The reference ranges of these concentrations are as follows: PGI, 70–165 μg/L; PGII, 3–15 μg/L; PGR, 7%-20%; G-17, 1-15 pmol/L. Serum PGI > 165 μg/L, serum PGII >15 μg/L, PGR > 20, and G17 > 15 pmol/L were regarded as elevated.
All statistical analyses were conducted using SPSS16.0 (SPSS Inc., Chicago, IL, United States). The serum levels of PGI, PGII, G-17, and PGR were compared between bleeding and non-bleeding groups by independent samples t-test. The proportions of peptic ulcer cases complicated by bleeding were compared between patient subgroups stratified according to upper-range cut-offs (e.g., greater or less than 15 pmol/L for G-17) by the Chi-square test. The efficacy of G-17 for predicting bleeding complication was assessed by ROC curve analysis. A P < 0.05 (two-tailed) was considered statistically significant for all tests.
Serum G-17 concentration was significantly higher in peptic ulcer patients with upper gastrointestinal bleeding compared to non-bleeding patients (t = 9.822, P < 0.01) (Table 2). Furthermore, serum G-17 was also significantly higher in gastric ulcer (GU) patients with bleeding and duodenal ulcer (DU) patients with bleeding compared to corresponding non-bleeding subgroups (both P < 0.01). Serum G-17 was slightly higher in DU patients without bleeding compared to GU patients without bleeding (P = 0.209), but did not differ between GU patients with bleeding and DU patients with bleeding (P = 0.940). Serum G-17 was also higher among patients with complex ulcer (CU) complicated by bleeding compared to patients with CU alone, but again the difference did not reach significance (P > 0.05). In contrast to G-17, there were no significant differences in serum PGI, PGII, and PGR between the bleeding and non-bleeding subgroups (all P > 0.05, Table 2). Thus, serum G-17 (but not PGI, PGII, or PGR) may be useful for distinguishing bleeding from non-bleeding peptic ulcer patients.
| Group | Number of cases (n) | PGI (μg/L) | PGII (μg/L) | PGR (PGI/II) | G-17 (pmol/L) | |
| Peptic ulcer group | GU | 45 | 197.90 ± 89.97 | 20.40 ± 13.70 | 11.46 ± 4.70 | 7.24 ± 8.43 |
| DU | 49 | 224.43 ± 80.60 | 23.19 ± 17.05 | 12.58 ± 5.33 | 9.17 ± 6.33 | |
| CU | 13 | 220.48 ± 107.82 | 24.09 ± 10.16 | 10.70 ± 5.86 | 13.11 ± 10.91 | |
| Total | 107 | 225.86 ± 91.10 | 21.68 ± 14.47 | 12.74 ± 5.51 | 13.03 ± 13.20 | |
| Peptic ulcer complicated with upper gastrointestinal bleeding group | GU | 20 | 240.52 ± 83.47 | 22.10 ± 15.30 | 15.15 ± 13.20 | 26.13 ± 13.68a |
| DU | 64 | 250.64 ± 85.70 | 19.22 ± 10.71 | 14.91 ± 5.32 | 25.84 ± 15.00a | |
| CU | 8 | 238.55 ± 95.15 | 18.30 ± 7.51 | 14.70 ± 6.31 | 19.32 ± 8.87 | |
| Total | 92 | 234.98 ± 90.67 | 19.65 ± 12.25 | 14.44 ± 7.39 | 25.34 ± 14.29a | |
Serum G-17 ranging from 1 to 15 pmol/L is considered normal. Therefore, we first compared bleeding incidence between peptic ulcer patients stratified by a serum G-17 cut-off of 15 pmol/L. Among peptic ulcer patients with serum G-17 > 15 pmol/L, approximately 73.2% exhibited bleeding compared to only 27.4% of patients with serum G-17 < 15 pmol/L (χ2 = 40.72, P<0.01) (Table 3). In contrast, the proportions of bleeding cases did not differ between groups stratified by similar upper limits in serum PGI, PGII, and PGR (χ2 = 0.395, χ2 = 0.009, and χ2 = 2.242, respectively, all P > 0.05).
| Serum G-17 level | Peptic ulcer, n (%) | Peptic ulcer complicated by bleeding, n (%) | Total, n | P value |
| Normal (≤ 15 pmol/L) | 85 (72.6) | 32 (27.4) | 117 | |
| Elevated (> 15 pmol/L) | 22 (26.8) | 60 (73.2) | 82 | |
| Total | 107 | 92 | 199 | 0.000 |
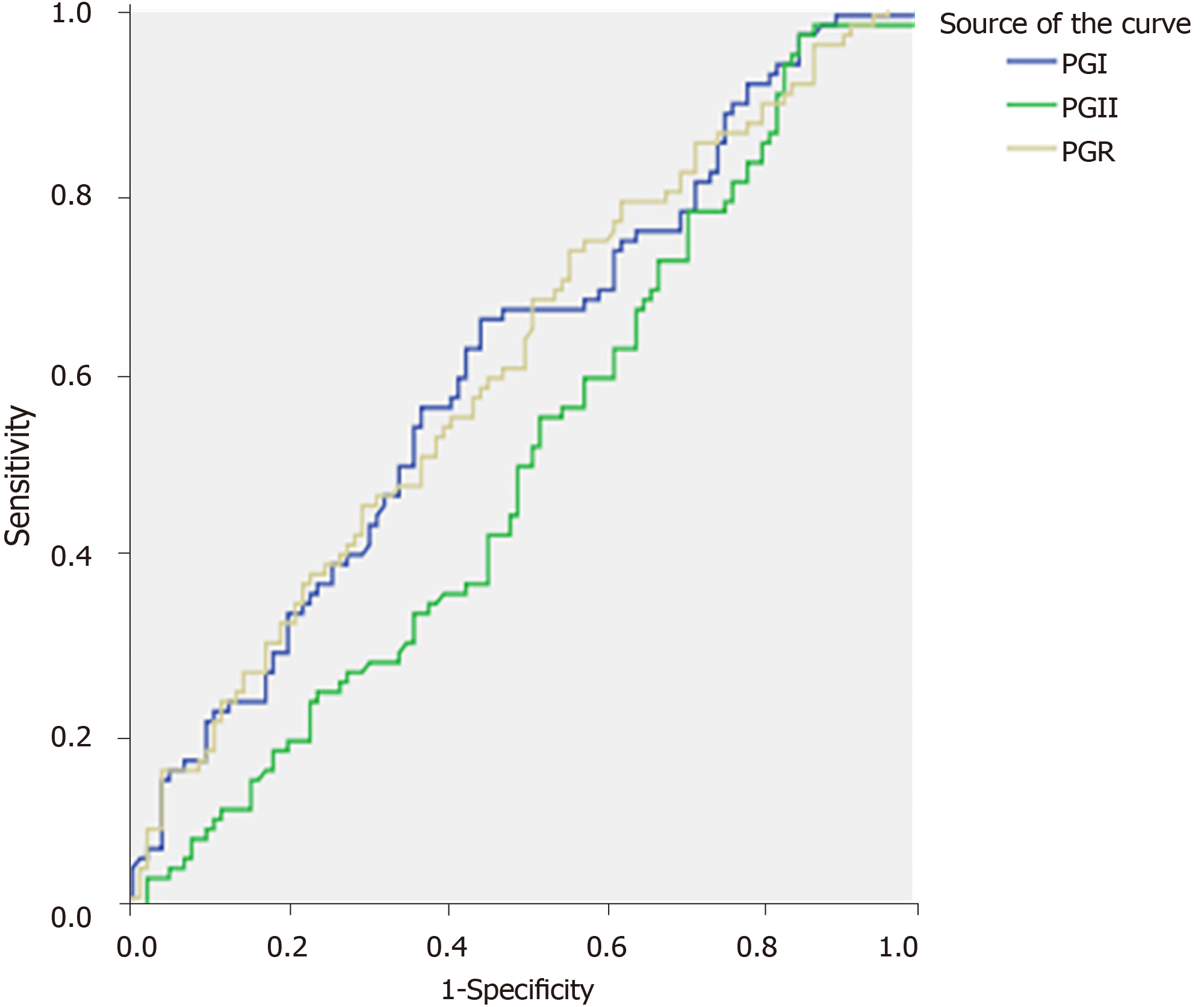
The area under the ROC curve (AUC) of serum G-17 for peptic ulcer patients with or without upper gastrointestinal bleeding was 0.866 ± 0.024. According to the Youden index, the optimal serum G-17 cut-off value for distinguishing bleeding from non-bleeding patients was 9.86 pmol/L, yielding 90.2% sensitivity and 68.2% specificity (Figure 1). In contrast, the AUC values for serum PGI, PGII, and PGR were only 0.599, 0.496, and 0.598, respectively, indicating low efficacy for distinguishing bleeding from non-bleeding peptic ulcer (Figure 2).
We report that serum gastrin concentration is significantly elevated above normal reference ranges in patients with gastric or duodenal ulcer, and is even higher among patients with peptic ulcer complicated by upper gastrointestinal bleeding. Indeed, the proportion of peptic ulcer patients with serum G-17 concentrations above the upper limit of normal (approximately 15 pmol/L) was significantly greater among those with bleeding compared to patients without bleeding (simple GU). Furthermore, ROC curve analysis revealed that G-17 > 9.86 pmol/L distinguished bleeding from non-bleeding GU patients with high sensitivity and moderate specificity, suggesting that G-17 evaluation prior to endoscopic examination could be employed to assess bleeding risk, thereby facilitating timely intervention, such as a higher proton pump inhibitor dose[7], and improving overall prognosis. Serum G-17 was also higher among complex ulcer (CU) patients with bleeding compared to CU patients without bleeding, although the difference did not reach statistical significance due to the small sample size. Hence, larger-scale studies are warranted to fully assess the efficacy of high G-17 for prediction of bleeding among GU patient subgroups, including prospective studies directly evaluating the association between baseline G-17 and future bleeding.
Approximately 10% of individuals will develop gastric or duodenal ulcer over a lifetime, but the majority can be completely cured with treatment. However, GU complicated by acute upper gastrointestinal bleeding increases treatment difficulty and can be life-threatening if uncontrolled[8]. Therefore, identifying predictive indices for upper gastrointestinal bleeding is critical for improving prognosis among more severely afflicted GU patients.
The incidence and severity of peptic ulcer are significantly associated with the production rates of gastric acid, gastrin, and pepsin[9]. Gastrin is a pre-hormone secreted by G-cells in the gastric antrum that is then converted to active molecules, mainly G-34 and G-17, of which G-17 accounts for approximately 80%–90% of the total bioactivity. The main physiological functions of gastrin are to stimulate gastric acid and PG secretion, regulate gastrointestinal motility, increase gastric mucosal blood flow, and promote gastric mucosal growth and nutrition[10]. However, excessive gastric acid secretion by high serum G-17 may destroy the gastric mucosa, leading to ulcer formation and possibly bleeding. In the presence of gastric acid, PG is rapidly converted into active pepsin, which can cause the dissolution of blood clots and thus impair the control of bleeding. Consequently, it is rational to speculate that high G-17 and high PG may predict the risk of bleeding among peptic ulcer patients. However, serum PG was unrelated to bleeding incidence, possibly because many factors can influence PG production aside from gastric acid secretion rate. In fact, the secretion of gastric acid, gastrin, and PG can be affected by multiple factors. For instance, HP infection increases gastrin secretion[11]. Hence, all patients enrolled in the present study were HP-positive to eliminate the effects of HP and related factors. In addition, long-term use of proton pump inhibitors may cause a compensatory increase in gastrin secretion[12], so patients receiving proton pump inhibitors were also excluded from the present study. Furthermore, peptic ulcer patients with other conditions associated with bleeding risk were eliminated, so the difference between groups likely reflects disease severity, further underscoring the importance of serum G-17 for evaluation of patient condition and prognosis. On the other hand, as yet unidentified factors influencing PG may have obscured differences between bleeding and non-bleeding patients.
Such PG measurements may still have clinical value. Pepsinogen can be classified as PGI or PGII according to immunogenicity, and these isoforms are generated by different regions of the gastric mucosa. Therefore, separate detection of serum PGI and PGII can provide an estimate of region-specific gastric acid secretion rate[13]. Activated pepsin can both enhance the risk of peptic ulcer and impair coagulation, potentially enhancing bleeding risk and diminishing treatment efficacy. In this study, however, serum PGI, PGII, and PGR were not associated with bleeding, possibly because PG secretion is affected to a greater extent by gastric mucosal atrophy, dysplasia, and other diseases than by ulcer (with or without bleeding)[14].
Although we controlled for multiple factors potentially influencing the secretion rates of G-17 and PG, factors such as drinking[15], smoking[16], age[17], and gastric mucosal lesions[18] may have differed between the groups. Thus, larger-scale studies allowing for more extensive subgroup analyses are required to validate the current finding that serum G-17 is a major predictive factor for bleeding among peptic ulcer patients.
In summary, serum G-17 is significantly elevated in peptic ulcer complicated by bleeding. Serum G-17 > 9.86 pmol/L distinguishes bleeding from non-bleeding cases with high sensitivity, suggesting its clinical utility for predicting bleeding risk.
Peptic ulcer is a relatively common chronic gastrointestinal disorder most frequently caused by Helicobacter pylori infection or long-term non-steroid anti-inflammatory drug administration. Severe cases are often complicated by bleeding, which can exacerbate symptoms, cause anemia, and lead to life-threatening hemorrhage. Hypersecretion of gastric acid increases the risk and severity of peptic ulcer. Gastric acid secretion is controlled by the hormones gastrin-17 (G-17), pepsinogen I (PGI), and pepsinogen II (PGII), suggesting that these factors may be predictive of bleeding among peptic ulcer patients.
If left untreated, peptic ulcer can result in gastrointestinal bleeding and gastric tumors. Therefore, accessible biomarkers such as serum factors predictive of bleeding risk are important tools for early diagnosis and treatment guidance, and may reduce the incidence of severe complications.
To examine if serum G-17, PGI, PGII, and (or) PGI/PGII ratio (PGR) can predict bleeding risk among peptic ulcer patients.
We compared serum G-17, PGI, PGII, and PGR between 199 peptic ulcer patients with bleeding (n = 92) or without bleeding (n = 107), and then assessed the efficacy of each factor for bleeding prediction by receiver operating characteristic curve analysis.
Serum G-17 was significantly higher among peptic ulcer patients with upper gastrointestinal bleeding, whereas serum PGI, PGII, and PGR did not differ significantly between bleeding and non-bleeding patients. A serum G-17 greater than 9.86 pmol/L distinguished bleeding from non-bleeding patients with 90.2% sensitivity and 68.2% specificity.
Elevated serum G-17 is predictive of bleeding risk among peptic ulcer patients. Lowering serum G-17 should be a major goal of clinical intervention.
Prospective studies are warranted to assess if elevated serum G-17 can predict bleeding complication prior to disease onset. The development of drugs able to regulate G-17 secretion is also desired to help reduce bleeding risk.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farid K S-Editor: Wang LL L-Editor: Webster JR P-Editor: Yu HG
| 1. | Schubert ML. Physiologic, pathophysiologic, and pharmacologic regulation of gastric acid secretion. Curr Opin Gastroenterol. 2017;33:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Schubert ML, Rehfeld JF. Gastric Peptides-Gastrin and Somatostatin. Compr Physiol. 2019;10:197-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Zhou B, Chen X, Huang B, Hu YH, Tang GR Zhang J, Lin Q. Changes in serum pepsinogen levels and their value as a predictor of treatment outcomes in children with pepticulcer. J Paediatr Child Health. 2019;55:1103-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, Sipponen P, Shimosegawa T. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Burkitt MD, Varro A, Pritchard DM. Importance of gastrin in the pathogenesis and treatment of gastric tumors. World J Gastroenterol. 2009;15:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Yuan L, Zhao JB, Zhou YL, Qi YB, Guo QY, Zhang HH, Khan MN, Lan L, Jia CH, Zhang YR, Ding SZ. Type I and type II Helicobacter pylori infection status and their impact on gastrin and pepsinogen level in a gastric cancer prevalent area. World J Gastroenterol. 2020;26:3673-3685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Tringali A, Manta R, Sica M, Bassotti G, Marmo R, Mutignani M. Comparing intravenous and oral proton pump inhibitor therapy for bleeding peptic ulcers following endoscopic management: a systematic review and meta-analysis. Br J Clin Pharmacol. 2017;83:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Rotondano G. Epidemiology and diagnosis of acute nonvariceal upper gastrointestinal bleeding. Gastroenterol Clin North Am. 2014;43:643-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Park SM, Park HS. G- and D-cell populations, serum and tissue concentrations of gastrin and somatostatin in patients with peptic ulcer diseases. Korean J Intern Med. 1993;8:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Germaná B, Di Mario F, Cavallaro LG, Moussa AM, Lecis P, Liatoupolou S, Comparato G, Carloni C, Bertiato G, Battiestel M, Papa N, Aragona G, Cavestro GM, Iori V, Merli R, Bertolini S, Caruana P, Franzé A. Clinical usefulness of serum pepsinogens I and II, gastrin-17 and anti-Helicobacterpylori antibodies in the management of dyspeptic patients in primary care. Dig Liver Dis. 2005;37:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Liu W, Sun Y, Yuan Y. Analysis of serum gastrin-17 and Helicobacter pylori antibody in healthy Chinese population. J Clin Lab Anal. 2020;34:e23518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Waldum HL, Hauso Ø, Fossmark R. The regulation of gastric acid secretion - clinical perspectives. Acta Physiol (Oxf). 2014;210:239-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Miraglia C, Moccia F, Russo M, Scida S, Franceschi M, Crafa P, Franzoni L, Nouvenne A, Meschi T, Leandro G, De' Angelis GL, Di Mario F. Non-invasive method for the assessment of gastric acid secretion. Acta Biomed. 2018;89:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Kwak MS, Kim N, Lee HS, Lee HE, Jung HC, Song IS. Predictive power of serum pepsinogen tests for the development of gastric cancer in comparison to the histologic risk index. Dig Dis Sci. 2010;55:2275-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Singer MV, Leffmann C, Eysselein VE, Calden H, Goebell H. Action of ethanol and some alcoholic beverages on gastric acid secretion and release of gastrin in humans. Gastroenterology. 1987;93:1247-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Fletcher DR, Shulkes A, Hardy KJ. The effect of cigarette smoking on gastric acid secretion and gastric mucosal blood flow in man. Aust N Z J Med. 1985;15:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Pilotto A, Vianello F, Di Mario F, Plebani M, Farinati F, Azzini CF. Effect of age on gastric acid, pepsin, pepsinogen group A and gastrin secretion in peptic ulcer patients. Gerontology. 1994;40:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Parhusip DH, Siregar GA, Dairi LB. The Difference of Serum Gastrin-17 Level Based on Gastritis Severity and Helicobacter Pylori Infection. Open Access Maced J Med Sci. 2019;7:1266-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |