Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10088
Peer-review started: April 27, 2021
First decision: June 13, 2021
Revised: June 14, 2021
Accepted: October 11, 2021
Article in press: October 11, 2021
Published online: November 26, 2021
Processing time: 208 Days and 11.8 Hours
Although small colorectal neoplasms (< 10 mm) are often easily resected endoscopically and are considered to have less malignant potential compared with large neoplasms (≥ 10 mm), some are invasive to the submucosa.
To clarify the clinicopathological features of small T1 colorectal cancers.
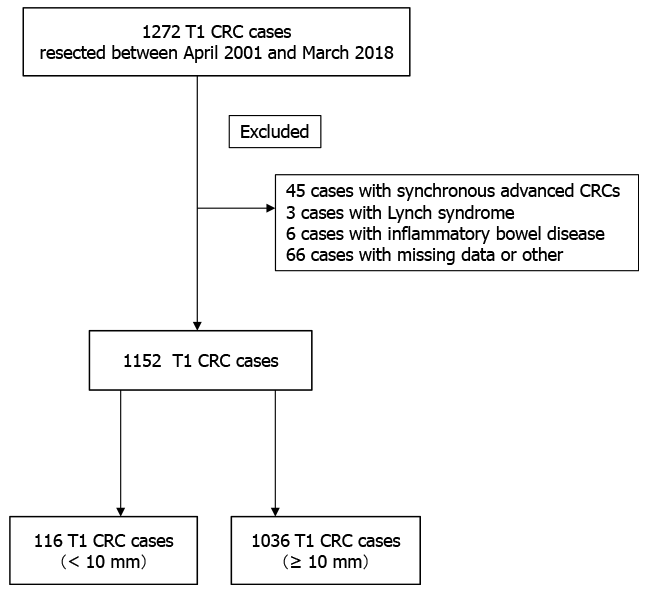
Of 32025 colorectal lesions between April 2001 and March 2018, a total of 1152 T1 colorectal cancers resected endoscopically or surgically were included in this study and were divided into two groups by tumor size: a small group (< 10 mm) and a large group (≥ 10 mm). We compared clinicopathological factors including lymph node metastasis (LNM) between the two groups.
The incidence of small T1 cancers was 10.1% (116/1152). The percentage of initial endoscopic treatment in small group was significantly higher than in large group (< 10 mm 74.1% vs ≥ 10 mm 60.2%, P < 0.01). In the surgical resection cohort (n = 798), the rate of LNM did not significantly differ between the two groups (small 12.3% vs large 10.9%, P = 0.70). In addition, there were also no significant differences between the two groups in pathological factors such as histological grade, vascular invasion, or lymphatic invasion.
Because there was no significant difference in the rate of LNM between small and large T1 colorectal cancers, the requirement for additional surgical resection should be determined according to pathological findings, regardless of tumor size.
Core Tip: This is a retrospective study to evaluate the clinicopathological features in T1 colorectal cancers. We compared clinicopathological factors including lymph node metastasis (LNM) between the two groups: A small group (< 10 mm) and a large group (≥ 10 mm). Since there was no significant difference in the rate of LNM followed by histological grade, vascular invasion, or lymphatic invasion, between small and large T1 colorectal cancers, the requirement for additional surgical resection should be determined according to pathological findings, regardless of tumor size.
- Citation: Takashina Y, Kudo SE, Ichimasa K, Kouyama Y, Mochizuki K, Akimoto Y, Maeda Y, Mori Y, Misawa M, Ogata N, Kudo T, Hisayuki T, Hayashi T, Wakamura K, Sawada N, Baba T, Ishida F, Yokoyama K, Daita M, Nemoto T, Miyachi H. Clinicopathological features of small T1 colorectal cancers. World J Clin Cases 2021; 9(33): 10088-10097
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10088.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10088
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cancer cause of death worldwide[1]. Lymph node metastasis (LNM) is present in approximately 10% of T1 CRCs that require surgical resection with lymph node dissection[2,3]. Therefore, risk stratification for LNM in T1 CRC is necessary. According to the current guidelines, the risk factors for LNM are lymphovascular invasion, histological differentiation, depth of submucosal invasion, and tumor budding. Surgical treatment is recommended if any of these factors are identified in the pathological diagnosis of endoscopically resected specimens[4-8], whereas follow-up by endoscopic resection alone would be acceptable when there are no risk factors. However, tumor size is not mentioned in these guidelines. Although tumor size was reported to be a risk factor for prognosis in advanced cancers, few reports have investigated the correlation between tumor size and clinicopathological features including the presence of LNM in T1 CRC[9,10]. Recently, the “resect and discard” strategy has emerged. In this approach, polyps smaller than 10 mm that are preoperatively diagnosed by magnifying narrow-band imaging do not need to be sent for path
A total of 32025 colorectal lesions (< 10 mm 21620 lesions, ≥ 10 mm 10405 lesions), excluding advanced cancers, were endoscopically or surgically resected at Showa University Northern Yokohama Hospital (Yokohama, Japan) between April 2001 and March 2018. Of these, 1272 were T1 CRCs. We excluded 45 patients who had synchronous advanced CRC, three patients with Lynch syndrome, six patients with inflammatory bowel disease, and 66 patients whose specimens were impossible to evaluate pathologically in detail because of damage or loss. In total, 1152 cases were included (Figure 1). Patient characteristics analyzed included age, sex, tumor location, tumor size, polypoid/non-polypoid growth, adenoma component, tumor morphology, initial treatment, depth of submucosal invasion, histological grade, vascular invasion, lymphatic invasion, tumor budding, and LNM. Surgical specimens were used as the gold standard for the presence of LNM. We classified tumor morphology into three types according to the Paris classification and Kudo’s classification: flat type (IIa, laterally spreading tumor), protruded type (Is, Ip, and Isp), and depressed type (IIc, IIa + IIc, IIc + IIa, Is + IIc, and Ip + IIc)[12].
All resected specimens were retrieved and immediately fixed in 10% buffered formalin and were observed with a focus on the pit pattern using a stereomicroscope. They were then cut at the point where the deepest invasion area could be exposed on the cut end surface. The other histological specimens were cut into parallel 2- to 3-mm-thick sections and stained with hematoxylin and eosin (H&E). Tumor size was measured after formalin fixation. All specimens were diagnosed on the basis of the 2019 World Health Organization Classification of Tumors[13] and the current Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines[6]. Histological grade was classified in view of the World Health Organization criteria as follows: well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, poorly differentiated adenocarcinoma (Por), and mucinous carcinoma (Muc). In this study, a Por/Muc component was considered present if any part of the lesion contained any of these features. The depth of submucosal invasion was classified according to the JSCCR classification as < 1000 μm (T1a) and ≥ 1000 μm (T1b)[6]. Vascular invasion was diagnosed by double staining with H&E and Victoria blue (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) and lymphatic invasion was diagnosed by H&E staining and immunostaining with D2-40 antibody (Dako North America Inc., Carpinteria, CA, United States). Tumor budding is defined as a cancer cell nest consisting of one or fewer than five cells that infiltrate the interstitium at the invasive margin of the cancer. On selecting the region where tumor budding is the greatest, the front of the tumor growth is observed at 200 × magnification to count the number of tumor buds: BD1, 0-4; BD2, 5-9; and BD3, ≥ 10[14].
Nominal and ordinal variables are expressed as frequencies and percentages. Continuous variables are reported as mean ± SD. Continuous variables were compared using Student t-tests, while dichotomous variables were compared using chi-squared or Fisher’s exact tests, as appropriate. All statistical analyses were performed using R for Windows 4.0.3. All P values were two sided, and P < 0.05 was considered statistically significant.
This study was approved by the institutional review board of Showa University Northern Yokohama Hospital (approval No. 19H057) and was registered with the University Hospital Medical Network Clinical Trials Registry (UMIN000043922). Written informed consent was obtained from all patients before treatment.
Patients’ characteristics are shown in Table 1. Of the included patients, 116 cases (10.1%) were included in the small group (tumors of less than 10 mm) and 1036 cases (89.9%) were included in the large group (tumors of 10 mm or larger). The mean age was 66.4 years. Seven hundred twenty-nine patients (63.2%) were male, and 788 patients (68.4%) had left-sided CRC. The number of patients with depressed type morphology was 280 (24.3%). The number of lesions that were initially selected for endoscopic treatment was 710 (61.6%). Seven hundred ninety-eight (69.3%) T1 CRCs were surgically resected. Vascular invasion was observed in 322 (28.0%) cases, and lymphatic invasion was observed in 342 (29.7%) cases. Among the operated cases, 11.0% (88/798) had LNM.
| Age, yr | 66.4 ± 11.6 |
| Sex (male/female) | 729 (63.3)/423 (36.7) |
| Location (left-sided/right-sided) | 788 (68.4)/364 (31.6) |
| Tumor size (mm) | 21.2 ± 13.3 |
| Polypoid/non-polypoid growth (polypoid/non-polypoid) | 714 (62.0)/438 (38.0) |
| Adenomatous component (±) | 466 (40.5)/686 (59.5) |
| Morphology (flat/ protruded/ depressed) | 397 (34.5)/475 (41.2)/280 (24.3) |
| Initial treatment (endoscopic/surgical) | 710 (61.6)/442 (38.4) |
| Surgical resection1 | 798 (69.3)/354 (30.7) |
| Depth of invasion (T1b/T1a) | 826 (71.7)/326 (28.3) |
| Histological grade (Por or Muc2/tub1 or tub2) | 58 (5.0)/1094(95.0) |
| Vascular invasion (±) | 322 (28.0)/830 (72.0) |
| Lymphatic invasion (±) | 342 (29.7)/810 (70.3) |
| Tumor budding (BD 2 or 3/BD 1) | 242 (21.0)/910 (79.0) |
Comparison of clinicopathological characteristics between < 10 mm and ≥ 10 mm tumors in total cohort T1 CRCs are shown in Table 2. Compared with T1 CRCs of ≥ 10 mm, T1 CRCs of < 10 mm had a significantly higher percentage of depressed type morphology (< 10 mm 51.7% vs ≥ 10 mm 21.2%, P < 0.01), a significantly lower percentage of polypoid growth (PG) (< 10 mm 43.1% vs ≥ 10 mm 64.1%, P < 0.01), and a significantly lower proportion of adenomatous component (< 10 mm 29.3% vs ≥ 10 mm 41.7%, P < 0.01). In terms of the initial treatment modality, the percentage of patients with T1 CRCs of less than 10 mm opting for endoscopic treatment was significantly higher (< 10 mm 74.1% vs ≥ 10 mm 60.2%, P < 0.01). Furthermore, the rate of T1b was higher in the large group than in the small group (< 10 mm 62.1% vs ≥ 10 mm 72.8%, P = 0.02). There were no significant differences in the rate of histological grade, vascular invasion, lymphatic invasion, or tumor budding.
| < 10 mm (n = 116) | ≥ 10 mm (n = 1036) | P value | |
| Age, yr | 66.8 ± 11.5 | 66.4 ± 11.6 | 0.72 |
| Sex (male/female) | 84 (72.4)/32 (27.6) | 645 (62.3)/391 (37.7) | 0.03 |
| Location (left-sided/right-sided) | 77 (66.4)/39 (33.6) | 710 (68.5)/326 (31.5) | 0.67 |
| Tumor size (mm) | 7.5 ± 1.2 | 22.5 ± 12.6 | 0.08 |
| Polypoid/non-polypoid growth (polypoid/non-polypoid) | 50 (43.1)/66 (56.9) | 664 (64.1)/372 (35.9) | < 0.01 |
| Adenomatous component (±) | 34 (29.3)/82 (70.7) | 432 (41.7)/604 (58.3) | < 0.01 |
| Morphology(flat/ protruded/ depressed) | 10 (8.6)/46 (39.7)/60 (51.7) | 387 (37.4)/429 (41.4)/220 (21.2) | < 0.01 |
| Initial treatment (endoscopic/surgical) | 86 (74.1)/30 (25.9) | 624 (60.2)/412 (39.8) | < 0.01 |
| Surgical resection1 | 73 (62.9)/43 (37.1) | 725 (70.0)/311 (30.0) | 0.14 |
| Depth of invasion (T1b/T1a) | 72 (62.1)/44 (37.9) | 754 (72.8)/282 (27.2) | 0.02 |
| Histological grade (Por or Muc2/tub1 or tub2) | 3 (2.6)/113 (97.4) | 55 (5.3)/981 (94.7) | 0.26 |
| Vascular invasion (±) | 35 (30.2)/81 (69.8) | 287 (27.7)/749 (72.3) | 0.59 |
| Lymphatic invasion (±) | 38 (32.8)/78 (67.2) | 304 (29.3)/732 (70.7) | 0.45 |
| Tumor budding (BD 2 or 3/BD 1) | 24 (20.7)/92 (79.3) | 218 (21.0)/818 (79.0) | 1.00 |
Comparison of clinicopathological characteristics between < 10 mm and ≥ 10 mm tumors in surgical resection cohort T1 CRCs are shown in Table 3. Of these 1152 Lesions, 798 T1 CRCs underwent initial or secondary surgical resection. There was no significant difference in the LNM rate between the two groups (< 10 mm 12.3% vs ≥ 10 mm 10.9%, P = 0.70). The small group showed a higher rate of depressed type morphology, a lower rate of polypoid growth, and a lower rate of smaller adeno
| < 10 mm (n = 73) | ≥ 10 mm (n = 725) | P value | |
| Age, yr | 66.0 ± 11.8 | 65.5 ± 11.2 | 0.71 |
| Sex (male/female) | 55 (75.3)/18 (24.7) | 438 (60.4)/287 (39.6) | 0.01 |
| Location (left-sided/right-sided) | 51 (69.9)/22 (30.1) | 516 (71.2)/209 (28.8) | 1.00 |
| Tumor size (mm) | 7.6 ± 1.1 | 22.3 ± 12.0 | < 0.01 |
| Polypoid/non-polypoid growth (polypoid/non-polypoid) | 26 (35.6)/47 (64.4) | 473 (65.2)/252 (34.8) | < 0.01 |
| Adenoma component (±) | 15 (20.5)/58 (79.5) | 236 (32.6)/489 (67.4) | 0.04 |
| Morphology (flat/ protruded/ depressed) | 4 (5.5)/ 24 (32.9)/ 45 (61.6) | 307 (42.3)/214 (29.5)/204 (28.1) | 0.03 |
| Initial treatment (endoscopic/surgical) | 43 (58.9)/30 (41.1) | 316 (43.6)/409 (56.4) | 0.01 |
| Depth of invasion (T1b/T1a) | 67 (91.8)/6 (8.2) | 631 (87.0)/94 (13.0) | 0.35 |
| Histological grade (Por or Muc1/tub1 or tub2) | 3 (4.1)/70 (95.9) | 47 (6.5)/678 (93.5) | 0.61 |
| Vascular invasion (±) | 32 (43.8)/41 (56.2) | 261 (36.0)/464 (64.0) | 0.20 |
| Lymphatic invasion (±) | 34 (46.6)/39 (53.4) | 270 (37.2)/455 (62.8) | 0.13 |
| Tumor budding (BD 2 or 3/BD 1) | 21 (28.8)/52 (71.2) | 193 (26.6)/532 (73.4) | 0.68 |
| Lymph node metastasis (±) | 9 (12.3)/64 (87.7) | 79 (10.9)/ 646 (89.1) | 0.70 |
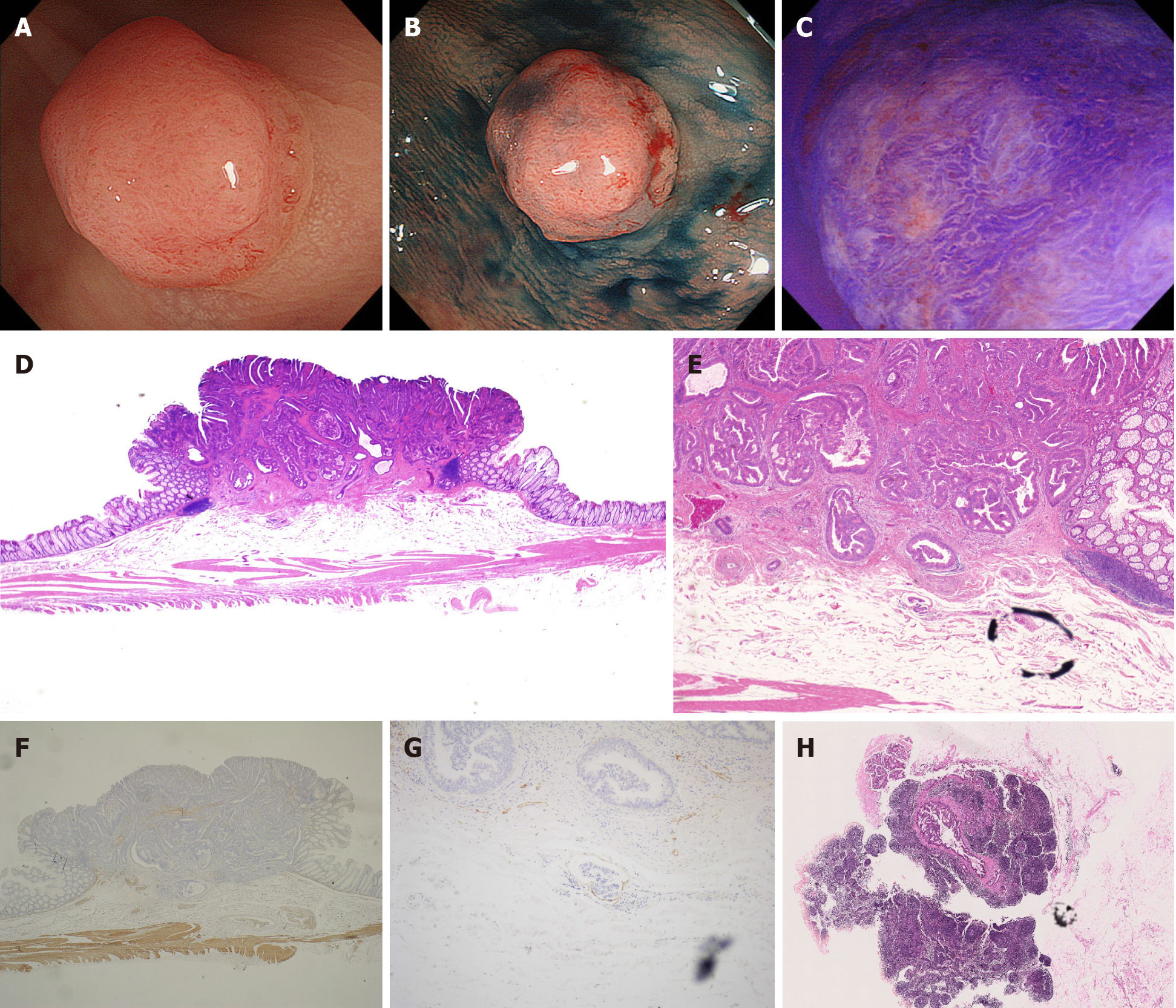
We present a typical case of small T1 CRC with LNM positivity in Figure 2. An 8-mm lesion with depressed type morphology was identified in the sigmoid colon. According to the magnification endoscopy findings, we predicted that the depth of invasion was T1b. Therefore, we selected surgical resection with lymph node dissection as the first-line treatment for this lesion. The final pathological findings were well to moderately differentiated adenocarcinoma, positive lymphovascular invasion, positive vascular invasion, 3750-μm depth of invasion, grade 2 tumor budding, and positive LNM. Despite the small lesion, it had risk factors for LNM and showed LNM positivity, and thus required surgical resection to achieve a cure. Of course, pre-treatment endoscopic diagnosis was important; however, if endoscopic resection was selected for this type of lesion, we should resect it with a negative margin and properly stratify the risk for LNM on the basis of the histopathological diagnosis.
LNM is present in approximately 10% of T1 CRC cases in which surgical resection with lymph node dissection is required to achieve a cure[15-18]. Therefore, we determine the need for additional surgical resection after endoscopic resection of T1 CRC according to the risk of LNM on the basis of the pathological factors. Although a consensus has been reached for several risk factors, including lymphovascular invasion, tumor differentiation, or tumor budding, no consensus has been reached for tumor size. Several reports investigated the relationship between tumor size and the rate of LNM in T1 CRC[19-21] with differing conclusions. Several claimed that tumor size is unrelated to LNM, while others reported that tumor size is related to LNM[22-27]. In this study, we concluded that tumor size alone not a risk factor for LNM.
Our findings revealed that the small group had higher rate of depressed type morphology. Kudo et al[28] recently reported the malignant potential of depressed type lesions. In their research, depressed type lesions showed a higher rate of LNM, followed by vascular invasion and lymphatic invasion, than other types of morphology (flat and protruded type). They speculated that the difference in the molecular phenotype by whole-exome sequencing and RNA sequencing was a potential reason for this observation. The small group showed a significantly higher rate of depressed type morphology in this study. This is a potential reason for why there were no significant differences in LNM between small and large T1 CRCs. Information on tumor morphology obtained by endoscopy is important and we should take care when performing resections, especially for such lesions even though they are small.
The "resect and discard" strategy using optical diagnosis is an attractive approach for endoscopists, pathologists, and patients, and enables a major reduction in the cost of screening and surveillance colonoscopy[29,30]. However, it has the potential risk to discard small, advanced neoplasia, which are lesions of less than 10 mm with advanced histology (high grade dysplasia, villous component, and adenocarcinoma). Notably, in T1 CRC, additional surgical resection after endoscopic resection is required according to the risk of LNM on the basis of the pathological findings of resected specimens to achieve a cure. More than 60% of T1 CRCs are misdiagnosed as adenoma by endoscopists according to a recent prospective study in the Netherlands[31]. In our study, small lesions occupied approximately 10.1% of total T1 CRCs, which had equal potential for metastasis to lymph nodes compared with large lesions. Therefore, careful observation by endoscopy should be undertaken when adopting the "resect and discard" strategy.
This study had several limitations. First, it was a retrospective analysis of patients treated at a single institution. Second, when evaluating the incidence of LNM, only patients who had undergone surgery were included. Patients treated by endoscopic resection alone were excluded because the incidence of LNM this group was not precisely assessed.
In conclusion, we investigated the clinicopathological features of small T1 CRCs and revealed that there was no significant difference in the rate of LNM, followed by the rate of vascular invasion, lymphatic invasion, or histological grade, between the small and large tumor groups. Therefore, requirements for additional surgical resection after endoscopic resection of T1 CRC should be determined on the basis of a careful pathological diagnosis, even if it is a small lesion.
Additional surgical resection of T1 colorectal cancer after endoscopic resection is determined according to the risk of lymph node metastasis (LNM) on the basis of the histopathological findings of resected specimens.
Clinicopathological features including the rate of LNM in small (< 10 mm) T1 colorectal cancer were unknown.
The purpose of this study was to clarify the clinicopathological characteristics of small (< 10 mm) T1 colorectal cancer compared with large (≥ 10 mm) tumors.
We retrospectively analyzed clinicopathological features, including the rate of LNM, of 1152 T1 colorectal cancers divided into two groups: small (< 10 mm) and large (≥ 10 mm) tumors.
Small T1 colorectal cancer had a similar rate of LNM, followed by a positive rate of histological grade and lymphovascular invasion, compared with large tumors.
Because there were no significant differences in the rate of LNM between small and large T1 colorectal cancers, the decision on whether to undertake secondary surgical resection should be determined according to pathological findings, regardless of tumor size.
Because this was a single-center retrospective study, prospective multicenter studies are required to validate these findings.
The authors would like to express great appreciation to all members of the Digestive Disease Center and the Department of Diagnostic Pathology, Showa University Northern Yokohama Hospital for their excellent effort.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ezenkwa US, Hasan A S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64436] [Article Influence: 16109.0] [Reference Citation Analysis (176)] |
| 2. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2289] [Article Influence: 208.1] [Reference Citation Analysis (1)] |
| 3. | Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, Hotta K, Saito Y, Matsuda T, Yamada K, Mitani T, Ohtsuka K, Chino A, Ide D, Imai K, Kishida Y, Nakamura K, Saiki Y, Tanaka M, Hoteya S, Yamashita S, Kinugasa Y, Fukuda M, Kudo T, Miyachi H, Ishida F, Itoh H, Oda M, Mori K. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology. 2021;160:1075-1084.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 950] [Article Influence: 237.5] [Reference Citation Analysis (16)] |
| 5. | National Health Commission of the People's Republic of China. [Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition)]. Zhonghua Wai Ke Za Zhi. 2020;58:561-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 6. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1310] [Article Influence: 262.0] [Reference Citation Analysis (1)] |
| 7. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 8. | Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D; ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64-vi72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 9. | Beppu K, Nagahara A, Terai T, Matsumoto K, Shibuya T, Sakamoto N, Osada T, Kawabe M, Otaka M, Ogihara T, Watanabe S. Clinicopathological characteristics of colorectal cancer less than 10 mm in diameter and invading submucosa and below. J Gastroenterol Hepatol. 2010;25 Suppl 1:S57-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Park SH, Oh SO, Park SS, Roh SJ, Han KS, Kim B, Hong CW, Kim BC, Sohn DK, Chang HJ, Park SC, Oh JH. Characteristics of minute T1 colorectal cancer in relevance to pathology and treatment. Ann Surg Treat Res. 2020;98:199-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Tsuji S, Takeda Y, Tsuji K, Yoshida N, Takemura K, Yamada S, Doyama H. Clinical outcomes of the "resect and discard" strategy using magnifying narrow-band imaging for small (< 10 mm) colorectal polyps. Endosc Int Open. 2018;6:E1382-E1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, Tanaka S, Watanabe H, Sung JJ, Feld AD, Inadomi JM, O'Brien MJ, Lieberman DA, Ransohoff DF, Soetikno RM, Triadafilopoulos G, Zauber A, Teixeira CR, Rey JF, Jaramillo E, Rubio CA, Van Gossum A, Jung M, Vieth M, Jass JR, Hurlstone PD. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 13. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2422] [Article Influence: 484.4] [Reference Citation Analysis (3)] |
| 14. | Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon. 2019;3:175-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 15. | Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, Maeda K, Hirai T, Kameyama M, Shirouzu K, Sugihara K. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013;15:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 18. | Sohn DK, Chang HJ, Park JW, Choi DH, Han KS, Hong CW, Jung KH, Kim DY, Lim SB, Choi HS, Jeong SY. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol. 2007;60:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Hu DY, Cao B, Li SH, Li P, Zhang ST. Incidence, risk factors, and a predictive model for lymph node metastasis of submucosal (T1) colon cancer: A population-based study. J Dig Dis. 2019;20:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Guo K, Feng Y, Yuan L, Wasan HS, Sun L, Shen M, Ruan S. Risk factors and predictors of lymph nodes metastasis and distant metastasis in newly diagnosed T1 colorectal cancer. Cancer Med. 2020;9:5095-5113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Brunner W, Widmann B, Marti L, Tarantino I, Schmied BM, Warschkow R. Predictors for regional lymph node metastasis in T1 rectal cancer: a population-based SEER analysis. Surg Endosc. 2016;30:4405-4415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Kye BH, Jung JH, Kim HJ, Kang SG, Cho HM, Kim JG. Tumor budding as a risk factor of lymph node metastasis in submucosal invasive T1 colorectal carcinoma: a retrospective study. BMC Surg. 2012;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Nakadoi K, Oka S, Tanaka S, Hayashi N, Terasaki M, Arihiro K, Shimamoto F, Chayama K. Condition of muscularis mucosae is a risk factor for lymph node metastasis in T1 colorectal carcinoma. Surg Endosc. 2014;28:1269-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T, Mochizuki H, Kato Y, Watanabe H, Koike M, Sugihara K. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Macias-Garcia F, Celeiro-Muñoz C, Lesquereux-Martinez L, Gude-Sampedro F, Uribarri-Gonzalez L, Abdulkader I, Alvarez-Castro A, Dominguez-Muñoz JE. A clinical model for predicting lymph node metastasis in submucosal invasive (T1) colorectal cancer. Int J Colorectal Dis. 2015;30:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Aytac E, Gorgun E, Costedio MM, Stocchi L, Remzi FH, Kessler H. Impact of tumor location on lymph node metastasis in T1 colorectal cancer. Langenbecks Arch Surg. 2016;401:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Kim B, Kim EH, Park SJ, Cheon JH, Kim TI, Kim WH, Kim H, Hong SP. The risk of lymph node metastasis makes it unsafe to expand the conventional indications for endoscopic treatment of T1 colorectal cancer: A retrospective study of 428 patients. Medicine (Baltimore). 2016;95:e4373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Kudo SE, Kouyama Y, Ogawa Y, Ichimasa K, Hamada T, Kato K, Kudo K, Masuda T, Otsu H, Misawa M, Mori Y, Kudo T, Hayashi T, Wakamura K, Miyachi H, Sawada N, Sato T, Shibata T, Hamatani S, Nemoto T, Ishida F, Niida A, Miyano S, Oshima M, Ogino S, Mimori K. Depressed Colorectal Cancer: A New Paradigm in Early Colorectal Cancer. Clin Transl Gastroenterol. 2020;11:e00269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Vleugels JLA, Koens L, Dijkgraaf MGW, Houwen B, Hazewinkel Y, Fockens P, Dekker E; DISCOUNT study group. Suboptimal endoscopic cancer recognition in colorectal lesions in a national bowel screening programme. Gut. 2020;69:977-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |