Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10355
Peer-review started: July 8, 2021
First decision: July 26, 2021
Revised: July 30, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 26, 2021
Processing time: 136 Days and 19.4 Hours
Venom-induced consumption coagulopathy (VICC) is characterized by coagulation dysfunction accompanied by decreased coagulation factor activity and fibrinogen (FBG) concentrations. We report a patient with VICC caused by snake bite who manifested persistent FBG deficiency without abnormal coagulation factor activity. This information may be helpful in diagnosing and treating VICC.
A 49-year-old man who had been bitten by a snake 13 h previously was admitted to the Emergency Department of our hospital with visible swelling of a finger and a bleeding puncture site. The provisional diagnosis was VICC, this being made based on persistent bleeding from the puncture site and subcutaneous hemo
Hemorrhagic snake venom can result in coagulation dysfunction characterized by persistent FBG deficiency without abnormal coagulation factor activity.
Core Tip: Venom-induced consumption coagulopathy (VICC) is characterized by decreased coagulation factor activity and fibrinogen (FBG) deficiency. Hemorrhage-inducing snake venom contains several ingredients that directly or indirectly consume fibrinogen through multiple mechanisms. We report a rare case with persistent afibrinogenemia without abnormal coagulation factor activity after snake bite. Our report may assist the diagnosis and treatment of FBG deficiency in patients with VICC.
- Citation: Xu MH, Li J, Han L, Chen C. Persistent fibrinogen deficiency after snake bite: A case report. World J Clin Cases 2021; 9(33): 10355-10361
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10355.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10355
Snake bite is a major and often neglected public health problem. An estimated 1.8 million people are bitten by snakes annually worldwide and more than 125000 of them die as a result[1]. In China, there are approximately 50 species of poisonous snakes and 10 venomous snakes and the death rate from snake bite is 5%–10%. Coagulation dysfunction is the most common consequence of snake bite, venom-induced consumption coagulopathy (VICC) being the most important form of such coagulation dysfunction. Coagulants in venom can cause significant declines in coagulation factors, platelet counts, and fibrinogen (FBG) concentrations, resulting in failure of blood to coagulate and severe bleeding[2,3]. Here, we report an uncommon case of snake-bite-induced VICC that was characterized by persistent FBG deficiency without abnormal coagulation factor activity. Treatment centered around continuous infusions of FBG was successful. Our findings may assist diagnosis and treatment of FBG deficiency in patients with VICC. To the best of our knowledge, no similar case has been reported. Because this is only a case report, the need for ethical approval was waived by the Ethics Committee of Tongji Hospital, which complies with the relevant institutional and national policies.
A 49-year-old man was bitten by a snake, with his chief complaint being bleeding for 13 h after that bite.
The patient was bitten on his right index finger by a snake (species unknown) while working on a construction site. The bite caused local swelling, pain, numbness, and bleeding, without dizziness, fever, diarrhea, consciousness disorders, or other symptoms. He was rushed to a local hospital for treatment, where he underwent puncture site drainage and antivenin injection, administration of coagulation factor supplements and other treatments. He was transferred to our hospital 13 h after the snake bite.
The patient was in good health, with no history of chronic or infectious diseases.
The patient had no history of smoking, drinking, or familial cancers.
On admission, the patient’s temperature was 36.9°C, heart rate 107 bpm, respiratory rate 25 breaths/min, blood pressure 91/63 mmHg and oxygen saturation in room air 98%. There was visible swelling of the right index finger and the puncture site was still oozing blood. The patient’s right upper limb had been coated with jidesheng sheyao (a local herbal medicine, specific ingredients unknown).
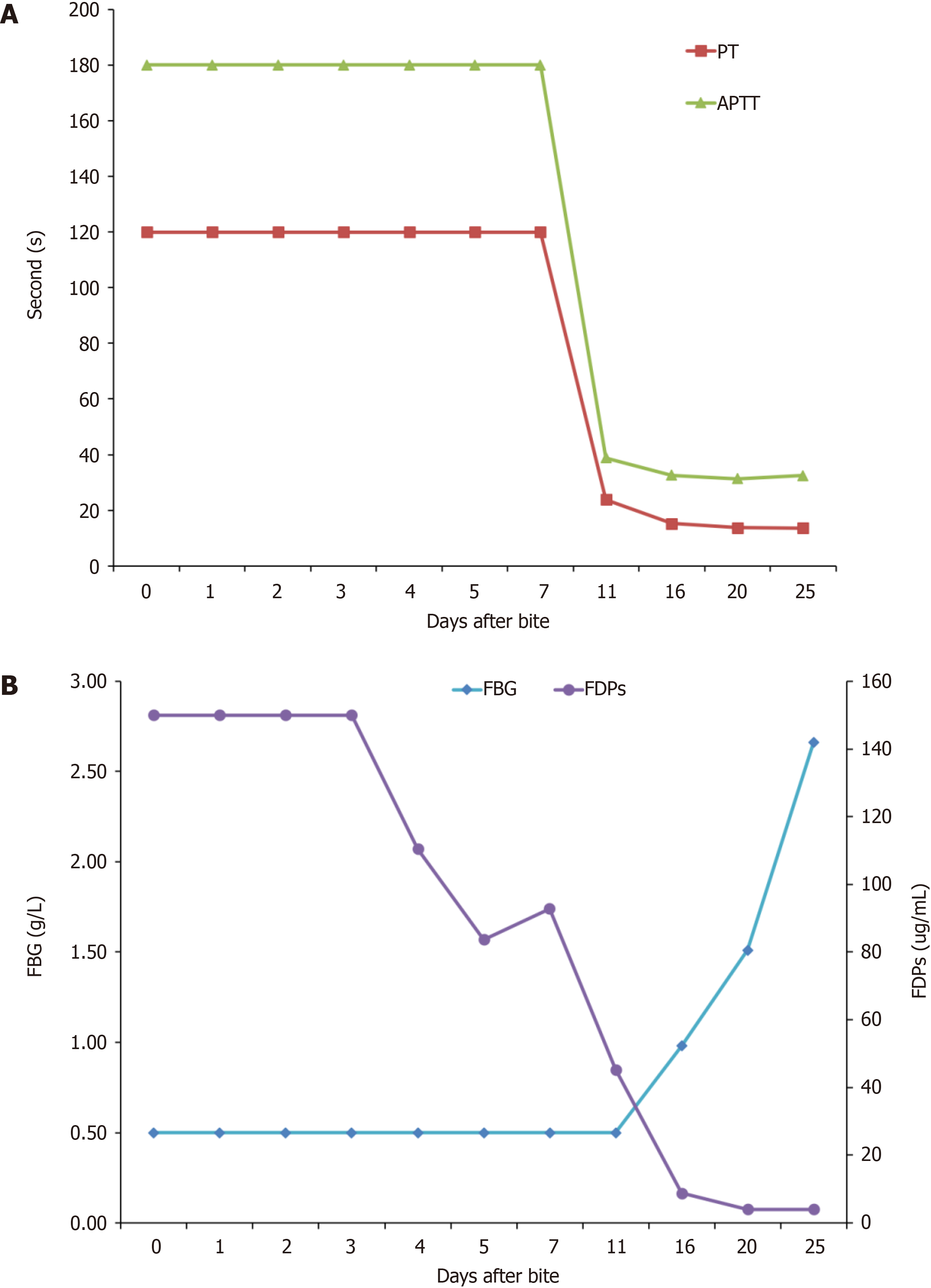
Laboratory tests for coagulation and fibrinolysis on admission showed a prothrombin time (PT) of > 120 s, international normalized ratio (INR) > 10, FBG < 0.5 g/L, activated partial thromboplastin time > 180 s, thrombin time (TT) > 240 s, D-dimer > 60 μg/mL FBG equivalent units, and FBG degradation products > 150 mg/mL. The timing and results of coagulation and fibrinolysis tests are shown in Table 1. Multiple markers were beyond the limits of detection for the first 7 d after the bite. Coagulation factor activity levels are shown in Table 2. Coagulation factor activity decreased slightly after the bite, normalizing within 4 d (Figure 1).
| Days after bite | PT | INR | FBG | APTT | TT | D-D | FDPs |
| 11.5-14.5 (s) | 0.5-1.2 | 2.0-4.0 (g/L) | 29-42 (s) | 14-19 (s) | < 0.5 (μg/L) | < 0.5 (μg/L) | |
| 0 | > 120 | > 10 | < 0.5 | > 180 | > 240 | > 60 | > 150 |
| 1 | > 120 | > 10 | < 0.5 | > 180 | > 240 | > 60 | > 150 |
| 2 | > 120 | > 10 | < 0.5 | > 180 | > 240 | > 60 | > 150 |
| 3 | > 120 | > 10 | < 0.5 | > 180 | > 240 | > 60 | > 150 |
| 4 | > 120 | > 10 | < 0.5 | > 180 | > 240 | > 60 | 110.5 |
| 5 | > 120 | > 10 | < 0.5 | > 180 | > 240 | > 60 | 83.7 |
| 7 | > 120 | > 10 | < 0.5 | > 180 | 236.3 | > 60 | 92.8 |
| 11 | 23.8 | 2.1 | < 0.5 | 38.8 | 33.3 | 27.53 | 45.3 |
| 16 | 15.2 | 1.19 | 0.98 | 32.6 | 18.7 | 2.35 | 8.8 |
| 20 | 13.7 | 1.05 | 1.51 | 31.4 | 18.2 | 0.28 | < 4.0 |
| 25 | 13.6 | 1.04 | 2.66 | 32.5 | 15.5 | < 0.22 | < 4.0 |
| Days after bite | II | V | VIII | X | XI | XII |
| 70%-120% | 70%-120% | 60%-150% | 70%-120% | 60%-150% | 50%-150% | |
| 0 | 55 | 41 | 246 | 59 | 85 | 51 |
| 4 | 76 | 86 | 150 | 69 | 60 | 44 |
| 7 | 82 | 79 | 94 | 73 | 79 | 49 |
| 10 | 95 | 107 | 127 | 89 | 74 | 68 |
| 15 | 89 | 101 | 112 | 99 | 83 | 72 |
| 20 | 93 | 86 | 134 | 84 | 96 | 85 |
| 25 | 113 | 94 | 129 | 105 | 95 | 103 |
Vascular ultrasonography revealed intermuscular vein thrombosis in the left upper extremity and superficial vein thrombosis in the left lower extremity.
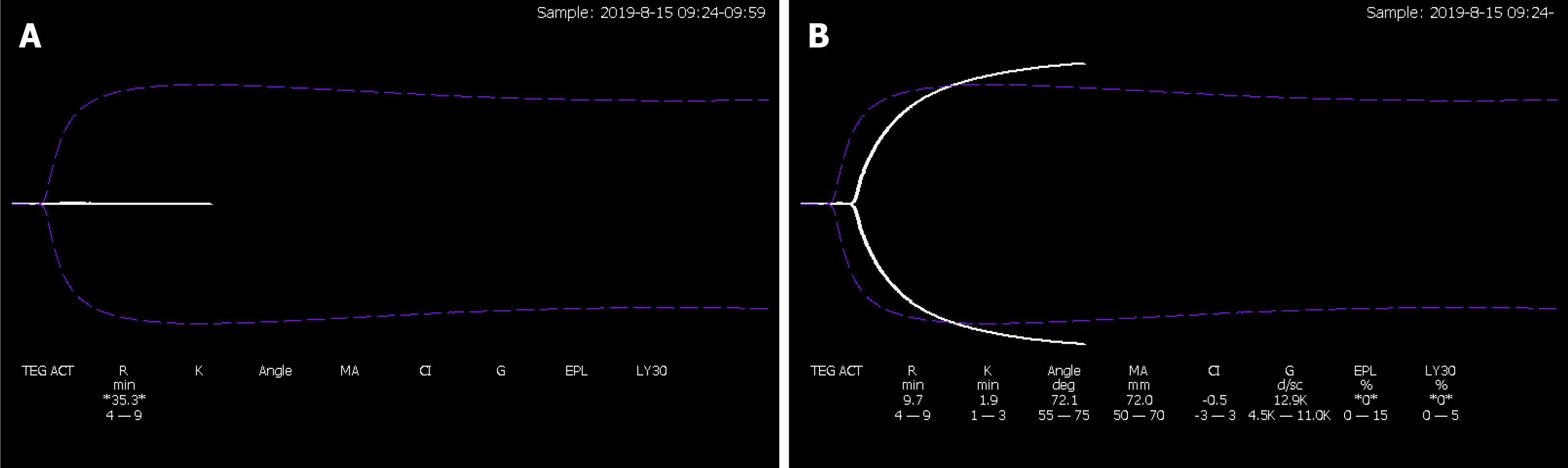
Five days after the bite, thromboelastography (TEG) showed R time, 35.3 min, and no coagulation curve was formed (Figure 2A). After addition of 2 g/L FBG preparation to whole blood samples in vitro, the coagulation curve pattern was close to normal (Figure 2B): R time, 9.7 min; K time, 1.9 min; α angle, 72.1, maximum amplitude, 72.0; coagulation index (CI), 0.5; and amplitude at 30 min, 0.0.
The final diagnosis of the presented case was snake-bite-related VICC characterized by persistent afibrinogenemia without abnormal coagulation factor activity.
The following treatment was administered. Cryoprecipitate (CRYO) 6 U, fresh frozen plasma (FFP) 600 mL, and human FBG 0.5 g were first injected intravenously 13 h after the bite. For 5 d thereafter, FFP, CRYO and FBG were administered daily as replacement therapy. Timing and specific doses are shown in Table 3.
| Days after bite | CRYO (u) | FFP (mL) | FBG (g) |
| 0 | 6 | 600 | 0.5 |
| 1 | 16 | 1600 | 0.5 |
| 2 | 30.5 | 3450 | 1.0 |
| 3 | 11 | 1900 | 0.5 |
| 4 | 5 | 600 | 1.0 |
| 5 | — | — | 2.0 |
| 7 | — | — | 2.0 |
| 9 | — | — | 2.0 |
| 12 | — | — | 1.0 |
During treatment, the swelling in the patient’s right upper limb improved significantly and his subcutaneous hemorrhage resolved by day 11. Twenty days after the bite, the results of TEG returned to normal. The patient’s afibrinogenemia lasted for 11 d, after which it began to improve, resolving completely by day 25 (Figure 1). All coagulation-related laboratory results returned to normal by 25 d after the bite. The patient was discharged 26 d after the bite and followed up for 12 mo after discharge. No further hemorrhagic events occurred.
Hemorrhagic snake venom can cause consumption of various coagulation factors and FBG through multiple mechanisms, leading to VICC[4,5]. VICC is often characterized by undetectable PT prolongation[6], increased INR, and decreased FBG, usually accompanied by a significant increase in D-dimer concentration. Decreases in FBG concentrations occur in all types of VICC[4].
There are three main protease families in hemorrhagic snake venom: Snake venom metalloproteinases (SVMPs), serine proteinases (SVSPs), and phospholipase A2. SVMPs promote conversion of prothrombin (II) into thrombin (IIa) and activate the fibrinolytic system, resulting in rapid consumption of FBG and various coagulation factors[1]. SVSPs commonly exhibit thrombin-like fibrinolytic functional activity. However, unlike thrombin, SVSPs are highly selective, acting directly on the α-chain of FBG and promoting polymerization of the resulting fibrin monomers[7]. The polymerization products are unstable and easily soluble by plasmin. Therefore, SVSPs only consume FBG and do not activate the coagulation pathway, leading to FBG deficiency only and a less severe type of VICC, in which other coagulation factors are generally unaffected.
One case report described a woman who was bitten by a rattlesnake and had an FBG deficiency and low platelet count[2]. In an Australian survey, 112 of 138 patients with complete VICC lacked FBG and factors V and VIII[3]. Our patient had persistent FBG deficiency without detectable changes in coagulation factor activity. To the best of our knowledge, this combination has not previously been reported. TEG showed that the coagulation curve was close to normal after addition of FBG preparation[8]. Given that our patient’s coagulation factors and platelet counts were normal, we believe that he had the type of coagulation dysfunction that is mediated via the above-mentioned SVSPs pathway. This type of dysfunction is characterized by FBG deficiency without abnormal coagulation factor activity. Thus, SVSPs were likely the main active components of the snake venom and responsible for the subsequent development of VICC. Our patient’s persistent afibrinogenemia may have been attributable to deposition into and subsequent slow release of some active components of snake venom from his hand, resulting in long-term toxicity. The specific mechanism of underlying persistent afibrinogenemia requires further elucidation.
The mainstays of treatment for VICC are antivenin and replacement therapy. The main replacement therapies are infusions of CRYO, FFP and FBG, and thus, directly supplementing these coagulation factors. In our case, early infusion of coagulation factors/plasma/FBG did not result in complete recovery of coagulation function, and TEG indicated that there was a serious deficiency of FBG. Therefore, continuous large doses of FBG were infused as follow-up treatment, achieving restoration of normal coagulation with FBG concentrations to 0.5–1.0 g/L and return of PT and INR to near normal range[4]. Thus, FBG infusion at an early stage has a positive effect, despite its concentration recovering slowly[9].
Hemorrhagic snake venom can result in coagulation dysfunction characterized by persistent FBG deficiency without abnormal coagulation factor activity. In patients with VICC, replacement therapy centered around FBG infusion can achieve restoration of coagulation function.
We thank Dr. Reynolds T, MBBS, FRACP, for editing the English text of a draft of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Nucci G, Joalsen I S-Editor: Gao CC L-Editor: Kerr C P-Editor: Li JH
| 1. | Slagboom J, Kool J, Harrison RA, Casewell NR. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br J Haematol. 2017;177:947-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Witham WR, McNeill C, Patel S. Rebound coagulopathy in patients with snakebite presenting with marked initial coagulopathy. Wilderness Environ Med. 2015;26:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Isbister GK, Scorgie FE, O'Leary MA, Seldon M, Brown SG, Lincz LF; ASP Investigators. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost. 2010;8:2504-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Berling I, Isbister GK. Hematologic effects and complications of snake envenoming. Transfus Med Rev. 2015;29:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Isbister GK. Snakebite doesn't cause disseminated intravascular coagulation: coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost. 2010;36:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Pongpit J, Limpawittayakul P, Juntiang J, Akkawat B, Rojnuckarin P. The role of prothrombin time (PT) in evaluating green pit viper (Cryptelytrops sp) bitten patients. Trans R Soc Trop Med Hyg. 2012;106:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost. 2009;35:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Nag I, Datta SS, De D, Pal P, Das SK. Role of thromboelastography in the management of snake bite: A case report from India. Transfus Apher Sci. 2017;56:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Valenta J, Stach Z, Michálek P. Envenoming by Crotalid Snake Chinese Moccasin Agkistrodon Acutus Bite - A Case Report. Prague Med Rep. 2015;116:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |