Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9997
Peer-review started: June 30, 2021
First decision: July 26, 2021
Revised: July 27, 2021
Accepted: September 23, 2021
Article in press: September 30, 2021
Published online: November 16, 2021
Processing time: 132 Days and 14.5 Hours
Chronic granulomatous disease (CGD) characterized by recurrent and severe bacterial and fungal infections is most common in childhood.
We reported a 24-d-old male infant who developed gastrointestinal symptoms as the first sign of CGD.
Gastrointestinal symptoms representing the first sign of CGD are very rare, and prompt diagnosis and treatment with broad-spectrum antibiotics were of crucial importance.
Core Tip: Most chronic granulomatous diseases (CGDs) present with life-threatening recurrent bacterial and fungal infections, usually diagnosed in childhood. We describe a newborn with CGD that initially showed gastrointestinal symptoms. CGD cases with gastrointestinal manifestations as the first sign are very rare. To the best of our knowledge, this might be the youngest case of confirmed CGD with gastrointestinal involvement.
- Citation: Meng EY, Wang ZM, Lei B, Shang LH. Gastrointestinal symptoms as the first sign of chronic granulomatous disease in a neonate: A case report. World J Clin Cases 2021; 9(32): 9997-10005
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9997.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9997
Chronic granulomatous disease (CGD) is a rare inherited primary immunodeficiency of the phagocytic system that leads to recurrent and severe bacterial and fungal infections, with a high mortality rate[1,2]. Most patients initially have an infection, which affects the lymph nodes, lungs, liver, bones and skin[3]. Recent studies have shown the high prevalence of gastrointestinal complications in patients with CGD[4,5]. CGD episodes in the neonatal period have some uncommon features and can easily be ignored. Herein, we describe a 24-d-old male infant with newly diagnosed CGD who presented with prolonged fever of unknown origin, persistent diarrhea and elevated C-reactive protein (CRP).
A 24-d-old Chinese male baby with a history of fever for 8 d and diarrhea for 10 d attended the Neonatology Department in our hospital.
The baby was treated with antibiotics at the local hospital, but he did not respond. He was fed with formula milk. The mother reported that the baby had increased fussiness, irritability, and unsatisfactory weight gain, while he continued to feed well and maintained good urine output. She denied any other symptoms or any medication use.
After birth, the infant received mechanical ventilation and piperacillin-tazobactam treatment in the Neonatal Intensive Care Unit due to meconium aspiration syndrome, and was discharged on the 10th day of life.
The primipara gave birth to the baby via a simple cesarean section at 38 wk of gestation. The baby weighed 3350 g at birth, with an APGAR Score of 6 points at 1 min and 7 points at 5 min after birth. No family history of CGD was reported. The newborn was cared for by his parents.
During the physical examination, the baby had a body temperature of 39.3°C, a heart rate of 152 bpm, respiratory rate of 40 breaths/min, weight of 3.92 kg, blood pressure of 65/36 mmHg, and an oxygen saturation of 95% in room air. The baby cried continuously and was not easily pacified. He was alert and appeared in distress. His abdomen was bulging, but there was no evident tenderness. The lung, heart, skin and nervous system examination results were all within the normal range.
A complete blood count suggested mild anemia and leukocytosis. Renal function test results and the measured values of serum electrolytes, glucose, phosphorus, direct and total bilirubin were all within the normal range. Total protein and albumin concentrations were decreased, while alanine aminotransferase and aspartate aminotransferase concentrations were slightly elevated. A routine stool test was normal. Blood, urine, stool and cerebrospinal fluid cultures indicated the absence of pathogens. Serum galactomannan and (1,3)-β-D-glucan (two fungal tests were negative, revealing no fungal infection. Only CRP level was significantly increased.
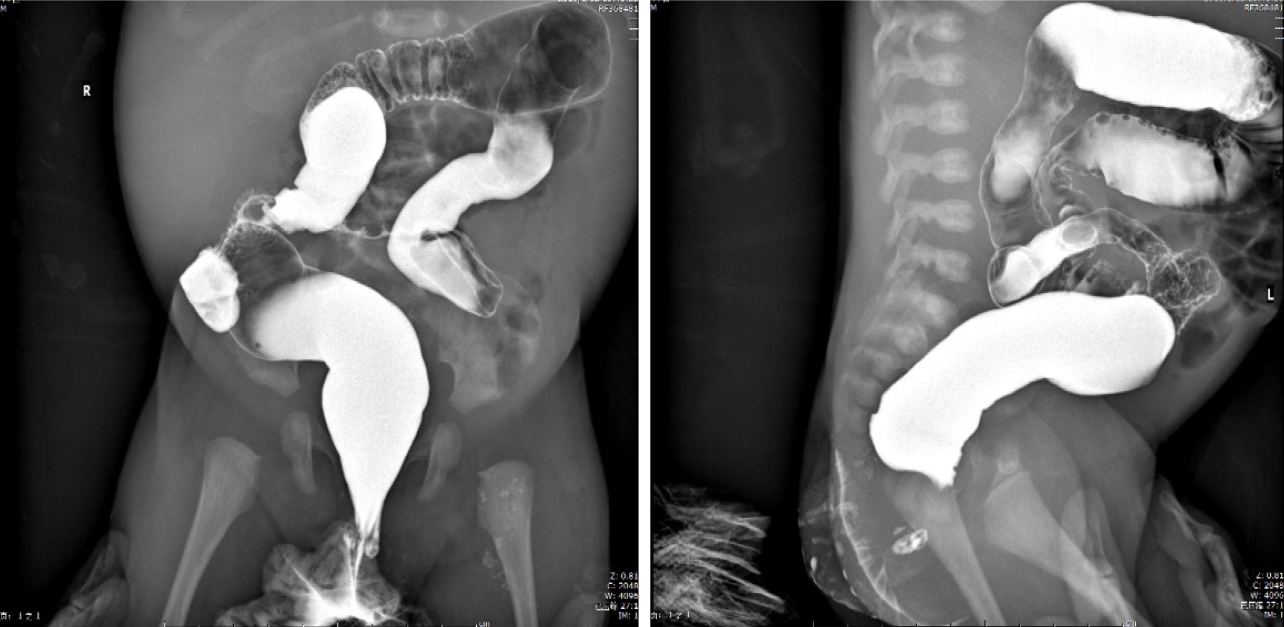
Chest X-ray showed increased bilateral markings. Mild flatulence was evident on abdominal X-ray images. Ultrasound examinations of the baby’s abdomen and brain were performed, and the results were normal.
The child was finally diagnosed with CGD with initial gastrointestinal symptoms.
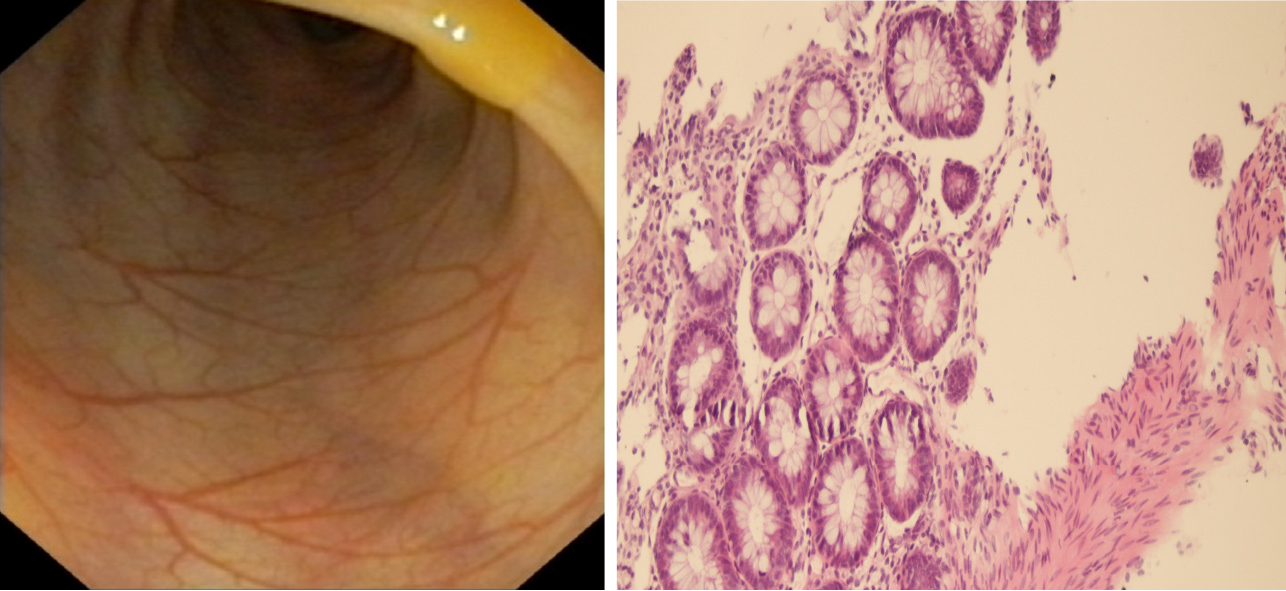
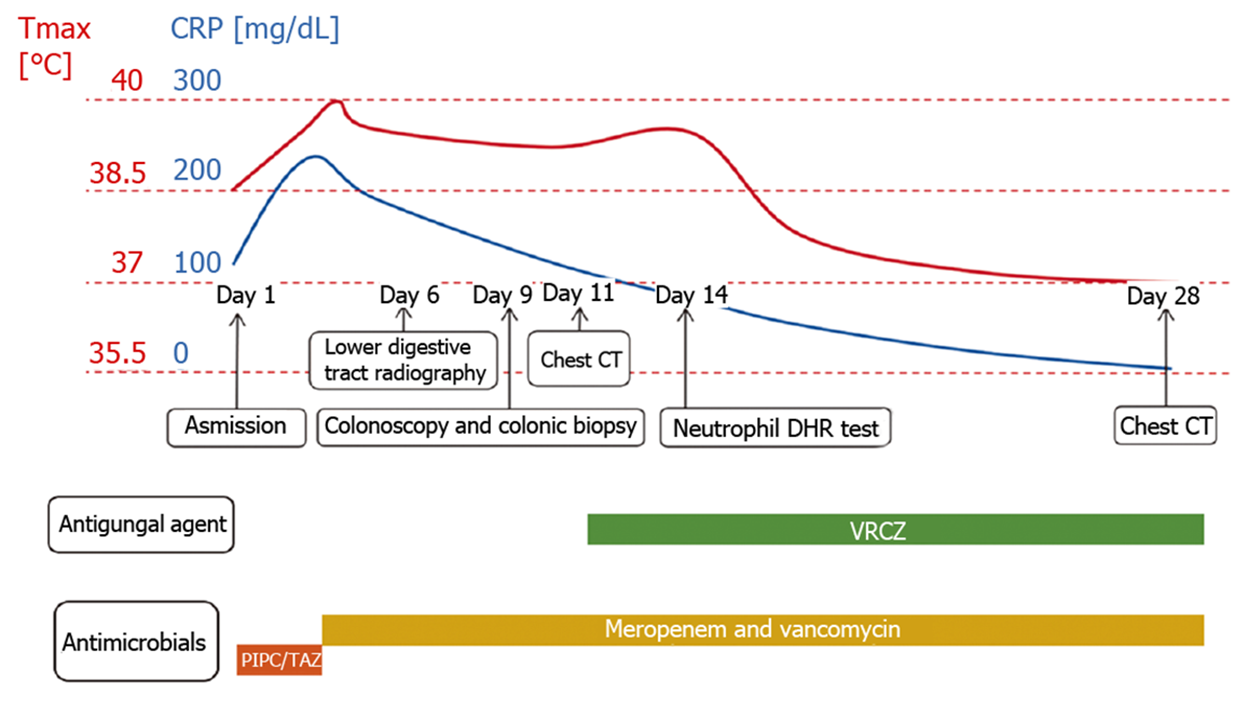
Piperacillin-tazobactam (300 mg/kg/d) was commenced under the diagnosis of sepsis. After 4 d of antibiotic treatment, fever and diarrhea did not improve, and the CRP level rose sharply to 239.45 mg/dL. This suggested that the newborn did not respond to piperacillin-tazobactam. Therefore, vancomycin and meropenem were intravenously injected 4 d after admission. X-ray was performed on the 6th day to check the lower gastrointestinal tract, and no abnormalities were observed (Figure 1). As inflammatory bowel disease (IBD) was highly suspected, a colonoscopy was conducted on day 9, revealing intestinal mucosal enteritis (Figure 2A). Histopathological examination of the intestinal tissue showed chronic mucosal inflammation without granulomas, fissures, or bowel wall thickening (Figure 2B). Therefore, the diagnosis of IBD was excluded.
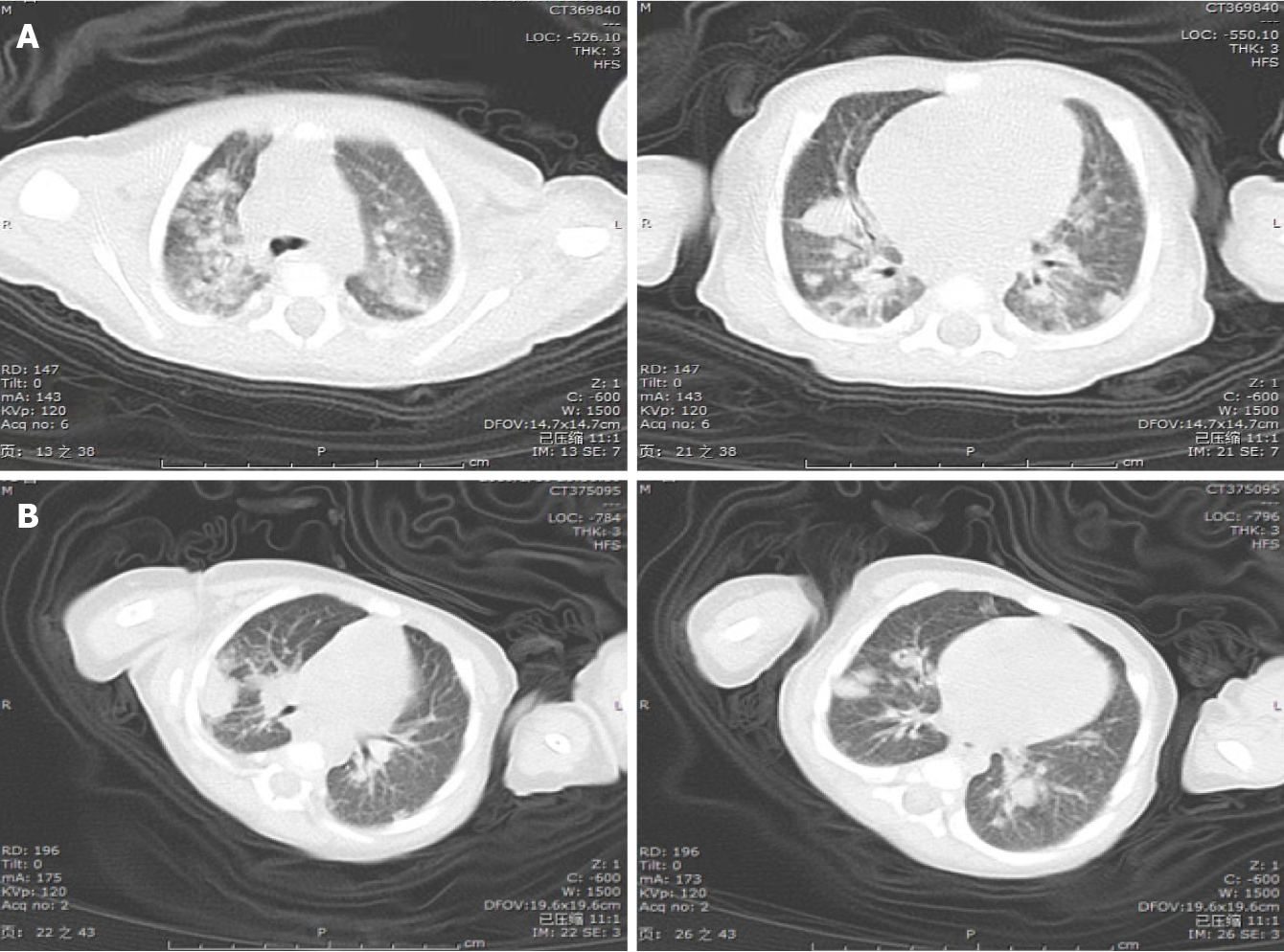
The infant still had a high fever (Tmax 40ºC) every day, but no obvious infection site was found. A computed tomography (CT) scan of the thorax was performed on day 11, revealing diffuse, scattered nodules and bilateral consolidation areas (Figure 3A). Due to suspicion of aspergillosis, voriconazole (20 mg/kg/d) was injected intravenously.
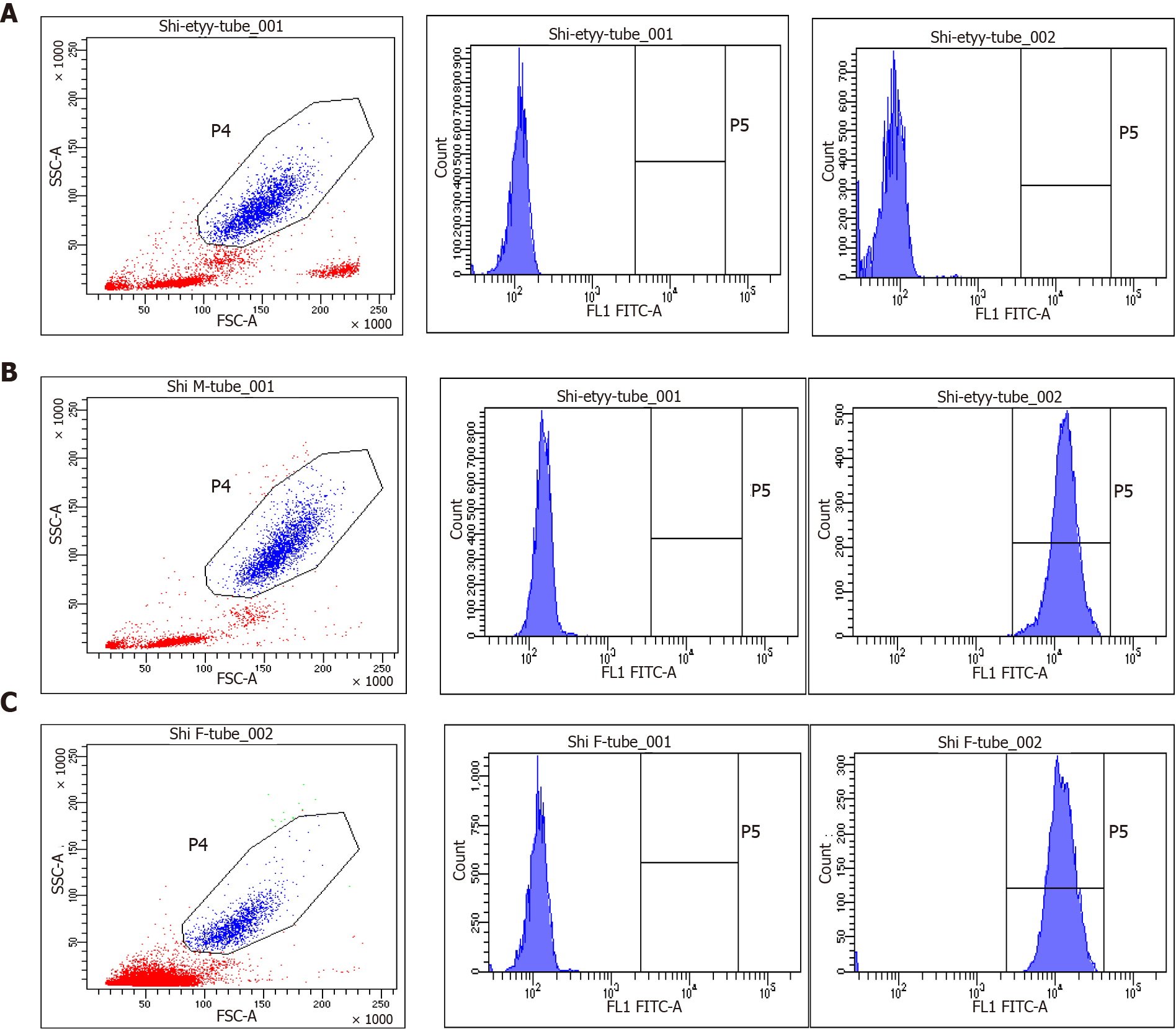
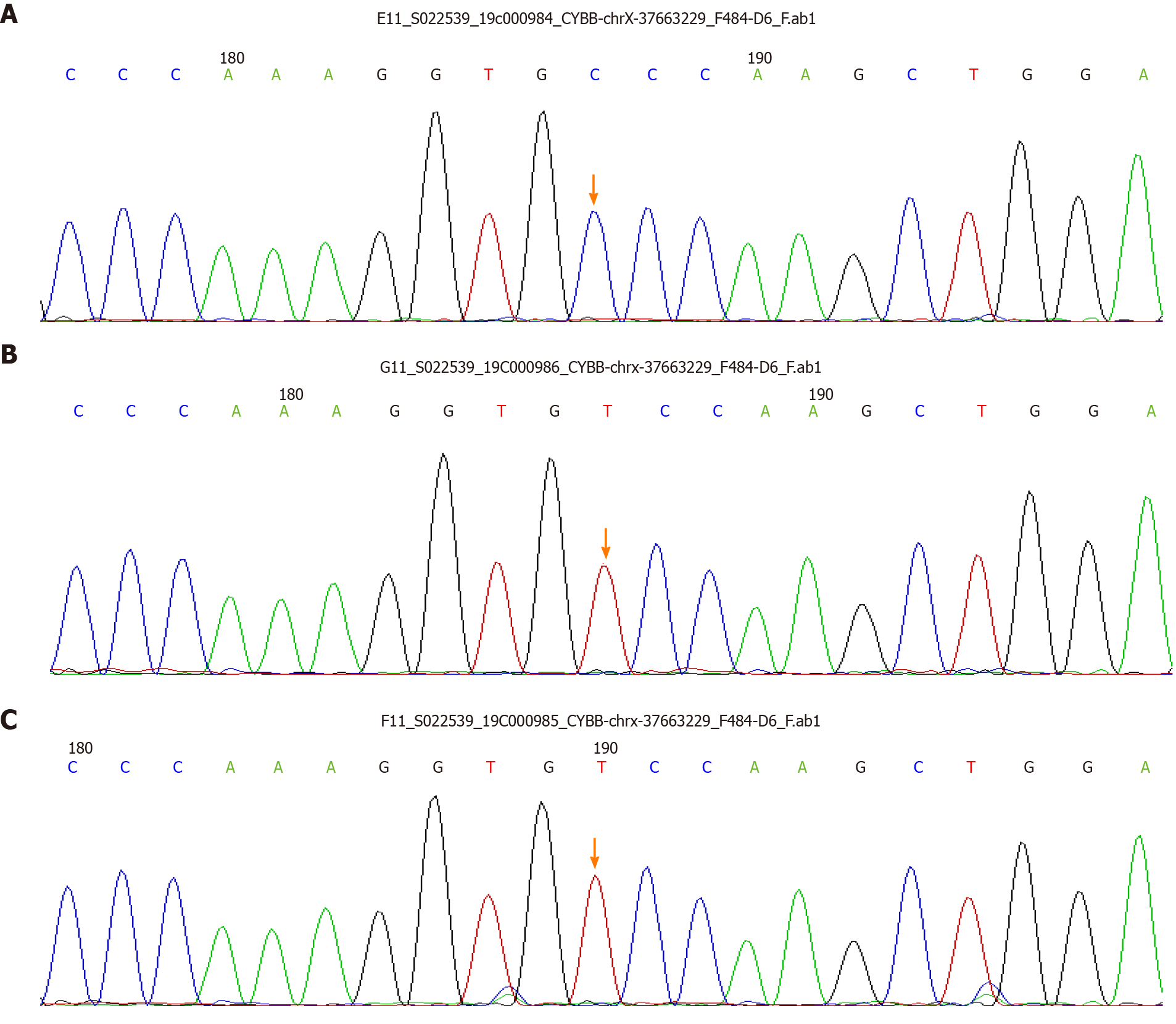
As primary immunodeficiencies were suspected, immunologic evaluations were performed. The neutrophil dihydrorhodamine (DHR) test revealed that the baby’s neutrophils lacked the ability to produce superoxide, and the ratio of activated neutrophils in his mother and father was 99.6% and 98.5%, respectively (Figure 4). The diagnosis of CGD was preliminarily confirmed, and subsequent genetic testing revealed a mutation in the CYBB gene (Figure 5).
After four weeks of combined anti-infection therapy with meropenem, vancomycin and voriconazole, the baby’s condition improved, and chest CT revealed a reduction in nodules and consolidations (Figure 3B). He was discharged on day 30. Following discharge, as the baby had a history of recurrent perianal abscesses, he received prophylactic trimethoprim-sulfamethoxazole, linezolid and voriconazole treatment. During the one-and-half-year follow-up, the child was in good condition except for recurrent perianal abscesses and infection. He underwent hematopoietic stem cell transplantation (HSCT) at another medical institution. The clinical time course of the patient is shown in Figure 6, and sequential laboratory data are shown in Table 1.
| Day 1 | Day 3 | Day 5 | Day 10 | Day 14 | Day 17 | Day 22 | Day 28 | |
| White blood cells [103/mL] | 15640 | 13620 | 4910 | 9180 | 10720 | 10740 | 8160 | 9320 |
| Hemoglobin [g/dL] | 11.2 | 9.3 | 8.3 | 7.3 | 9.9 | 9.5 | 9.5 | 9 |
| Platelets [107/mL] | 17.8 | 19.5 | 18.5 | 11.5 | 11.1 | 24.1 | 34.2 | 28.2 |
| CRP [mg/dL] | 121.59 | 239.45 | 193.53 | 124.33 | 82.52 | 52.3 | 26.72 | 5.8 |
| CSF white blood cells [103/mL] | 10 | 33 | 0 | |||||
| CSF glucose [mmol/L] | 3.1 | 2 | 2.8 | |||||
| CSF protein [mg/dL] | 7.9 | 17.7 | 9.6 |
As stated in the United States and European cohorts[1,3], the prevalence of CGD has been reported to range between 1/200000-250000 live births. However, the actual incidence might be even higher as estimations also tend to depend on clinical expertise. To date, fewer cases have been reported in China, which reveals that the disease has not yet been widely recognized in China.
Due to the inability to generate superoxide and to destroy certain infectious pathogens, CGD is one of the most common primary immunodeficiency diseases. The majority of patients are diagnosed before they are 5 years old[1,2]. Only 35 neonatal CGD cases have been reported in the English literature to date[6]. The main clinical features included pneumonia or pulmonary abscess or pleural effusion, diarrhea, perianal abscess, skin infection, aspergillus infection and tuberculosis infection. Pneumonia is the most common form of infection, although abscesses and lymphangitis are also frequently observed. Infections are mainly caused by five pathogens: Staphylococcus aureus, Serratia marcescens, Burkholderia cepacia, Nocardia and
Approximately half of CGD patients have IBD which may be indistinguishable from Crohn’s disease[5,12,13]. Inflammatory granulomatous colitis can also be accompanied by obstructive disease, diarrhea, malabsorption, perianal abscess, or other complications. The baby in this report first presented with fever, diarrhea and elevated CRP. Consequently, he was thought to be suffering from IBD, for which colonoscopy was performed, revealing no evidence of colitis or related complications. We did not consider CGD until a chest CT performed one week after admission revealed diffuse, scattered bilateral nodules and consolidation areas. Early diagnosis and prompt treatment for these conditions are crucial for optimal outcome in affected patients. It is important to recognize that CGD can present with gastrointestinal manifestations without any evidence of infection. Diarrhea in this baby was a specific symptom of CGD, but not the finding of concomitant infection.
As primary immunodeficiencies were suspected, immunologic evaluations were performed. Flow cytometry is currently the most widely used technique, as it is rapid, sensitive and multiparametric. DHR is the most effective indicator for reactive oxygen species (ROS) measurement by flow cytometry[14]. The DHR assay revealed that the superoxide-generating ability of neutrophils in this baby was absent, and his parents were healthy. Based on the above results, the baby was initially diagnosed with CGD. Further gene sequencing data confirmed the diagnosis. The DHR assay has become the main diagnostic method for CGD, which can be used to identify patients with mild disease and CGD-related gene carriers and may help differentiate the X-linked recessive (XR) and autosomal recessive (AR) inheritance[1].
Genetic mutations of CGD occur in one of the six following genes: CYBB (encoding gp91), NCF1 (p47p hox), NCF2 (p67phox), CYBA (p22 phox), NCF4 (p40phox) and CYBC1 (EROS). XR-CGD is caused by mutation of the CYBB gene in 65% of all CGD patients. AR-CGD has been shown to be associated with mutations of NCF1, NCF2 and CYBA genes encoding p47phox, p67phox or P22phox[15]. Furthermore, the geographic context and social/cultural background, which influence the frequency of consanguineous marriage, change the balance between XR-CGD and AR-CGD and determine the relative distribution of the two inheritance forms in different countries. XR-CGD is the most common form in Europe, United States and Japan[3,16,17]. AR-CGD is the most predominant form in the Middle East, North Africa, and western, central, and southern Asia[18]. However, the study by Marks et al[5] suggested no significant difference in any demographic characteristics between the X-linked and autosomal groups. A large sample single-center study in China showed that XR-CGD accounted for 81.6% of all CGD[19].
Gastrointestinal manifestations in CGD have been reported to occur at greater rates in patients with X-linked disease[20]. The baby had a CYBB coding region hemizygous variant C. 997T > C, resulting in a serine to proline amino acid 333 substitution (p.S333P), which was a previously reported missense variant. Family validation analysis showed that there was no mutation in the paternal and maternal locus. The mutation was spontaneous and pathogenic. Clinicians have noted a milder clinical course for AR-CGD patients, leading to longer survival compared to X-linked CGD patients, although the exact cause was unclear[20]. The deficiency of gp91phox still appears to be one of the steady determinants of mortality[21]. Unfortunately, the baby had a hemizygous variant in the CYBB gene (encoding gp91), and the presentation in the neonatal period was related to the effect of the variant on ROS production. It was evident that this variant was associated with a marked decrease in ROS production, which might explain his early manifestations.
At present, HSCT is the only definitive treatment to cure CGD and reverse organ dysfunction[22]. Timing, donor selection and conditioning regimens remain the key points of this therapy. Furthermore, surviving CGD patients with gastrointestinal manifestations who received HSCT seem to thrive and have better outcomes. Colitis in CGD patients may be a consideration in favoring HSCT[23,24]. Our patient presented with gastrointestinal manifestations so he may achieve a good outcome through HSCT. Fortunately, the patient underwent HSCT recently, and the treatment effect was good.
The diagnosis of CGD in the neonatal period tends to be delayed due to the heterogeneity of clinical manifestations. The male neonate in this report initially presented with CGD-associated IBD. His abnormal DHR test and the detection of CYBB gene mutation confirmed the diagnosis. This case highlights the importance of a thorough medical history review and complete laboratory examination in evaluating patients. To the best of our knowledge, this might be the youngest confirmed case of CGD with gastrointestinal involvement as the first sign.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Afzal MS, Crocé LS, Farhadi R S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore). 2000;79:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1081] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 2. | Wolach B, Gavrieli R, de Boer M, van Leeuwen K, Berger-Achituv S, Stauber T, Ben Ari J, Rottem M, Schlesinger Y, Grisaru-Soen G, Abuzaitoun O, Marcus N, Zion Garty B, Broides A, Levy J, Stepansky P, Etzioni A, Somech R, Roos D. Chronic granulomatous disease: Clinical, functional, molecular, and genetic studies. The Israeli experience with 84 patients. Am J Hematol. 2017;92:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O'Brien S, Hilligoss DM, Malech HL, Gallin JI, Holland SM. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn's disease. Am J Gastroenterol. 2009;104:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Ling W, Xiao-wen C, Yang-shan O, Li T, Wei Z, Juan H. A case report of neonatal-onset chronic granulomatosis disease with aspergillus infection and literature review. Zhonghua Xinshengerke Zazhi. 2019;34:42-46. [DOI] [Full Text] |
| 7. | Davoodi P, Wright SA, Brown EV, Perry JR. Rare diagnosis in a neonate who presents with fever. Clin Pediatr (Phila). 2015;54:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Greenberg DE, Goldberg JB, Stock F, Murray PR, Holland SM, Lipuma JJ. Recurrent Burkholderia infection in patients with chronic granulomatous disease: 11-year experience at a large referral center. Clin Infect Dis. 2009;48:1577-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Blumental S, Mouy R, Mahlaoui N, Bougnoux ME, Debré M, Beauté J, Lortholary O, Blanche S, Fischer A. Invasive mold infections in chronic granulomatous disease: a 25-year retrospective survey. Clin Infect Dis. 2011;53:e159-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Dellepiane RM, Tortorano AM, Liotto N, Laicini E, Di Landro G, Carnelli V, Pietrogrande MC. Invasive Aspergillus nidulans infection in a patient with chronic granulomatous disease. Mycoses. 2008;51:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Khangura SK, Kamal N, Ho N, Quezado M, Zhao X, Marciano B, Simpson J, Zerbe C, Uzel G, Yao MD, DeRavin SS, Hadigan C, Kuhns DB, Gallin JI, Malech HL, Holland SM, Heller T. Gastrointestinal Features of Chronic Granulomatous Disease Found During Endoscopy. Clin Gastroenterol Hepatol. 2016;14:395-402.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Ramanuja S, Wolf KM, Sadat MA, Mahoney SJ, Dinauer MC, Nelson RP Jr. Newly diagnosed chronic granulomatous disease in a 53-year-old woman with Crohn disease. Ann Allergy Asthma Immunol. 2005;95:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Metzger JC, Kurz E, von Spee-Mayer C, Kolck G, Bogumil A, Galle PR, Zimmermann T. [Chronic granulomatous disease as a rare differential diagnosis of inflammatory bowel disease]. Z Gastroenterol. 2018;56:1507-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Alvarez-Larrán A, Toll T, Rives S, Estella J. Assessment of neutrophil activation in whole blood by flow cytometry. Clin Lab Haematol. 2005;27:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 254] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Martire B, Rondelli R, Soresina A, Pignata C, Broccoletti T, Finocchi A, Rossi P, Gattorno M, Rabusin M, Azzari C, Dellepiane RM, Pietrogrande MC, Trizzino A, Di Bartolomeo P, Martino S, Carpino L, Cossu F, Locatelli F, Maccario R, Pierani P, Putti MC, Stabile A, Notarangelo LD, Ugazio AG, Plebani A, De Mattia D; IPINET. Clinical features, long-term follow-up and outcome of a large cohort of patients with Chronic Granulomatous Disease: an Italian multicenter study. Clin Immunol. 2008;126:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Kobayashi S, Murayama S, Takanashi S, Takahashi K, Miyatsuka S, Fujita T, Ichinohe S, Koike Y, Kohagizawa T, Mori H, Deguchi Y, Higuchi K, Wakasugi H, Sato T, Wada Y, Nagata M, Okabe N, Tatsuzawa O. Clinical features and prognoses of 23 patients with chronic granulomatous disease followed for 21 years by a single hospital in Japan. Eur J Pediatr. 2008;167:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Köker MY, Camcıoğlu Y, van Leeuwen K, Kılıç SŞ, Barlan I, Yılmaz M, Metin A, de Boer M, Avcılar H, Patıroğlu T, Yıldıran A, Yeğin O, Tezcan I, Sanal Ö, Roos D. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132:1156-1163.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Xu H, Tian W, Li SJ, Zhang LY, Liu W, Zhao Y, Zhang ZY, Tang XM, Wang M, Wu DQ, Shi JS, Ding Y, Zhao XD, Yang XQ, Jiang LP. Clinical and molecular features of 38 children with chronic granulomatous disease in mainland china. J Clin Immunol. 2014;34:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Liese J, Kloos S, Jendrossek V, Petropoulou T, Wintergerst U, Notheis G, Gahr M, Belohradsky BH. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J Pediatr. 2000;137:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, Zarember KA, Malech HL, Holland SM, Gallin JI. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600-2610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Cole T, Pearce MS, Cant AJ, Cale CM, Goldblatt D, Gennery AR. Clinical outcome in children with chronic granulomatous disease managed conservatively or with hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;132:1150-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Arnon. Reem. M, R. B. Gastrointestinal Abnormalities among Patients with Chronic Granulomatous Disease. J Clin Cell Immunol. 2014;5:1-6. |
| 24. | Freudenberg F, Wintergerst U, Roesen-Wolff A, Albert MH, Prell C, Strahm B, Koletzko S, Ehl S, Roos D, Tommasini A, Ventura A, Belohradsky BH, Seger R, Roesler J, Güngör T. Therapeutic strategy in p47-phox deficient chronic granulomatous disease presenting as inflammatory bowel disease. J Allergy Clin Immunol. 2010;125:943-946.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |