Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10052
Peer-review started: February 19, 2021
First decision: March 28, 2021
Revised: April 15, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: November 26, 2021
Processing time: 276 Days and 3.9 Hours
The liver is the main target organ for hepatitis viruses and the vital organ for alcohol metabolism. These two factors of viral hepatitis and alcohol abuse in combination can exert dual harmful actions, leading to enhanced damage to the liver. Epidemiological studies have revealed a higher prevalence of hepatitis C virus (HCV) infection among alcoholics than the general population. The interaction of alcohol with viral hepatitis [e.g., hepatitis B virus (HBV), HCV] and the underlying mechanisms are not fully understood. The effects of alcohol on viral hepatitis include promoted viral replication, weakened immune response, and increased oxidative stress. Clinically, alcohol abuse is correlated with an increased risk of developing end-stage liver cirrhosis and hepatocellular car
Core Tip: (1) Alcohol adversely affects hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in the liver via promoting viral replication, increasing oxidative stress, suppressing the viral immune responses, etc.; (2) The interaction of alcohol with viral hepatitis contributes to an increased risk of developing HBV- or HCV-induced liver fibrosis, end-stage cirrhosis, and even deadly liver cancer; and (3) Individuals with HBV or HCV infection should abstain from alcohol to slow the disease progression.
- Citation: Xu HQ, Wang CG, Zhou Q, Gao YH. Effects of alcohol consumption on viral hepatitis B and C. World J Clin Cases 2021; 9(33): 10052-10063
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10052.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10052
Hepatitis B virus (HBV) and hepatitis C virus (HCV) are the two most common types of viral infections in the liver, with approximately 257 million and 71 individuals living with HBV and HCV worldwide, respectively, and as many as 780000 HBV- and 400000 HCV-related deaths annually[1-3]. Epidemiologic studies suggest that approximately 10% to 15% of patients with HCV infection are coinfected with HBV[4]. HBV infection is particularly prevalent in China, and HBV-related deaths account for 63% of deaths from liver cirrhosis and other chronic liver diseases, and 53% of deaths from liver cancer[5]. Alcohol abuse is among the high-risk factors contributing to the accelerated progression of chronic hepatitis B (CHB) and chronic hepatitis C (CHC) into more severe liver diseases such as liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[6-8].
Alcohol consumption is very common across different cultures and religions worldwide. Indeed, in 2016, as high as 57% of the population aged 15 and older consumed alcohol. The use and misuse of alcohol are leading risk factors for a broad spectrum of health problems worldwide, directly influencing health-associated targets of the sustainable development goals including those for infectious diseases (viral hepatitis, human immunodeficiency, tuberculosis), maternal and child health, mental health, noncommunicable diseases, injuries, and poisonings.
Alcohol is mainly metabolized and converted into more toxic acetaldehyde in the liver. Combined alcohol abuse and viral hepatitis may lead to more severe hepatic damage. To date, the mechanisms underlying the interaction of alcohol with viral hepatitis (e.g., HBV, HCV) are not fully understood. In addition, the effects of alcohol intake on the progression of HBV- or HCV-associated liver disease have not been accurately examined.
Herein, we performed a literature review and synthesized existing studies on the complex interaction of alcohol with HBV and HCV infections.
Excessive alcohol use is related to an increased risk of spreading the infection of viruses, including HBV and HCV[9]. In fact, HCV infection is more prevalent in patients with alcohol use disorders (AUDs) than the general population. CHC is associated with excessive current and previous alcohol drinking[10]. The World Health Organization HCV elimination strategy also highlights the requirement for addressing alcohol intake as a co-morbidity among patients with HCV infection[11]. The prevalence of hepatitis C varies ranges from 4.6% to 55.5% in alcoholics[12-18]. A systematic review of 133 publications showed that the prevalence of HCV infection is up to 30-fold higher in alcoholics compared with the general population[18]. Meanwhile, alcohol abuse is more prevalent in patients with HCV infection who also have a longer duration of alcohol use than the general population. A systematic review of 11 previous studies including 286641 patients with CHC reported that 22.3% of the patients identified with AUDs, a much greater proportion than the general population[19]. A previous international study showed that 28%, 32%, and 50% of HCV patients with decompensated cirrhosis had AUD in British Columbia, New South Wales, and Scotland, respectively[20]. Rosman et al[21] reported that anti-HCV is significantly more prevalent in alcoholics than non-drinkers, indicting alcohol misuse as an independent risk factor associated with HCV infection. Although direct evidence is lacking, it has been postulated that alcohol use enhances the acquisition of HCV after exposure to the virus[22,23].
Unlike the relatively high incidence of HCV infection in alcohol drinkers, the prevalence of HBV infection in this population is still inconclusive. Some previous studies have revealed a higher frequency of HBV markers in the serum of chronic alcoholics[24-26] as well as of patients with alcoholic hepatitis and liver cirrhosis[27] compared with the general population. A study from Taiwan reported a link of alcohol drinking with a lower prevalence of hepatitis B surface antigen (HBsAg)[28], which is not consistent with the study by Rosman et al[21], indicating that the prevalence of anti-HCV is significantly greater in alcoholics than in non-drinkers, whereas there is no significant difference in the prevalence of HBV between alcohol drinkers and non-drinkers. These previous findings suggest that alcohol abuse is an independent risk factor for HCV but not HBV infection[21], but the reason for the difference remains unclear. Lin et al[29] proposed that HBV infection can play an important role in the evolution of the aldehyde dehydrogenase 2 (ALDH2*2) allele in the Chinese Han population. Geographical areas with a particularly high prevalence of HBV infection are located in Eastern Asia, especially in China. In addition, 30%-40% of Asian populations are deficient in ALDH2, a key enzyme in detoxifying the ethanol metabolite acetaldehyde. Variation in the ALDH gene influences drinking behavior and the risk of alcoholism development through acetaldehyde formation. In the long-term HBV endemic in China, non-alcohol drinking HBV carriers may have evolutionary advantages over alcohol-drinking HBV carriers. Accordingly, HBV carriers who do not drink or drink in moderation may have better chances of surviving than those who drink more alcohol. It has also been hypothesized that the ALDH2*2 allele, through the natural selection of liver diseases, becomes the adaptive gene among Chinese Han population, resulting in a better chance of surviving and facilitating transmission of the virus to the next generation. In summary, HBV selects ALDH gene mutations, which can affect drinking behavior, making HBV-infected people not good at drinking alcohol, and providing more opportunities for long-term survival and transmission of HBV.
Both alcohol and HBV/HCV infection are associated with liver injury, and the interactions of alcohol with hepatitis B and C infections are complex. Although the exact underlying mechanisms are not fully understood, studies have provided evidence that alcohol can increase the replication of HBV/HCV, oxidative stress, and cytotoxicity but decrease antiviral immune responses in the liver[30].
Existing in vitro and in vivo studies have shown that alcohol intake can increase the replication of HBV, and possibly HCV, as well their host hepatocytes. Larkin et al[31] found that the serum viral DNA load and levels of HBsAg increased by nearly 6-fold in mice treated with alcohol compared with those given the control diet. In the same study, elevated levels of HBV-RNA as well as surface, core, and X antigens were also observed after alcohol treatment, especially in the pericentral regions of the liver in mice. In in vitro studies, Ganesan et al[32] reported that the levels of HBV RNA, covalently closed circular DNA, and HBsAg were increased in response to alcohol treatment in HepG2.2.15 cells. Recently, Lin et al[29] showed that hepatitis B viral load was higher in alcoholic than non-alcoholic HBV patients. Alcohol can promote HBV transcription by regulating some key factors [e.g., peroxisome proliferator-activated receptor alpha, farnesoid-X-receptor alpha, cytochrome P450 2E1 (CYP2E1)] as well as the hypoxia-inducible factor-1α-dependent pathway, which have been proposed as potential molecular mechanisms whereby excessive alcohol consumption increases the replication of HBV in the liver[33-35]. In addition to the direct effect of alcohol on the replication of HBV, alcohol can act on lipid rafts with pivotal roles in viral entry and other processes of the viral life cycle, leading to indirect effects on HBV replication[23].
Unlike HBV, the effect of alcohol on the replication of HCV remains a subject of debate due to conflicting results from different studies. Ran et al[36] showed that alcohol treatment promoted HCV replication in Huh7 cells. Sobhanimonfared et al[37] showed that acute alcohol treatment was associated with increased HCV replication, while chronic alcohol treatment led to decreased HCV replication in Huh 7 cells. By contrast, Plumlee et al[38] found that high concentrations of acute alcohol inhibited HCV replication in cell culture. Cromie et al[39] showed that even a small amount of alcohol can lead to an increase in serum HCV RNA in patients with HCV infection. In a meta-analysis, conclusive results were not reached regarding the effect of alcohol consumption on serum HCV-RNA levels[40].
In the cases of HBV and HCV infections, eliciting antiviral immune responses, including innate and adaptive immune responses, are important host defenses in the control of viruses. Excessive alcohol intake adversely affects the antiviral immune system in response to hepatitis viruses, leading to unfavorable outcomes of HBV or HCV infection. In terms of innate immune responses, alcohol exerts inhibitory effects on the antiviral activity of natural killer cells. In addition, long-term alcohol consumption appears to affect innate immune responses to hepatitis virus infection in the production of some important cytokines including interferon (IFN)-α, IFN-γ, tumor necrosis factor-α, transforming growth factor β, and interleukin 10[41-45]. In the adaptive immune responses to hepatitis viruses, alcohol reduces the number of B cells, particularly circulating B cells[46-48]. Alcohol may suppress the adaptive immune response of B cells by reducing the number of B cells, subsequently decreasing the production of antibodies against HBV antigens, thereby leading to persistent HBV infection and development of CHB. Data from animal and human studies have clearly shown that alcohol reduces the number of T cells, alters their patterns, suppresses their activation, and promotes the apoptosis of T cells[49]. Alcohol has also been shown to affect dendritic cells, which are critically important immune cells in adaptive immune responses to virus infection in patients with HCV infection[50,51].
Chronic alcohol intake induces the microsomal ethanol-oxidizing system, including that of CYP450. Among the variants of CYP450, CYP2E1 is markedly affected by chronic alcohol consumption, with activity that increases in response to alcohol. Free radicals (e.g., superoxide, hydroxyl radicals, hydrogen peroxide) are generated in ethanol metabolic pathways involving CYP2E1, and excessive oxygen radicals cause oxidative stress. Rigamonti et al[52] showed that moderate alcohol consumption (< 50 g/d) and heavy alcohol drinking (> 50 g/d) increase the risk of developing oxidative stress 3-fold and up to 24-fold, respectively. Oxidative stress can activate nuclear factor kappa B[53], playing a key role in hepatic inflammation, liver injury and regeneration, and the development of HCC[54-56]. Moreover, CYP2E1-associated oxidative stress increases the ethanol-induced transactivation of HBV[57].
Oxidative stress is a negative effect exerted by reactive oxygen species, highly reactive oxygen intermediates that can chemically modify the structure of various molecules and thus pose a threat to the living cell. High levels of reactive oxygen species induce oxidative DNA damage, such as 8-hydroxy-2-deoxyguanosine (8-OHdG), an important biomarker for oxidative stress. Wong et al[58] showed that HBV- and HCV-induced hepatic inflammation and alcohol consumption can induce oxidative stress, with alcohol consumption correlated with 8-OHdG. The accumulation of 8-OHdG associated with alcohol in hepatocytes may establish a possible link between HBV infection and hepatic carcinogenesis[59]. These findings provide an explanation for the enhanced progression of disease in hepatitis patients with alcohol abuse.
In addition, the interaction of alcohol with hepatitis virus may involve activation of the unfolded protein response[60], promotion of hepatic steatosis[61], increase in iron storage[62,63], and induction of hepatocytes apoptosis[64-66]. Moreover, in the progression of liver disease, these mechanisms can interact with each other. For example, higher levels of oxidative stress affect innate immunity, resulting in the more rapid spread of the virus and progression to end-stage liver disease.
Alcohol intake has a synergistic effect with viral hepatitis on the liver disease progression. For example, a case control study found synergism between positive HBsAg or HCV RNA and heavy alcohol drinking[67]. Among patients coinfected with HBV and/or HCV, alcoholic patients tended to be younger and had a higher male-to-female ratio, worse performance status, more severe liver cirrhosis, more advanced cancer stage, and higher tumor burden compared to non-alcoholic patients[68]. In terms of HCC, the values of multivariate odd ratios (ORs) [95% confidence intervals (CIs)] were 15.3 (4.3-54.4), 12.6 (2.5-63.1), 4.5 (1.4-14.8), and 4.3 (1.9-9.9) for anti-HCV, HBsAg, heavy alcohol intake (> 80 mL ethanol per day), and diabetes mellitus, respectively. There were synergistic interactions between heavy alcohol intake and chronic viral hepatitis (OR: 53.9, 95%CI: 7.0-415.7)[69].
A study of 1113 Japanese patients with CHB revealed that the prevalence of hepatitis B e antigen (HBeAg) tended to be higher and decrease more slowly with age in heavy drinkers (> 60 g alcohol per day) than in nondrinkers, indicating that alcohol misuse may delay the loss of HBeAg[70]. Other studies in the Japanese population found that alcohol intake, particularly excessive alcohol intake (> 60 g alcohol per day), can increase viral inflammatory changes in the liver in patients with persistent HBs-antigenemia[71] or HBsAg carriers[72]. Li et al[65] documented that interactions of alcohol and HB synergistically promote high-fat diet-induced hepatic steatosis in mice. The same study showed that alcohol consumption is associated with an increased risk of developing hepatic steatosis in HBV-infected patients.
Alcohol abuse in patients with CHB is correlated with an elevated risk of developing liver cirrhosis and HCC. In agreement with the above findings, Lin et al[73] indicated that heavy alcohol consumption significantly increased the risk of HCC in patients with HBV-related liver cirrhosis. It was also shown that HCC occurred in 28.8%, 15.8%, and 10.4%, respectively, in cirrhotic patients with HBV infection and alcoholism, cirrhotic patients with HBV infection, and cirrhotic patients with alcoholism. In addition, the 10-year cumulative incidences of HCC were 52.8%, 39.8%, and 25.6%, respectively, and the annual incidences of HCC were 9.9%, 4.1%, and 2.1%, respectively, in cirrhotic patients with HBV infection and alcoholism, cirrhotic patients with HBV infection, and cirrhotic patients with alcoholism[73]. Notably, these incidences were significantly greater in cirrhotic patients with HBV infection and alcoholism than in those in patients with HBV infection or alcoholism alone[73]. Heavy alcohol consumption accelerates the progression of liver disease into liver cirrhosis and eventually into HCC with a 1.3- to 8.4-fold increased risk[74]. A longitudinal study of healthy blood donors with positive HBsAg in Japanese population spanning the period between 1972 and 1975 demonstrated that alcohol intake > 27 g/d was associated with more than a 5-fold increase in the relative risk (RR) of developing HCC[75]. A prospective cohort study of 610 patients with consecutive positive HBsAg noted that cumulative alcohol intake of 500 kg and more was significantly associated with the rate of carcinogenesis, with a RR (95%CI) of 8.37 (2.70-25.93, P = 0.0002)[76]. In addition, alcohol intake was reported to affect the mortality of HBV-related liver disease in a study by Ribes et al[77], in which 2352 HBsAg-positive patients were followed up for 20 years, and lifetime chronic consumption of alcohol more than 60 g/d was associated with a 6-fold increase in the risk of death from liver cirrhosis and HCC. Moreover, patients with HCC caused by CHB and chronic alcohol consumption are approximately 10 years younger than patients with HCC caused by CHB alone[78,79], suggesting that heavy alcohol drinking increases both the mobility and mortality of HCC in CHB patients.
It is worth noting that, compared with heavy alcohol drinking, the influence of mild to moderate alcohol drinking on HBV-induced liver fibrosis, cirrhosis, and HCC among patients infected with HBV remains unclear. A cross-sectional study of CHB patients showed that the incidence of advanced liver fibrosis in those patients who reported drinking alcohol (1-20 g alcohol per day) was similar to that in those who abstained from alcohol[80]; hence, alcohol consumption should be kept to a minimum in patients with HBV infection[81]. A study of 1045 hepatitis B patients showed that the prevalence of advanced liver fibrosis among patients with mild to moderate alcohol intake (26, 18.8%) was comparable to that of non-drinkers (190, 21.0%) (P = 0.57)[80]. The large-scale, prospective cohort REVEAL-HBV study of more than 3500 patients (aged 30-65 years) in Taiwan showed that male sex, older age, seropositivity for HBeAg, and habitual alcohol consumption are significantly correlated with the development of HCC[82]. Taken together, these studies suggest that light-to-moderate habitual alcohol consumption appears to have, at best, a modest correlation with the progression of HBV-induced liver disease[74]; this effect was not always significant, particularly in studies with a relatively small sample size. In addition, more accurate epidemiological and pathophysiological data obtained from larger cohort studies are required in order to examine and trace the risk of developing liver cirrhosis and HCC in patients with HBV infection and light-to moderate alcohol intake.
Alcohol intake is an independent risk factor associated with the progression of HCV infection and its related liver disease[83,84]. Multiple lines of evidence have shown worsened outcome of patients with chronic HCV and heavy alcohol use, although the definition of heavy alcohol use is somewhat different. For example, alcohol intake (40 g ethanol per day or more) is associated with the more rapid progression of HCV-induced liver diseases, including HCV-induced liver fibrosis and cirrhosis, compared to patients who consumed lower levels of alcohol[85]. A meta-analysis showed that the RR of progression to liver cirrhosis was 2.33-fold (95%CI: 1.67-3.26) in patients with heavy alcohol intake (240-560 g per week) compared to those with less heavy alcohol intake among patients with chronic HCV infection[86]. Decompensated cirrhosis in patients with hepatitis C is independently correlated with AUD in British Columbia [hazard ratio (HR): 1.92, 95%CI: 1.76-2.10], New South Wales (HR: 3.68, 95%CI: 3.38-4.00), and Scotland (HR: 3.88, 95%CI: 3.42-4.40)[20]. Heavy alcohol intake increases the risk of HCC in patients with HCV infection[87,88]. Studies from Japan noted an increased risk of developing HCC in HCV patients who drank more than 65 g alcohol daily for over 5 years (RR: 3.04, 95%CI: 1.31-7.09)[89,90]. Similar to the findings in HBV-infected patients, heavy alcohol abuse is associated with an increased risk of developing HCC at a younger age in patients with HCV infection. Among HCV-positive patients who reported drinking alcohol (> 46 g/d), HCC occurred in patients at an average of 26 ± 6 years, younger than 31 ± 9 years for those who consumed alcohol less than 46 g/d[91]. Moreover, comparative analysis of tumor characteristics of HCC patients revealed that the tumors of heavy alcohol drinkers were significantly more anaplastic (5% with well-differentiated HCC vs 45% of nondrinkers) with increased extracapsular, capsular, and portal vein invasion and intrahepatic meta
Compared with heavy alcohol drinking, light-to-moderate alcohol intake has also been shown to promote the progression of HCV-related liver disease[92-94]. Monto et al[92] showed the incremental effects of alcohol on liver fibrosis. Although the liver fibrosis was overall more severe in HCV patients who drank heavily than in those who did not drink, there was a range of disease in each category of alcohol intake (light, moderate, and heavy). Another study of 1574 patients with hepatitis C and alcohol consumption was assessed in three groups of patients: No alcohol intake, moderate alcohol intake (0–49 g/d), and heavy alcohol intake (50 g/d or more). As a result, the rate of progression of fibrosis in fibrosis units per year increased from 0.125 (95%CI: 0.111-0.143) in the no alcohol intake group, to 0.143 (95%CI: 0.118-0.160) in the moderate alcohol intake group, and to 0.167 (95%CI: 0.133-0.174) in the heavy alcohol intake group[95]. In addition, there was a synergistic effect of HCV infection and the consumption of alcohol (< 40 g/d) with the development of HCC[96]. A prospective study showed that light-to-moderate alcohol intake (median alcohol intake: 15 g/d) increases the risk of developing HCC in patients with HCV-related liver cirrhosis (HR for alcohol consumption: 3.43, 95%CI: 1.49-7.92, P = 0.004)[97].
In summary, no safe level of alcohol intake has been established for patients with HCV. Even light-to-moderate alcohol use can exert a synergistic effect with viral hepatitis, leading to the rapid progression of liver disease.
IFN-based therapy is less effective in alcohol drinkers than in control patients, even after abstinence from alcohol for a period of time. As such, it has been recommended that patients with chronic infection of HCV should restrict alcohol intake to < 10 g/d, and abstinence from alcohol should be encouraged in patients with presence of liver cirrhosis or prior to IFN therapy[98]. Direct-acting antivirals are highly effective for the treatment of HCV infection, and alcohol intake is unlikely to alter achievement of sustained virologic suppression among patients with direct-acting antivirals treatment[99].
In terms of effects of alcohol drinking on the clinical efficacy of anti-HBV treatment, relative studies are limited. Hosaka et al[100] showed that alcohol consumption (> 200 kg) is a risk factor for cumulative HCC incidence rates at 5 years in patients with CHB treated with entecavir (HR = 2.21, 95%CI: 1.18-4.16, P = 0.013).
In addition to the effects of alcohol intake on antiviral therapy, liver fibrosis, cirrhosis, and liver cancer, it has not been reported whether it can cause chronic acute liver failure and whether other pathogens could be involved in chronic hepatitis patients. At the same time, there is no detailed report on whether other related decompensation complications can occur more easily or earlier in patients with HBV infection and alcoholism compared to patients with HBV infection or alcoholism alone. Future research efforts should focus on addressing the above issues.
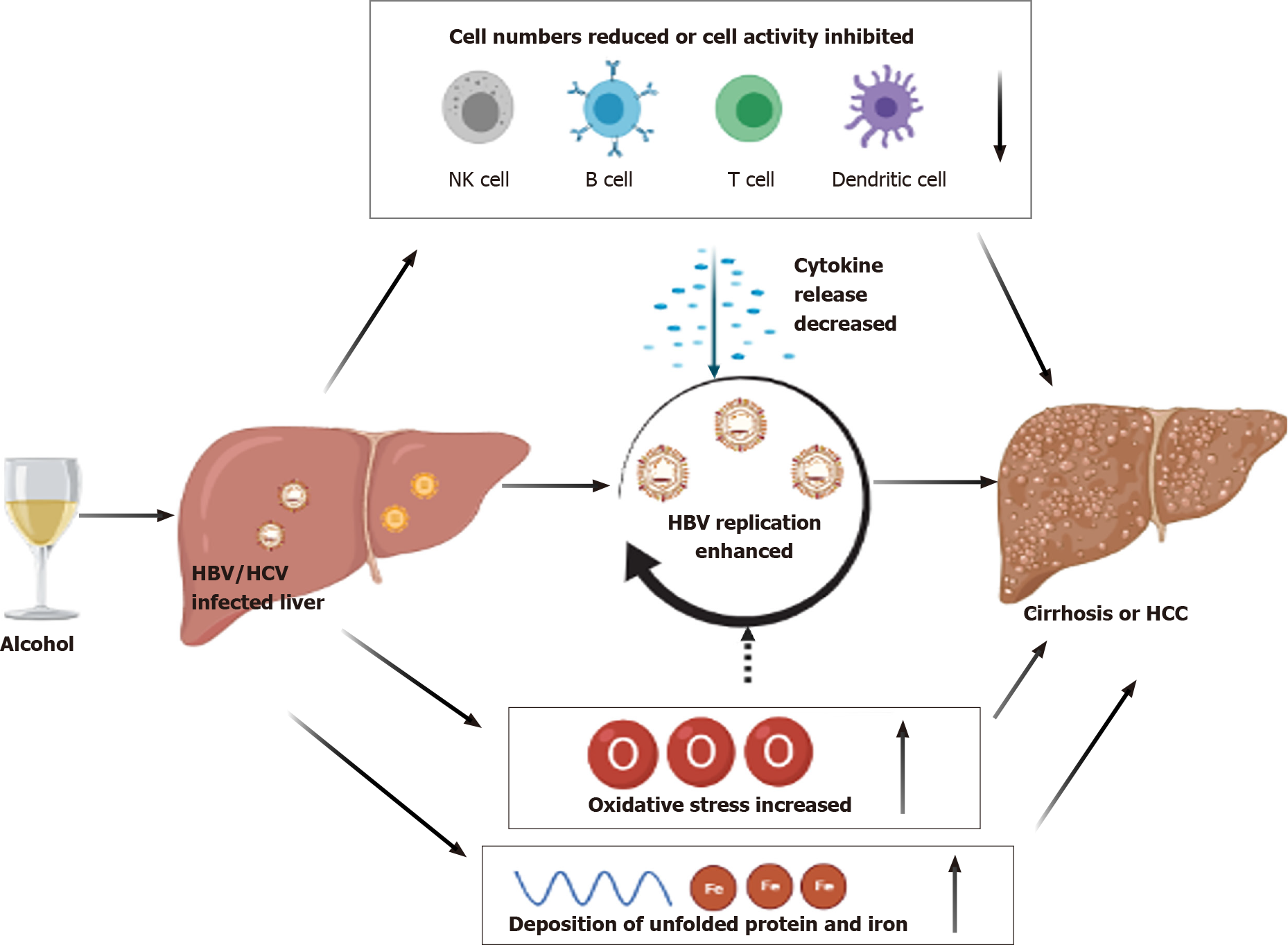
Taken together, the existing studies indicate that alcohol adversely affects HBV and HCV infections in the liver by promoting viral replication and oxidative stress and suppressing viral immune responses. Considering the findings that the interaction of alcohol with viral hepatitis (e.g., HBV, HCV) contributes to the increased risk of developing HBV- or HCV-induced liver fibrosis, end-stage cirrhosis, and even deadly liver cancer, such as HCC, it is highly recommended that individuals with HBV or HCV infection abstain from alcohol to slow disease progression. In addition, these findings may have broader implications that abstaining from alcohol is needed for all individuals to protect the liver (Figure 1).
Figure 1 was created with BioRender.com.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lin W, Mrzljak A, Singeap AM S-Editor: Yan JP L-Editor: Filipodia P-Editor: Li X
| 1. | World Health Organization. Global hepatitis report. 2017. April 2017. [cited 10 February 2021]. Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. |
| 2. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1989] [Article Influence: 198.9] [Reference Citation Analysis (3)] |
| 3. | Tun W, Vu L, Adebajo SB, Abiodun L, Sheehy M, Karlyn A, Njab J, Ahonsi B, Issa BK, Idogho O. Population-based prevalence of hepatitis B and C virus, HIV, syphilis, gonorrhoea and chlamydia in male injection drug users in Lagos, Nigeria. Int J STD AIDS. 2013;24:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Kawagishi N, Suda G, Onozawa M, Kimura M, Maehara O, Ohara M, Izumi T, Umemura M, Ito J, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N. Comparing the risk of hepatitis B virus reactivation between direct-acting antiviral therapies and interferon-based therapies for hepatitis C. J Viral Hepat. 2017;24:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. WHO Global Health Estimates 2015: deaths by cause, age, sex, by country and by region, 2000–2015. 2016. [cited 10 February 2021]. Available from:https://www.who.int/healthinfo/global_burden_disease/estimates_regional_2000_15/en/. |
| 6. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1578] [Article Influence: 175.3] [Reference Citation Analysis (2)] |
| 7. | Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The Effects of Hepatic Steatosis on the Natural History of HBV Infection. Clin Liver Dis. 2019;23:433-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol. 2019;25:3527-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 9. | Cortes VF, Taveira A, Cruz HM, Reis AA, Cezar JS, Silva BS, D'Assunção CF, Lampe E, Villar LM. Prevalence of Hepatitis B and C virus infection among alcoholic individuals: importance of screening and vaccination. Rev Inst Med Trop Sao Paulo. 2017;59:e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Taylor AL, Denniston MM, Klevens RM, McKnight-Eily LR, Jiles RB. Association of Hepatitis C Virus With Alcohol Use Among U.S. Adults: NHANES 2003-2010. Am J Prev Med. 2016;51:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. 17 May 2016. [cited 10 February 2021]. Available from: https://www.who.int/publications-detail-redirect/WHO-HIV-2016.06. |
| 12. | Befrits R, Hedman M, Blomquist L, Allander T, Grillner L, Kinnman N, Rubio C, Hultcrantz R. Chronic hepatitis C in alcoholic patients: prevalence, genotypes, and correlation to liver disease. Scand J Gastroenterol. 1995;30:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Shimizu S, Kiyosawa K, Sodeyama T, Tanaka E, Nakano M. High prevalence of antibody to hepatitis C virus in heavy drinkers with chronic liver diseases in Japan. J Gastroenterol Hepatol. 1992;7:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Novo-Veleiro I, Calle Cde L, Domínguez-Quibén S, Pastor I, Marcos M, Laso FJ. Prevalence of hepatitis C virus infection in alcoholic patients: cohort study and systematic review. Alcohol Alcohol. 2013;48:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Nishiguchi S, Kuroki T, Yabusako T, Seki S, Kobayashi K, Monna T, Otani S, Sakurai M, Shikata T, Yamamoto S. Detection of hepatitis C virus antibodies and hepatitis C virus RNA in patients with alcoholic liver disease. Hepatology. 1991;14:985-989. [PubMed] |
| 16. | Mendenhall CL, Seeff L, Diehl AM, Ghosn SJ, French SW, Gartside PS, Rouster SD, Buskell-Bales Z, Grossman CJ, Roselle GA. Antibodies to hepatitis B virus and hepatitis C virus in alcoholic hepatitis and cirrhosis: their prevalence and clinical relevance. The VA Cooperative Study Group (No. Hepatology. 1991;14:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 17. | Kwon SY, Ahn MS, Chang HJ. Clinical significance of hepatitis C virus infection to alcoholics with cirrhosis in Korea. J Gastroenterol Hepatol. 2000;15:1282-1286. [PubMed] |
| 18. | Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | World Health Organization. Global status report on alcohol and health 2018. 27 Sep 2018. [cited 10 February 2021]. Available from: https://apps.who.int/iris/handle/10665/274603. |
| 20. | Alavi M, Janjua NZ, Chong M, Grebely J, Aspinall EJ, Innes H, Valerio HM, Hajarizadeh B, Hayes PC, Krajden M, Amin J, Law MG, George J, Goldberg DJ, Hutchinson SJ, Dore GJ. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Rosman AS, Waraich A, Galvin K, Casiano J, Paronetto F, Lieber CS. Alcoholism is associated with hepatitis C but not hepatitis B in an urban population. Am J Gastroenterol. 1996;91:498-505. [PubMed] |
| 22. | Balasubramanian S, Kowdley KV. Effect of alcohol on viral hepatitis and other forms of liver dysfunction. Clin Liver Dis. 2005;9:83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Dolganiuc A. Alcohol and Viral Hepatitis: Role of Lipid Rafts. Alcohol Res. 2015;37:299-309. [PubMed] |
| 24. | Goudeau A, Maupas P, Dubois F, Coursaget P, Bougnoux P. Hepatitis B infection in alcoholic liver disease and primary hepatocellular carcinoma in France. Prog Med Virol. 1981;27:26-34. [PubMed] |
| 25. | Hislop WS, Follett EA, Bouchier IA, MacSween RN. Serological markers of hepatitis B in patients with alcoholic liver disease: a multi-centre survey. J Clin Pathol. 1981;34:1017-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Orholm M, Aldershvile J, Tage-Jensen U, Schlichting P, Nielsen JO, Hardt F, Christoffersen P. Prevalence of hepatitis B virus infection among alcoholic patients with liver disease. J Clin Pathol. 1981;34:1378-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Zarski JP, Thelu MA, Moulin C, Rachail M, Seigneurin JM. Interest of the detection of hepatitis C virus RNA in patients with alcoholic liver disease. Comparison with the HBV status. J Hepatol. 1993;17:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ; Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 913] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 29. | Lin YP, Cheng TJ. Why can't Chinese Han drink alcohol? Med Hypotheses. 2002;59:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Tikhanovich I, Kuravi S, Campbell RV, Kharbanda KK, Artigues A, Villar MT, Weinman SA. Regulation of FOXO3 by phosphorylation and methylation in hepatitis C virus infection and alcohol exposure. Hepatology. 2014;59:58-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Larkin J, Clayton MM, Liu J, Feitelson MA. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice. Hepatology. 2001;34:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Ganesan M, Krutik VM, Makarov E, Mathews S, Kharbanda KK, Poluektova LY, Casey CA, Osna NA. Acetaldehyde suppresses the display of HBV-MHC class I complexes on HBV-expressing hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2019;317:G127-G140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 33. | Min BY, Kim NY, Jang ES, Shin CM, Lee SH, Park YS, Hwang JH, Jeong SH, Kim N, Lee DH, Kim JW. Ethanol potentiates hepatitis B virus replication through oxidative stress-dependent and -independent transcriptional activation. Biochem Biophys Res Commun. 2013;431:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 34. | Bar-Yishay I, Shaul Y, Shlomai A. Hepatocyte metabolic signalling pathways and regulation of hepatitis B virus expression. Liver Int. 2011;31:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Quasdorff M, Protzer U. Control of hepatitis B virus at the level of transcription. J Viral Hepat. 2010;17:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Ran M, Chen H, Liang B, Liao W, Jiang J, Huang J, Ning C, Zang N, Zhou B, Liao Y, Liu H, Qin F, Yang Q, Li J, Ho W, Liang H, Ye L. Alcohol-induced autophagy via upregulation of PIASy promotes HCV replication in human hepatoma cells. Cell Death Dis. 2018;9:898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Sobhanimonfared F, Bamdad T, Roohvand F. Cross talk between alcohol-induced oxidative stress and HCV replication. Arch Microbiol. 2020;202:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Plumlee CR, Lazaro CA, Fausto N, Polyak SJ. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol J. 2005;2:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Cromie SL, Jenkins PJ, Bowden DS, Dudley FJ. Chronic hepatitis C: effect of alcohol on hepatitic activity and viral titre. J Hepatol. 1996;25:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Anand BS, Thornby J. Alcohol has no effect on hepatitis C virus replication: a meta-analysis. Gut. 2005;54:1468-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Ran M, Huang J, Liang H, Jiang J, Liang B, Ning C, Zang N, Liao W, Liu H, Qin F, Yang Q, Ho W, Ye L, Chen H. Alcohol attenuates anti-HCV function of IFN-λ1 through up-regulation of PLASy expression in human hepatic cells. J Med Virol. 2018;90:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O'Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003;27:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Perlemuter G, Lettéron P, Carnot F, Zavala F, Pessayre D, Nalpas B, Bréchot C. Alcohol and hepatitis C virus core protein additively increase lipid peroxidation and synergistically trigger hepatic cytokine expression in a transgenic mouse model. J Hepatol. 2003;39:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Kim WH, Hong F, Jaruga B, Hu Z, Fan S, Liang TJ, Gao B. Additive activation of hepatic NF-kappaB by ethanol and hepatitis B protein X (HBX) or HCV core protein: involvement of TNF-alpha receptor 1-independent and -dependent mechanisms. FASEB J. 2001;15:2551-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Cook RT, Waldschmidt TJ, Cook BL, Labrecque DR, McLatchie K. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clin Exp Immunol. 1996;103:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Matos LC, Batista P, Monteiro N, Ribeiro J, Cipriano MA, Henriques P, Girão F, Carvalho A. Lymphocyte subsets in alcoholic liver disease. World J Hepatol. 2013;5:46-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Pasala S, Barr T, Messaoudi I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015;37:185-197. [PubMed] |
| 50. | Szabo G, Wands JR, Eken A, Osna NA, Weinman SA, Machida K, Joe Wang H. Alcohol and hepatitis C virus--interactions in immune dysfunctions and liver damage. Alcohol Clin Exp Res. 2010;34:1675-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Osna NA, Ganesan M, Kharbanda KK. Hepatitis C, innate immunity and alcohol: friends or foes? Biomolecules. 2015;5:76-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Rigamonti C, Mottaran E, Reale E, Rolla R, Cipriani V, Capelli F, Boldorini R, Vidali M, Sartori M, Albano E. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology. 2003;38:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Schmid RM, Adler G. NF-kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology. 2000;118:1208-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Baeuerle PA. Pro-inflammatory signaling: last pieces in the NF-kappaB puzzle? Curr Biol. 1998;8:R19-R22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 55. | Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor kappa B in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994;269:30429-30435. [PubMed] |
| 56. | Tai DI, Tsai SL, Chen YM, Chuang YL, Peng CY, Sheen IS, Yeh CT, Chang KS, Huang SN, Kuo GC, Liaw YF. Activation of nuclear factor kappaB in hepatitis C virus infection: implications for pathogenesis and hepatocarcinogenesis. Hepatology. 2000;31:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Gitto S, Vitale G, Villa E, Andreone P. Update on Alcohol and Viral Hepatitis. J Clin Transl Hepatol. 2014;2:228-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Wong RH, Yeh CY, Hsueh YM, Wang JD, Lei YC, Cheng TJ. Association of hepatitis virus infection, alcohol consumption and plasma vitamin A levels with urinary 8-hydroxydeoxyguanosine in chemical workers. Mutat Res. 2003;535:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Lin YT, Liu W, He Y, Wu YL, Chen WN, Lin XJ, Lin X. Hepatitis B Virus X Protein Increases 8-Oxo-7,8-Dihydro-2'-Deoxyguanosine (8-Oxodg) Level via Repressing MTH1/ MTH2 Expression in Hepatocytes. Cell Physiol Biochem. 2018;51:80-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Hernandez C, Blanc EB, Pène V, Le-Grand B, Villaret M, Aoudjehane L, Carpentier A, Conti F, Calmus Y, Podevin P, Garlatti M, Rouach H, Rosenberg AR. Impact of hepatitis C virus and alcohol, alone and combined, on the unfolded protein response in primary human hepatocytes. Biochimie. 2020;168:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 773] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 62. | Izumi N, Enomoto N, Uchihara M, Murakami T, Ono K, Noguchi O, Miyake S, Nouchi T, Fujisawa K, Marumo F, Sato C. Hepatic iron contents and response to interferon-alpha in patients with chronic hepatitis C. Relationship to genotypes of hepatitis C virus. Dig Dis Sci. 1996;41:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Piperno A, Vergani A, Malosio I, Parma L, Fossati L, Ricci A, Bovo G, Boari G, Mancia G. Hepatic iron overload in patients with chronic viral hepatitis: role of HFE gene mutations. Hepatology. 1998;28:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai MM. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691-3697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 65. | Gao B. Interaction of alcohol and hepatitis viral proteins: implication in synergistic effect of alcohol drinking and viral hepatitis on liver injury. Alcohol. 2002;27:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Pianko S, Patella S, Sievert W. Alcohol consumption induces hepatocyte apoptosis in patients with chronic hepatitis C infection. J Gastroenterol Hepatol. 2000;15:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Donato F, Tagger A, Chiesa R, Ribero ML, Tomasoni V, Fasola M, Gelatti U, Portera G, Boffetta P, Nardi G. Hepatitis B and C virus infection, alcohol drinking, and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC Study. Hepatology. 1997;26:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Lee YH, Hsu CY, Hsia CY, Huang YH, Su CW, Chiou YY, Lin HC, Huo TI, Lee SD. Alcoholism worsens the survival of patients with hepatitis B virus and C virus-related hepatocellular carcinoma. Hepatol Int. 2013;7:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 533] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 70. | Nomura H, Hayashi J, Kajiyama W, Kashiwagi S. Alcohol consumption and seroconversion from hepatitis B e antigen in the Okinawa Japanese. Fukuoka Igaku Zasshi. 1996;87:237-241. [PubMed] |
| 71. | Murata T, Takanari H, Watanabe S, Tanaka T, Suzuki S. Enhancement of chronic viral hepatitic changes by alcohol intake in patients with persistent HBs-antigenemia. Am J Clin Pathol. 1990;94:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi A, Tani S, Goto M. An epidemiologic study of effects of alcohol in the liver in hepatitis B surface antigen carriers. Am J Epidemiol. 1988;128:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, Lo GH, Chen YS, Yen YC, Hu JT, Yu ML, Lee PH, Lin JT, Yang SS. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol. 2013;58:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Iida-Ueno A, Enomoto M, Tamori A, Kawada N. Hepatitis B virus infection and alcohol consumption. World J Gastroenterol. 2017;23:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Oshima A, Tsukuma H, Hiyama T, Fujimoto I, Yamano H, Tanaka M. Follow-up study of HBs Ag-positive blood donors with special reference to effect of drinking and smoking on development of liver cancer. Int J Cancer. 1984;34:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N, Kumada H. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 314] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 77. | Ribes J, Clèries R, Rubió A, Hernández JM, Mazzara R, Madoz P, Casanovas T, Casanova A, Gallen M, Rodríguez C, Moreno V, Bosch FX. Cofactors associated with liver disease mortality in an HBsAg-positive Mediterranean cohort: 20 years of follow-up. Int J Cancer. 2006;119:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Ohnishi K, Iida S, Iwama S, Goto N, Nomura F, Takashi M, Mishima A, Kono K, Kimura K, Musha H, Kotota K, Okuda K. The effect of chronic habitual alcohol intake on the development of liver cirrhosis and hepatocellular carcinoma: relation to hepatitis B surface antigen carriage. Cancer. 1982;49:672-677. [PubMed] |
| 79. | Pereira FE, Gonçalves CS, Zago Mda P. The effect of ethanol intake on the development of hepatocellular carcinoma in HBsAg carriers. Arq Gastroenterol. 1994;31:42-46. [PubMed] |
| 80. | Ong A, Wong VW, Wong GL, Chan HL. The effect of caffeine and alcohol consumption on liver fibrosis - a study of 1045 Asian hepatitis B patients using transient elastography. Liver Int. 2011;31:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Hagström H. Alcohol Consumption in Concomitant Liver Disease: How Much is Too Much? Curr Hepatol Rep. 2017;16:152-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2360] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 83. | Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 84. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 739] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 85. | Wiley TE, McCarthy M, Breidi L, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 302] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 86. | Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Grebely J, Dore GJ. What is killing people with hepatitis C virus infection? Semin Liver Dis. 2011;31:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 88. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C, Porru S, Nardi G. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 89. | Kubo S, Kinoshita H, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Kuroki T. High malignancy of hepatocellular carcinoma in alcoholic patients with hepatitis C virus. Surgery. 1997;121:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Aizawa Y, Shibamoto Y, Takagi I, Zeniya M, Toda G. Analysis of factors affecting the appearance of hepatocellular carcinoma in patients with chronic hepatitis C. A long term follow-up study after histologic diagnosis. Cancer. 2000;89:53-59. [PubMed] |
| 91. | Noda K, Yoshihara H, Suzuki K, Yamada Y, Kasahara A, Hayashi N, Fusamoto H, Kamada T. Progression of type C chronic hepatitis to liver cirrhosis and hepatocellular carcinoma--its relationship to alcohol drinking and the age of transfusion. Alcohol Clin Exp Res. 1996;20:95A-100A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Monto A, Patel K, Bostrom A, Pianko S, Pockros P, McHutchison JG, Wright TL. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004;39:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | Hézode C, Lonjon I, Roudot-Thoraval F, Pawlotsky JM, Zafrani ES, Dhumeaux D. Impact of moderate alcohol consumption on histological activity and fibrosis in patients with chronic hepatitis C, and specific influence of steatosis: a prospective study. Aliment Pharmacol Ther. 2003;17:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Brogniez V, Nyssen-Behets C, Grégoire V, Reychler H, Lengelé B. Implant osseointegration in the irradiated mandible. A comparative study in dogs with a microradiographic and histologic assessment. Clin Oral Implants Res. 2002;13:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2158] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 96. | Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 97. | Vandenbulcke H, Moreno C, Colle I, Knebel JF, Francque S, Sersté T, George C, de Galocsy C, Laleman W, Delwaide J, Orlent H, Lasser L, Trépo E, Van Vlierberghe H, Michielsen P, van Gossum M, de Vos M, Marot A, Doerig C, Henrion J, Deltenre P. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J Hepatol. 2016;65:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Schiff ER. Hepatitis C and alcohol. Hepatology. 1997;26:39S-42S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 100. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 540] [Article Influence: 45.0] [Reference Citation Analysis (0)] |