Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10064
Peer-review started: July 14, 2021
First decision: August 8, 2021
Revised: August 22, 2021
Accepted: September 30, 2021
Article in press: September 30, 2021
Published online: November 26, 2021
Processing time: 130 Days and 21.6 Hours
Human life expectancy increases as society becomes more developed. This increased life expectancy poses challenges associated with the rapid aging of the population. Sarcopenia, an age-related disease, has become a worldwide health issue. Patients with sarcopenia experience decreases in muscle mass and function, becoming frail and eventually bedridden. Type 2 diabetes mellitus (T2DM) is also a major health issue; the incidence of T2DM increases with aging. T2DM is associated with reduced muscle strength and poor muscle quality and may contribute to acceleration of the aging process, augmenting age-related sarcopenia. Recent studies indicate that elderly patients with diabetes are at an increased risk for sarcopenia. Therefore, these older diabetic patients with sarcopenia need specific anti-diabetic therapies targeting not only glycemic control but also sarcopenia, with the goal of preventing sarcopenia in pre-sarcopenic patients. Presently, various types of hypoglycemic drugs are available, but which hypoglycemic drugs are better suited for geriatric T2DM patients with sarcopenia remains undetermined. In this review, we discuss the association between diabetes and sarcopenia in geriatric patients, and how anti-diabetic drugs may influence sarcopenia outcomes. This review will guide clinical workers in the selection of drugs best suited for this patient population.
Core Tip: Elderly patients with diabetes are at an increased risk for sarcopenia. Therefore, these older diabetic patients with sarcopenia need specific anti-diabetic therapies targeting not only glycemic control but also sarcopenia, with the goal of preventing sarcopenia in pre-sarcopenic patients. We herein discuss the association between diabetes and sarcopenia in geriatric patients, and how anti-diabetic drugs may influence sarcopenia outcomes.
- Citation: Ma XY, Chen FQ. Effects of anti-diabetic drugs on sarcopenia: Best treatment options for elderly patients with type 2 diabetes mellitus and sarcopenia. World J Clin Cases 2021; 9(33): 10064-10074
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10064.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10064
Diabetes mellitus (DM), a chronic metabolic disease, has reached epidemic status and is considered one of the major threats to human health in the 21st century. In 2017, the International Diabetes Federation estimated that 425 million individuals worldwide have DM; this number is expected to increase to 629 million by 2045[1,2]. Approximately 90% of these individuals have type 2 DM (T2DM)[3], with the highest prevalence of T2DM observed in older adults[4]. Sarcopenia, which, in addition to the traditional microvascular and macrovascular complications, has emerged as a third category of T2DM-associated complications in geriatric individuals with diabetic syndromes, results in considerable disability[5]. Sarcopenia manifests primarily as decreased skeletal muscle mass. Combined with decreased bone mineral content and deteriorating bone quality, sarcopenia causes physical frailty and increases the risk for complications, which decrease quality of life and increase mortality[6]. A progressive decrease in muscle mass occurs at an annual rate of 1%-2% after 30 years of age, accelerates to 1.5%-3% per year after 60 years of age, and progresses even more rapidly after 75 years of age[7]. Because muscle is the main site of glucose consumption, reduced muscle mass leads to increased insulin resistance. Sarcopenia causes insulin resistance, which, in turn, exacerbates the loss of skeletal muscle[8]. Because sarcopenia is multifactorial, the diabetic geriatric population needs specific treatment parameters for both the initial and maintenance therapy using anti-diabetic agents. Therefore, prescribing anti-diabetic agents in such individuals should be conducted to lower not only hyperglycemic levels but also to treat and possibly prevent sarcopenia in pre-sarcopenic patients. In this review, we evaluate the relationship between diabetes and sarcopenia in elderly patients and discuss how certain anti-diabetic agents may play specific roles in influencing disease outcomes.
In 1989, Rosenberg first introduced the term “sarcopenia” in reference to age-related loss of skeletal muscle mass and volume[9]. Currently, sarcopenia is defined as an involuntary loss of skeletal muscle and used as predictor of physical disability/mortality. Muscle mass accounts for 75% of body-cell mass and 45% of body mass[10]. Once people reach 60 years of age, they lose 1.5%-3% of their muscle mass per year. Therefore, aging is associated with adverse changes in body composition. Sarcopenia, a common disorder in the elderly, contributes to functional decline, disability, frailty, and falls[11]. Because of the aging of the population, sarcopenia has become a worldwide health concern. In China, the prevalence of sarcopenia in people aged 60 years and older is 10.6% (11.3% in men and 9.8% in women)[12]. In Japan, the prevalence of sarcopenia among community-dwelling older adults aged 65-89 years is 21.8% in men and 22.1% in women[13]. Low muscle mass in the legs is associated with muscle weakness, poor lower-extremity performance, and loss of mobility in older adults[14-16]. In the United States, community-dwelling older adults diagnosed with sarcopenia have a 1.29-fold higher risk for all-cause mortality[17]. The pathogenesis of sarcopenia is poorly understood, although altered hypoxic signaling, oxidative stress, and adipokines may be involved in sarcopenic processes. Age-related, chronic, low-grade inflammation has also been recognized as an important causative factor in sarcopenia[18].
T2DM is a heterogeneous, multifactorial, polygenic, endocrine, metabolic, chronic, and age-related disease characterized by obesity, insulin resistance, and hyperglycemia[19]. Aging is characterized by a progressive loss of physiological integrity, leading to impaired function. This deterioration is the primary risk factor for major human pathologies including cancer, cardiovascular disorders, neurodegenerative diseases, and diabetes[20]. Nevertheless, our understanding of how cellular aging contributes to the pathogenesis of diabetes is incomplete, and currently, there are no therapies targeting cellular aging in diabetes. Insulin resistance has been shown to induce the expression of aging markers, suggesting that β-cell aging could accelerate progression toward diabetes[21-23]. Therefore, reversing cellular aging may be a potential approach in novel anti-T2DM therapies.
Although the mechanisms underlying the association between T2DM and sarcopenia are currently unknown, mitochondrial dysfunction, muscle protein degradation, and autophagy may be associated with loss of skeletal muscle mass and strength in patients with diabetes[24]. Sarcopenia contributes to functional impairment, and elderly diabetic patients are two times more likely to develop sarcopenia than those without diabetes[25]. The Health, Aging, and Body Composition Study showed that annual decline in appendicular muscle mass in patients with diabetes is approximately 0.2 kg/year (1%/year), while decline in appendicular lean mass in non-diabetic persons is 0.15 kg/year (0.7%/year)[26]. A recent prospective study showed that poor glycemic control is associated with sarcopenia and that chronic inflammation combined with mitochondrial dysfunction and oxidative stress play important roles in muscle atrophy[27]. Interactions among these factors may involve several intracellular signaling pathways that affect the balance between protein synthesis and degradation and induction of apoptosis; these two aspects are involved in the primary pathology of significant muscle mass loss[28].
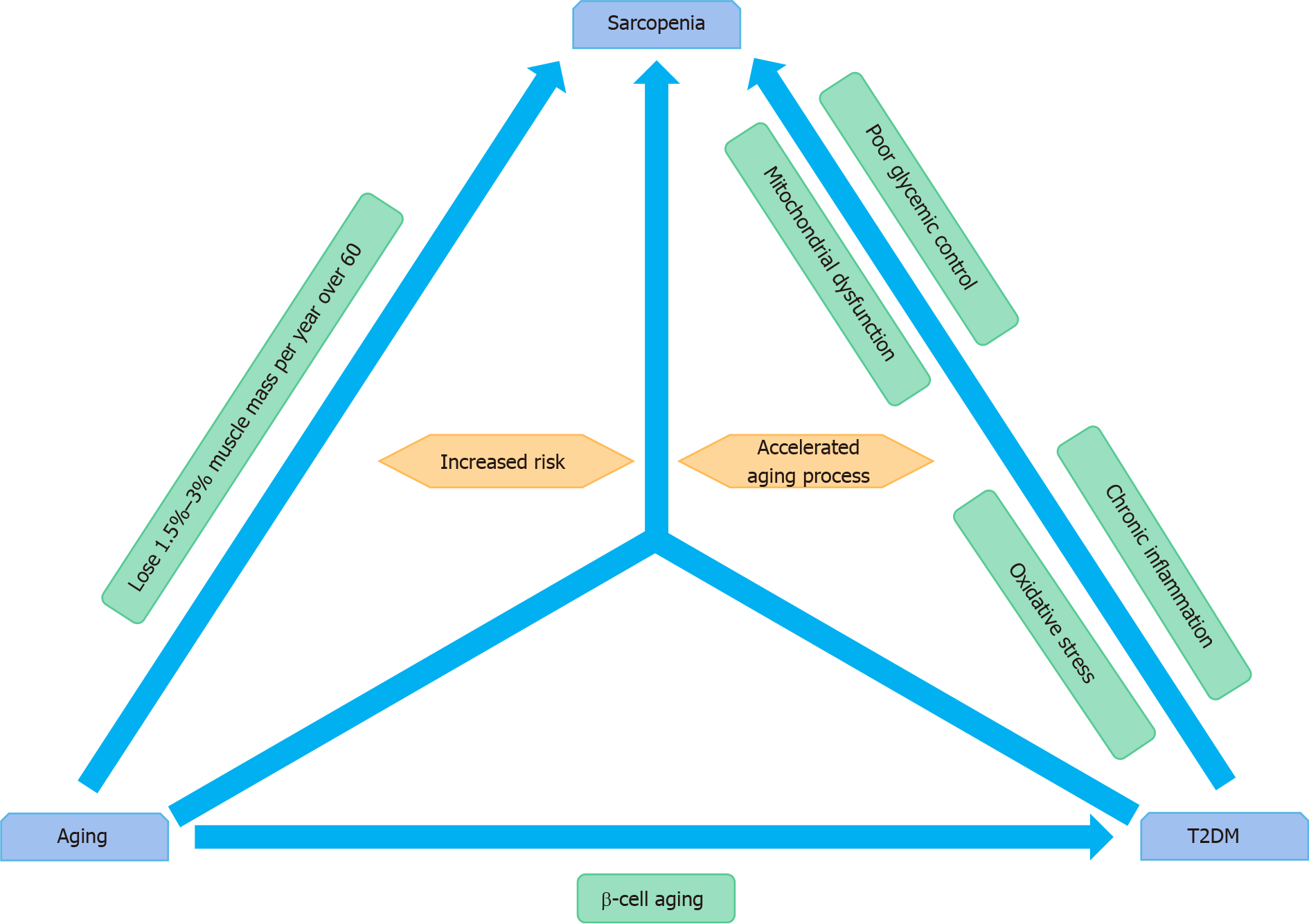
Sarcopenia, one of the most serious health-related problems among elderly adults with diabetes, impairs the activities of daily living, increasing the risk of mortality[29]. Recent studies have reported that elderly patients with diabetes are at an increased risk for sarcopenia[24,26]. Additionally, microinflammation and insulin resistance may be central in the sarcopenia pathogenesis[24]. Previous research has shown that older adults with T2DM have an accelerated loss of muscle mass and strength compared with those of adults without diabetes[26]. Diabetes, which is associated with reduced muscle strength and poor muscle quality, involves an accelerated aging process that intensifies age-related sarcopenia[30]. The results from the Korean Sarcopenic Obesity Study showed that the prevalence of sarcopenia in patients with diabetes is considerably higher than in non-diabetic individuals. In patients older than 60 years, individuals with and without diabetes demonstrated a significant difference in the prevalence of sarcopenia; this was observed for both sexes[9] and agreed with findings obtained in another study[31]. In the general population, studies have shown that with aging, men lose more skeletal muscle mass than do women, even though men have a higher starting skeletal muscle mass compared with that of women[32]; however, women with diabetes are at a particularly high risk for the loss of skeletal muscle mass[26]. The relationship among aging, T2DM, and sarcopenia is illustrated in Figure 1.
Evaluating the use of anti-diabetic agents in older T2DM patients with sarcopenia is important in order to determine which anti-diabetic drugs may alleviate sarcopenia or pose a decreased risk for the progression of sarcopenia. Currently available anti-diabetic agents include biguanides (metformin), insulin secretagogues (sulfonylureas and glinides), alpha-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase 4 (DPP4) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose cotransporter-2 inhibitor (SGLT2i), and insulin. While all of these agents have shown beneficial hypoglycemic effects, we will discuss their potential utility in geriatric patients with sarcopenia (Table 1).
| Anti-diabetic drugs | Effect on sarcopenia | Good option or poor option |
| Biguanides | Positive[31,33-36,38-40]/negative[41-44] | Unclear |
| Insulin secretagogues | Negative[46-48] | Poor |
| -Glucosidase inhibitors | No data | No data |
| Thiazolidinediones | Positive[35,53-56] | Careful use |
| Dipeptidyl peptidase IV inhibitors | Positive[57-60]/neutral[62] | Good |
| Glucagon-like peptide-1 receptor agonists | Positive[63,70,71]/negative[62] | Unclear |
| Sodium-glucose cotransporter-2 inhibitor | Positive[72,73]/unclear[6,74] | Unclear |
| Insulin | Positive/unclear[75] | Unclear |
Metformin (1,1-dimethylbiguanide hydrochloride) lowers blood glucose levels by sensitizing the liver to the effects of insulin, thereby suppressing hepatic glucose output. According to updated guidelines, metformin is considered a first-line treatment for T2DM, especially in elderly diabetic patients. In a study conducted in Iran, 51 individuals newly diagnosed with T2DM (21 men and 30 women) were treated using 1000 mg metformin twice daily for 6 mo; 41 participants (80.4%) completed the study. The results obtained in that study indicated that by week 24, the lean-to-fat ratio increased in the participants, with men showing significantly greater changes compared with those of women. The administration of metformin for 3 mo showed favorable effects on body composition, insulin sensitivity, and glucose homeostasis. This finding suggests that a metformin-based therapy may postpone the development of sarcopenia and may be particularly effective in women with T2DM, who are at an increased risk for the loss of skeletal muscle mass[31]. A two-site, randomized, double-blind, placebo-controlled clinical trial investigating the effects of metformin, combined with a progressive resistance-training program, showed that individuals aged 65 and older, treated using 1700 mg metformin per day for 16 wk, showed increased muscle hypertrophy and strength gains, thereby maintaining functional independence[33]. Another study showed that participants with risk factors for T2DM, treated using 850 mg metformin twice a day for 2 mo, showed a decrease in fat weight and increase in lean weight[34]. In the osteoporotic fractures in men study, 151 diabetic men were treated with insulin sensitizers, and 111 diabetic men were treated without insulin sensitizers, with a follow-up of 3.5 ± 0.7 years. Analysis of specific insulin sensitizers revealed that diabetic men treated using metformin, or using metformin coupled with thiazolidinediones, had significantly reduced total and appendicular lean mass loss compared with that of men with untreated diabetes, or that of diabetic men treated without insulin sensitizers[35]. Metformin increases the activity of adenosine monophosphate-activated protein kinase, which inhibits the mechanistic target of rapamycin (mTOR)[36], a key factor in muscle growth[37]. Notably, metformin is currently being evaluated in clinical trials for the improvement of muscle function in patients with Duchenne muscular dystrophy[38,39].
Metformin-mediated mechanisms are also being evaluated in animal models. One study has shown that the percentage of centronucleated myofibers in metformin-treated mice is lower than that in control mice at 4 d post-injury. Moreover, at 7 d post-injury, control myofibers show a larger cross-sectional area (500 ha2) than that obtained from metformin-conditioned mice. These data suggest that metformin-treated mice were fully regenerated at 7 d post-injury, indicating that metformin may be used for the maintenance of muscle stem cells during repeated regeneration cycles in disease and aging[40]. However, other studies have suggested that metformin is ineffective in the recovery of muscle mass and strength[41-44]. One study evaluated aged (23 mo) and young (3 mo) male mice fed a low-fat diet without or with the addition of 0.5% metformin for up to 8 wk. The results of that study showed that in aged mice, long-term treatment with metformin does not alter the decreased relative muscle mass that exhibits hyperactive mTORC1 signaling during the fasted state. However, the same treatment paradigm using metformin reduced fasted muscle mTORC1 signaling in young mouse muscle[41]. Consistent with the results obtained in this study, another study demonstrated that 0.5% metformin administered for 6 wk shows limited effects on restoring normal metabolic and growth signaling in aged adipose tissue and muscle, respectively[42]. Other studies have suggested that metformin may negatively impact the mitochondrial function in skeletal muscle[43,44]. Thus, it is unclear whether metformin exerts positive, negative, or negligible effects on muscle mass and strength.
Sulfonylureas are insulin secretagogues that are typically divided into first- and second-generation drugs. Glinides possess a mechanism-of-action that is similar to that of sulfonylureas, but act in a plasma-glucose concentration-dependent manner with a shorter circulating half-life than that of sulfonylureas. Sulfonylureas and glinides, which are ATP-sensitive potassium channel blockers, stimulate insulin release from pancreatic beta cells[45]. Several studies have shown that drugs in this class may be associated with muscle atrophy because they induce a muscle type-dependent atrophy in mice[46]. Additionally, from October 6, 2011 through June 29, 2012, the Food and Drug Administration Adverse Event Reporting System received 1697582 reports of adverse events in human patients. Muscle atrophy was reported in 0.27% of reports on glibenclamide/glyburide. A data-mining analysis, performed by calculating the proportional reporting ratio, revealed a significant association between muscle atrophic events and the use of glyburide[46]. This may occur because hypoglycemia can induce “in vitro” apoptosis and autophagic cell death, and high rates of hypoglycemia characterizing glibenclamide use are a precipitating factor in inducing atrophy “in vivo” in human patients[47,48]. These findings indicate that insulin secretagogues should not be used as first-line therapy in older T2DM patients with sarcopenia.
Alpha-glucosidase inhibitors are known to lower postprandial glucose by inhibiting the breakdown of complex carbohydrates in the intestine. However, at present, no studies have been used to examine the relationship between alpha-glucosidase inhibitors and sarcopenia.
Thiazolidinediones (pioglitazone and rosiglitazone) can improve insulin sensitivity by enhancing insulin-mediated glucose disposal via activation of peroxisome proliferator-activated receptor gamma. In 2010, the European Medicines Agency suspended the use of rosiglitazone because of cardiotoxicity; thus, it is not commonly used in China, especially in elderly patients. Recent studies have shown that insulin resistance and mitochondrial dysfunction play an important role in loss of muscle mass[49]. T2DM[50,51] and aging-related sarcopenia[52] are characterized by fatty muscle. One study[53] showed that human satellite cells possess adipogenic potential, which may explain the origin of mature adipocytes within myofibers or within the intermuscular space. Treatment with rosiglitazone does not induce fat conversion in human satellite cells but does considerably enhance the adipogenic potential of these cells, which is triggered by the addition of a specific medium permissive of adipogenesis. Thus, thiazolidinediones, which can increase fatty acid disposal and oxidation in skeletal muscle[54], can reduce increases in intramyocellular triglyceride content and prevent the development of fat cells within muscle fibers[53]. Several studies have shown that peroxisome proliferator-activated receptor gamma agonists exert beneficial effects on muscle performance in older diabetic patients[35,55,56]. However, elderly individuals may have numerous common conditions affecting their health, complicated by coronary heart disease and cardiac insufficiency; thus, prescribing these types of drugs to elderly patients should be undertaken with caution.
DPP4 inhibitors, which are second-line treatment for T2DM, are widely employed because of their safety and effectiveness in glycemic control. Rizzo et al[57] reported in a cross-sectional study that elderly diabetic patients treated with DPP4 inhibitors show low levels of inflammatory parameters, high GLP-1 activity during the postprandial state, and high skeletal muscle mass (SMM) and strength compared with those of patients treated with sulfonylurea[57]. Another study showed that changes in the skeletal muscle index (SMI) of patients treated and not treated using DPP4 inhibitors were 0.04 ± 0.03 and -0.12 ± 0.03, respectively, and that this difference was clinically significant[58]. The findings obtained in this study indicate that DPP4 inhibitors can protect against the loss of muscle mass in Japanese patients with T2DM. Moreover, these 20 patients (11 men and 9 women) with DPP4 inhibitors coupled with sitagliptin for 24 wk significantly reduced total body-fat mass (FM) but not fat-free mass (FFM)[59]. Numerous factors may account for this protective effect. The soluble form of DPP4 induces inflammation, and inflammation can be prevented by DPP4 inhibition[60], which is the presumed cause of sarcopenia[61]. Also, inhibitors of DPP4, a GLP-1 degradation enzyme, are associated with alleviation in the reduction of muscle mass in diabetic and elderly diabetic patients[57,58]. However, another study showed that neither FM nor SMM changed following a 6-mo treatment with teneligliptin in 21 T2DM patients on hemodialysis[62]. Therefore, the effect of DPP4 inhibition on sarcopenia is likely protective or neutral, indicating that DPP4 inhibitors are safe to use in elderly T2DM patients with sarcopenia.
GLP-1 is a 30-amino acid peptide incretin hormone synthesized and secreted by intestinal endocrine L-cells in the small intestine in response to eating. GLP-1 performs numerous physiological actions via its receptor, GLP-1R[63]; these actions include promoting glucose-induced insulin secretion, increasing β-cell survival, inhibiting glucagon production, delaying gastric emptying, and regulating appetite[63]. GLP-1R expression in muscle tissues and cells is controversial[64]. GLP-1 can also induce insulin-independent vasodilation and may stimulate nitric oxide synthase phosphorylation in endothelial cells[65]. GLP-1RA may exert anti-sarcopenic effects. A previous study has shown that the GLP-1RA, Ex-4, attenuates muscle atrophy in dexamethasone-induced mouse model of muscle atrophy and in chronic renal disease-derived model of muscle atrophy. Additionally, a long-acting GLP-1RA, dulaglutide, shows a therapeutic effect in DBA/2J-mdx mice, which are used to model Duchenne muscular dystrophy[63].
GLP-1RA have revolutionized the management of T2DM in elderly adults with T2DM who tend to develop sarcopenia and frailty as a result of poor energy intake. Increases in GLP-1 expression may represent a compensatory response to FFM loss, intended to enhance vascular/metabolic coupling in muscle via synergistic effect on nitric oxide and insulin signaling pathways. GLP-1 expression is the strongest predictor of FFM loss, as was shown using the multivariate model[63]. This finding indicates that targeting GLP-1 may potentially be used to reduce FFM loss under hypoxic conditions. This can be achieved using DPP-4 inhibitors or GLP-1RA such as exendin-4; this pharmacological approach is now commonly used in the treatment of diabetes[66]. Inflammation can induce muscle atrophy by regulating the nuclear factor kappa B signaling pathway[67,68], while suppression of inflammation reverses muscle atrophy[68,69]. Indeed, GLP-1RA have shown anti-inflammatory effects in numerous different diseases that involve inflammation[70]. A 24-wk treatment using 3.0 mg liraglutide in overweight and obese elderly patients with T2DM was shown as safe, and none of the patients receiving this treatment became sarcopenic[71]. Unexpectedly, 5 patients showed an improvement in SMI, which was mainly due to an increase in fat-free mass of the legs and arms. Liraglutide reduces body weight and shows particular efficaciousness in reducing fat mass while supporting the stability of trophic SMM. This observation suggests that liraglutide affects muscle by preventing the breakdown of muscle proteins[71]. In another study, however, 6-mo treatment using dulaglutide combined with insulin therapy in T2DM patients on hemodialysis significantly reduced FM and SMM but did achieve significantly improved glycemic control and decreased the insulin dose. Therefore, dulaglutide should be used with caution in these patients because it may promote sarcopenia[62]. In conclusion, it is unclear whether GLP-1RA exert positive or negative effects on muscle mass and strength in patients with T2DM.
SGLT2i is a new type of anti-diabetic drug for the treatment of individuals with T2DM. Because of its protective effects on the cardiovascular system and kidneys, it is currently widely prescribed in this patient population. In a previous study, the effects of SGLT2i luseogliflozin on muscle atrophy were investigated in Db/Db mice using cross-sectional areas of the soleus and plantaris muscles. After 8 wk of treatment with luseogliflozin, the cross-sectional areas of the soleus muscle obtained from Db/Db mice not treated with SGLT-2i were significantly smaller than those obtained from Db/Db mice that were treated with SGLT-2i. This may have occurred because of suppression of increased foxo1 expression, which is associated with muscle atrophy in the skeletal muscle of Db/Db mice[72]. However, SGLT-2i shows the opposite effects in humans. A study conducted in Japan showed that the SMI of 37 obese T2DM patients treated with SGLT2 (tofogliflozin) was significantly reduced in both men and women. Although skeletal muscle was significantly decreased, SMI, assessed after such reductions, was sufficiently high and far enough from the cutoff values used in the Asian criteria for sarcopenia[73]. Another report from Japan showed that in T2DM patients treated with luseogliflozin for 52 wk, SMI decreased over the course of the treatment; these changes, however, did not reach the level of statistical significance[6]. These two studies included young obese patients, suggesting that it may not be advantageous to administer SGLT2 inhibitors to older T2DM patients at risk for sarcopenia. SGLT2i should also be used with caution in elderly adults with diabetes because these drugs can increase the risk for both dehydration and sarcopenia[74]. Currently, it is unclear whether SGLT2i exert positive or negative effects on sarcopenia. Further investigations are required in order to maintain adequate levels of skeletal muscle mass during treatment with SGLT2-i in T2DM patients.
Insulin, a powerful anabolic signal in proteins, may prevent sarcopenia in patients with T2DM. Insulin is known to stimulate muscle-protein synthesis in young adults, but not in older individuals or animals[75]. However, the beneficial effects of insulin on sarcopenia have not been yet confirmed in clinical settings.
Anti-diabetic drugs and resistance exercise also exert beneficial effects in T2DM patients with sarcopenia. One study assessed the effects of modified plant-based Mediterranean diet, circuit resistance training, and empagliflozin, separately and in combination, on the body composition and physical function of older individuals with T2DM. The results of that study showed that these interventions were effective in delaying the progression from diabetes to sarcopenia and/or frailty[76]. Another study showed that a combined exercise-metformin intervention therapy benefitted older individuals by promoting muscle hypertrophy and strength gains, thereby maintaining functional independence[33].
Sarcopenia is an increasingly common problem in the elderly, especially in geriatric patients with T2DM and in those receiving treatment with anti-diabetic agents. Therefore, it is important to assess appropriately a patient’s condition before administering anti-diabetic drugs. Elderly patients are at a much higher risk than younger patients for the side effects of anti-diabetic drugs. This review will aid clinicians in their selection of appropriate anti-diabetic drugs for the treatment of geriatric T2DM patients with, or at risk for, sarcopenia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Endocrinology and Metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Osailan A S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2490] [Article Influence: 311.3] [Reference Citation Analysis (0)] |
| 2. | Chen F, Wei G, Wang Y, Liu T, Huang T, Wei Q, Ma G, Wang D. Risk factors for depression in elderly diabetic patients and the effect of metformin on the condition. BMC Public Health. 2019;19:1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1255] [Cited by in RCA: 1540] [Article Influence: 128.3] [Reference Citation Analysis (4)] |
| 4. | Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37:278-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1205] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 5. | Sinclair AJ, Abdelhafiz AH, Rodríguez-Mañas L. Frailty and sarcopenia - newly emerging and high impact complications of diabetes. J Diabetes Complications. 2017;31:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Sasaki T, Sugawara M, Fukuda M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig. 2019;10:108-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 479] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Krentz AJ, Viljoen A, Sinclair A. Insulin resistance: a risk marker for disease and disability in the older person. Diabet Med. 2013;30:535-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 10. | Nair KS. Age-related changes in muscle. Mayo Clin Proc. 2000;75 Suppl:S14-S18. [PubMed] |
| 11. | Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Hai S, Wang H, Cao L, Liu P, Zhou J, Yang Y, Dong B. Association between sarcopenia with lifestyle and family function among community-dwelling Chinese aged 60 years and older. BMC Geriatr. 2017;17:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, Kayama H, Aoyama T, Arai H. Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 14. | Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 603] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 15. | Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 990] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 16. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8401] [Article Influence: 560.1] [Reference Citation Analysis (0)] |
| 17. | Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7:290-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 18. | Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 19. | Hayden MR. Type 2 Diabetes Mellitus Increases The Risk of Late-Onset Alzheimer's Disease: Ultrastructural Remodeling of the Neurovascular Unit and Diabetic Gliopathy. Brain Sci. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10570] [Cited by in RCA: 10248] [Article Influence: 854.0] [Reference Citation Analysis (0)] |
| 21. | Aguayo-Mazzucato C, Andle J, Lee TB Jr, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019;30:129-142.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 22. | Aguayo-Mazzucato C, Midha A. β-cell senescence in type 2 diabetes. Aging (Albany NY). 2019;11:9967-9968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Aguayo-Mazzucato C, van Haaren M, Mruk M, Lee TB Jr, Crawford C, Hollister-Lock J, Sullivan BA, Johnson JW, Ebrahimi A, Dreyfuss JM, Van Deursen J, Weir GC, Bonner-Weir S. β Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance. Cell Metab. 2017;25:898-910.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 24. | Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 753] [Article Influence: 68.5] [Reference Citation Analysis (1)] |
| 25. | Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 536] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 26. | Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman AB; Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993-1997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 571] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 27. | Choi MH, Ow JR, Yang ND, Taneja R. Oxidative Stress-Mediated Skeletal Muscle Degeneration: Molecules, Mechanisms, and Therapies. Oxid Med Cell Longev. 2016;2016:6842568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509-1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 29. | Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, Inagaki N, Uemoto S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 30. | Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 31. | Aghili R, Malek M, Valojerdi AE, Banazadeh Z, Najafi L, Khamseh ME. Body composition in adults with newly diagnosed type 2 diabetes: effects of metformin. J Diabetes Metab Disord. 2014;13:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000;89:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1672] [Cited by in RCA: 1908] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 33. | Long DE, Peck BD, Martz JL, Tuggle SC, Bush HM, McGwin G, Kern PA, Bamman MM, Peterson CA. Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials. 2017;18:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Rodríguez-Moctezuma JR, Robles-López G, López-Carmona JM, Gutiérrez-Rosas MJ. Effects of metformin on the body composition in subjects with risk factors for type 2 diabetes. Diabetes Obes Metab. 2005;7:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Lee CG, Boyko EJ, Barrett-Connor E, Miljkovic I, Hoffman AR, Everson-Rose SA, Lewis CE, Cawthon PM, Strotmeyer ES, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Study Research Group. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381-2386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 609] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 37. | Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1847] [Cited by in RCA: 2303] [Article Influence: 164.5] [Reference Citation Analysis (0)] |
| 38. | Hafner P, Bonati U, Rubino D, Gocheva V, Zumbrunn T, Gueven N, Fischer D. Treatment with L-citrulline and metformin in Duchenne muscular dystrophy: study protocol for a single-centre, randomised, placebo-controlled trial. Trials. 2016;17:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Hafner P, Bonati U, Erne B, Schmid M, Rubino D, Pohlman U, Peters T, Rutz E, Frank S, Neuhaus C, Deuster S, Gloor M, Bieri O, Fischmann A, Sinnreich M, Gueven N, Fischer D. Improved Muscle Function in Duchenne Muscular Dystrophy through L-Arginine and Metformin: An Investigator-Initiated, Open-Label, Single-Center, Proof-Of-Concept-Study. PLoS One. 2016;11:e0147634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Pavlidou T, Marinkovic M, Rosina M, Fuoco C, Vumbaca S, Gargioli C, Castagnoli L, Cesareni G. Metformin Delays Satellite Cell Activation and Maintains Quiescence. Stem Cells Int. 2019;2019:5980465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Dungan CM, Li Z, Wright DC, Williamson DL. Hyperactive mTORC1 signaling is unaffected by metformin treatment in aged skeletal muscle. Muscle Nerve. 2016;53:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Mennes E, Dungan CM, Frendo-Cumbo S, Williamson DL, Wright DC. Aging-associated reductions in lipolytic and mitochondrial proteins in mouse adipose tissue are not rescued by metformin treatment. J Gerontol A Biol Sci Med Sci. 2014;69:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Wessels B, Ciapaite J, van den Broek NM, Nicolay K, Prompers JJ. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS One. 2014;9:e100525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Kane DA, Anderson EJ, Price JW 3rd, Woodlief TL, Lin CT, Bikman BT, Cortright RN, Neufer PD. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med. 2010;49:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 46. | Mele A, Calzolaro S, Cannone G, Cetrone M, Conte D, Tricarico D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol Res Perspect. 2014;2:e00028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, Prasadan K, Shiota C, Gittes GK. Hypoglycemia reduces vascular endothelial growth factor A production by pancreatic beta cells as a regulator of beta cell mass. J Biol Chem. 2013;288:8636-8646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | de la Cadena SG, Hernández-Fonseca K, Camacho-Arroyo I, Massieu L. Glucose deprivation induces reticulum stress by the PERK pathway and caspase-7- and calpain-mediated caspase-12 activation. Apoptosis. 2014;19:414-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, Lattanzio F. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 306] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 51. | Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 52. | Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 254] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | De Coppi P, Milan G, Scarda A, Boldrin L, Centobene C, Piccoli M, Pozzobon M, Pilon C, Pagano C, Gamba P, Vettor R. Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia. 2006;49:1962-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Cha BS, Ciaraldi TP, Park KS, Carter L, Mudaliar SR, Henry RR. Impaired fatty acid metabolism in type 2 diabetic skeletal muscle cells is reversed by PPARgamma agonists. Am J Physiol Endocrinol Metab. 2005;289:E151-E159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond). 2007;31:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Skov V, Glintborg D, Knudsen S, Tan Q, Jensen T, Kruse TA, Beck-Nielsen H, Højlund K. Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PLoS One. 2008;3:e2466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Rizzo MR, Barbieri M, Fava I, Desiderio M, Coppola C, Marfella R, Paolisso G. Sarcopenia in Elderly Diabetic Patients: Role of Dipeptidyl Peptidase 4 Inhibitors. J Am Med Dir Assoc. 2016;17:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 58. | Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab Res Rev. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Kato H, Nagai Y, Ohta A, Tenjin A, Nakamura Y, Tsukiyama H, Sasaki Y, Fukuda H, Ohshige T, Terashima Y, Sada Y, Kondo A, Sasaoka T, Tanaka Y. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;109:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Wronkowitz N, Görgens SW, Romacho T, Villalobos LA, Sánchez-Ferrer CF, Peiró C, Sell H, Eckel J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim Biophys Acta. 2014;1842:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 61. | Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 411] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 62. | Yajima T, Yajima K, Takahashi H, Yasuda K. The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis. J Diabetes Complications. 2018;32:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 63. | Hong Y, Lee JH, Jeong KW, Choi CS, Jun HS. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J Cachexia Sarcopenia Muscle. 2019;10:903-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 64. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2461] [Cited by in RCA: 2648] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 65. | Ding L, Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin. 2012;33:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Wandrag L, Siervo M, Riley HL, Khosravi M, Fernandez BO, Leckstrom CA, Martin DS, Mitchell K, Levett DZH, Montgomery HE, Mythen MG, Stroud MA, Grocott MPW, Feelisch M; Caudwell Xtreme Everest Research Group. Does hypoxia play a role in the development of sarcopenia in humans? Redox Biol. 2017;13:60-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Jackman RW, Cornwell EW, Wu CL, Kandarian SC. Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol. 2013;98:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Yu R, Chen JA, Xu J, Cao J, Wang Y, Thomas SS, Hu Z. Suppression of muscle wasting by the plant-derived compound ursolic acid in a model of chronic kidney disease. J Cachexia Sarcopenia Muscle. 2017;8:327-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Ham DJ, Murphy KT, Chee A, Lynch GS, Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin Nutr. 2014;33:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Lee YS, Jun HS. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediators Inflamm. 2016;2016:3094642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 71. | Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, Rondanelli M. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res. 2016;28:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 72. | Okamura T, Hashimoto Y, Osaka T, Fukuda T, Hamaguchi M, Fukui M. The sodium-glucose cotransporter 2 inhibitor luseogliflozin can suppress muscle atrophy in Db/Db mice by suppressing the expression of foxo1. J Clin Biochem Nutr. 2019;65:23-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | Kamei S, Iwamoto M, Kameyama M, Shimoda M, Kinoshita T, Obata A, Kimura T, Hirukawa H, Tatsumi F, Kohara K, Nakanishi S, Mune T, Kaku K, Kaneto H. Effect of Tofogliflozin on Body Composition and Glycemic Control in Japanese Subjects with Type 2 Diabetes Mellitus. J Diabetes Res. 2018;2018:6470137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Yabe D, Nishikino R, Kaneko M, Iwasaki M, Seino Y. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015;14:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 75. | Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 76. | Buch A, Eldor R, Kis O, Keinan-Boker L, Dunsky A, Rubin A, Lopez A, Sofer Y, Osher E, Marcus Y, Stern N. The effect of circuit resistance training, empagliflozin or "vegeterranean diet" on physical and metabolic function in older subjects with type 2 diabetes: a study protocol for a randomized control trial (CEV-65 trial). BMC Geriatr. 2019;19:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |