Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9903
Peer-review started: June 6, 2021
First decision: June 25, 2021
Revised: July 9, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 16, 2021
Processing time: 156 Days and 8.6 Hours
Visceral leishmaniasis (VL) is a parasitic disease caused by Leishmania and transmitted by infected sand flies. VL has a low incidence in China, and its clinical presentation is complex and atypical. This disease is easily misdiagnosed and can become life-threatening within a short period of time. Therefore, early, rapid and accurate diagnosis and treatment of the disease are essential.
A 25-year-old male patient presented with the clinical manifestations of irregular fever, hepatosplenomegaly, increased polyclonal globulin, and pancytopenia. The first bone marrow puncture biopsy did not provide a clear diagnosis. In order to relieve the pressure and discomfort of the organs caused by the enlarged spleen and to confirm the diagnosis, splenectomy was performed, and hemophagocytic syndrome was diagnosed by pathological examination of the spleen biopsy. Following bone marrow and spleen pathological re-diagnosis and metagenomic next-generation sequencing (mNGS) technology detection, the patient was finally diagnosed with VL. After treatment with liposomal amphotericin B, the body temperature quickly returned to normal and the hemocytes recovered gradually. Post-treatment re-examination of the bone marrow puncture and mNGS data showed that Leishmania was not detected.
As a fast and accurate detection method, mNGS can diagnose and evaluate the efficacy of treatment in suspicious cases of leishmaniasis.
Core Tip: Visceral leishmaniasis (VL) is an easily overlooked parasitic disease because of its low incidence and atypical clinical manifestations. Here, we report a case of imported VL. Initially, no Leishmania was found in the bone marrow or by spleen biopsy. Leishmania was finally confirmed by metagenomic next-generation sequencing (mNGS) analysis of peripheral blood. Finally, after treatment with amphotericin B, the patient recovered well, and various indicators gradually returned to normal during the follow-up period. As a rapid and accurate detection method, mNGS can be used as an alternative method to diagnose and evaluate suspicious cases.
- Citation: Lin ZN, Sun YC, Wang JP, Lai YL, Sheng LX. Next-generation sequencing technology for diagnosis and efficacy evaluation of a patient with visceral leishmaniasis: A case report. World J Clin Cases 2021; 9(32): 9903-9910
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9903.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9903
Visceral leishmaniasis (VL), also known as Kala-azar, is a parasitic disease caused by visceral Leishmania parasites in the human body, transmitted by sand flies. Its clinical manifestations are long-term irregular fever, weight loss, progressive hepatosplenomegaly and pancytopenia. It is distributed around the world and is one of the deadliest neglected tropical diseases[1]. Given the diversity of its clinical manifestations, it is often misdiagnosed, even in countries in which it is relatively common[2,3]. In recent years, owing to increased population mobility, imported cases have been reported more frequently in China. The lack of experience with VL by medical staff in non-epidemic areas can easily lead to missed diagnosis and misdiagnosis.
Herein, we report a case of imported VL that was initially misdiagnosed as hemophagocytic syndrome (HPS) on pathology, and subsequently correctly diagnosed using metagenomic next-generation sequencing (mNGS) technology and pathological follow-up. After treatment, mNGS was used to assist in the evaluation of the therapeutic efficacy.
A 25-year-old man presented with pancytopenia and hepatosplenomegaly for 5 mo.
At 5 mo prior, the patient presented with a 6-d history of fever and was admitted to Ningbo Development District Central Hospital in Ningbo, China. Physical examination showed splenomegaly. Laboratory testing was significant for pancytopenia with splenomegaly, as evidenced by leukocyte count of 2.5 × 109 cells/L (normal range: 3.5-9.5 × 109 cells/L), hemoglobin level of 111 g/L (normal range: 130-175 g/L), platelet count of 78.0 × 109/L (normal range: 125-350 × 109/L), and C-reactive protein level of 55.80 mg/L (normal range: 0-5 mg/L). Bone marrow puncture examination was performed, and showed active hyperplasia but no other obvious abnormalities. The patient was diagnosed with splenomegaly and pancytopenia. Anti-infective treatment was administered, and the patient’s body temperature returned to normal. The patient had no fever and reported no other discomfort after discharge from the hospital.
The patient’s medical history was unremarkable.
The patient was born in Longnan, Gansu Province, which is one of the endemic areas of VL in China. He lived there for 18 years before moving to Ningbo, where he has lived for the past 6 years and worked in a factory. The patient denies exposure to radioactive and toxic substances. His family had no similar medical history.
The patient’s body temperature was 37.2°C, and he showed physical signs of anemia. The liver was palpable and intense splenomegaly was observed, with the spleen’s lower margin being located 5 cm inferior to the umbilicus.
Abnormal laboratory test results included the following: leukocyte count of 1.6 × 109 cells/L (normal range: 3.5-9.5 × 109 cells/L); neutrophil percentage of 69.8% (normal range: 40%-75%); lymphocyte percentage of 13.5% (normal range: 20%-50%); hemoglobin content of 86 g/L (normal range: 130-175 g/L); platelet count of 42 × 109 cells/L (normal range: 125-350 × 109 cells/L); total bilirubin of 10.0 μmol/L (normal range: 3.4-20.5 μmol/L); albumin of 23.4 g/L (normal range: 40-55 g/L); albumin/globulin ratio of 0.32 (normal range: 1.2-2.3); alanine aminotransferase of 43 U/L (normal range: 9-50 U/L); aspartate aminotransferase of 45 U/L (normal range: 15-40 U/L); creatinine of 65 µmol/L (normal range: 57-97 μmol/L); lactic acid dehydrogenase of 243 U/L (normal range: 120-250 U/L); triglycerides of 2.02 mmol/L (normal range: 0-1.7 mmol/L); coagulase original time of 14.2 s (normal range: 9.4-12.5 s); active partial clotting enzyme time of 36.7 s (normal range: 25.1-36.5 s); fibrinogen of 2.4 g/L (normal range: 2-4 g/L); immunoglobulin (Ig)G of 73.84 g/L (normal range: 6.8-17.4 g/L); serum ferritin of 466.02 ng/mL (normal range: 23.9-336.2 ng/mL); erythrocyte sedimentation rate of 114 mm/h (normal range: 0-15 mm/h); light chains: Kappa of 13.20 g/L (normal range: 5.74-12.76 g/L) and Lambda of 6.71 g/L (normal range: 2.69-6.38 g/L). Finally, blood immunofixation electrophoresis revealed increased polyclonal globulin.
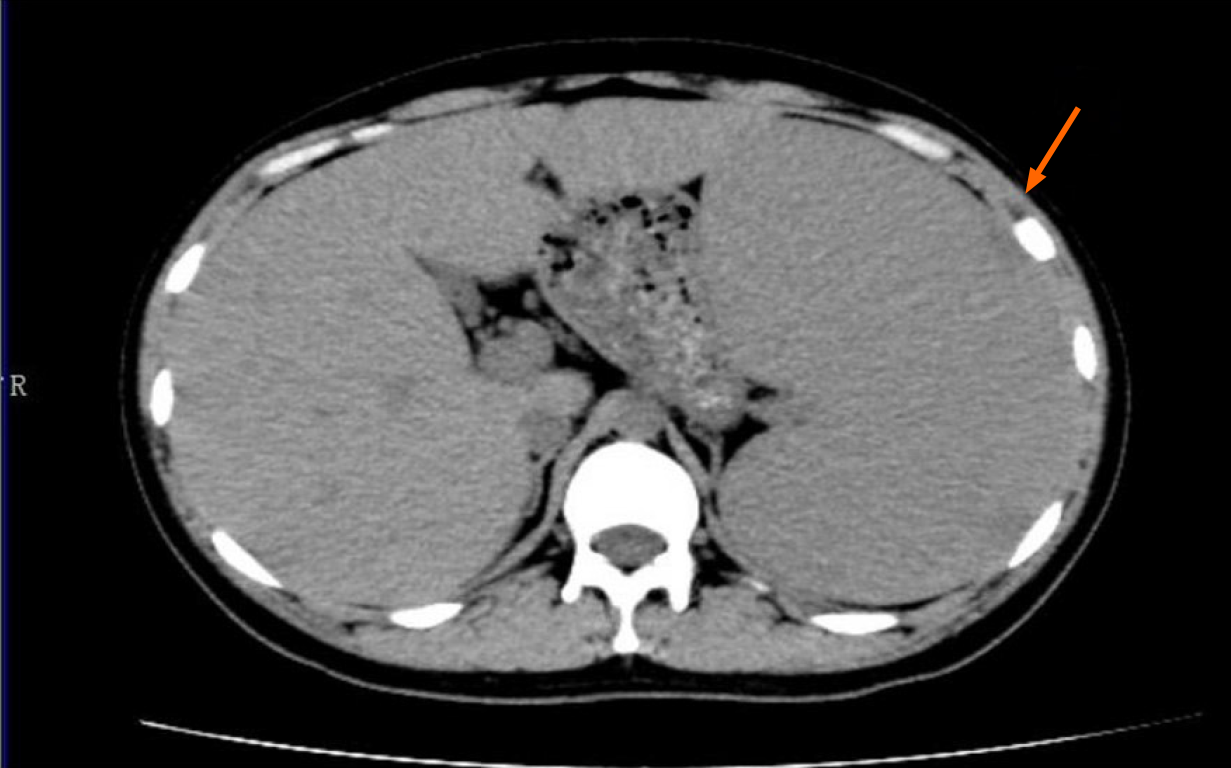
Enhanced computed tomography of the upper abdomen showed splenomegaly and displacement of viscera in the right upper abdomen under pressure (Figure 1).
A bone marrow puncture was performed, with bone marrow smears. The proportion of plasma cells was increased (8.5%), immature plasma cells were rare (1.5%), and atypical lymphocytes were visible. When these results were combined with the clinical findings, lymphoma could not be excluded. The bone marrow immunotype was CD138 (+) and CD38 (+), the proportion of nucleated cells was 6.3%, and the patient was CD45 (+), CD19 (+), CD56 part (+), CD20 (-) and CD117 (-), which was considered to indicate a high proportion of plasma cells. Given that the diagnosis was unclear, and the massively enlarged spleen was causing a gradual decline in platelets, laparoscopic exploration and splenectomy were performed under general anesthesia. Grossly, the spleen was 28 cm × 15.5 cm × 8 cm in size, with normal surface. Microscopically, the red pulp was enlarged, the blood was congested, histiocytosis was accompanied by phagocytosis of blood cells, and the plasma cells were proliferating in clusters. In combination with the immunohistochemistry findings detailed above, hematopoietic lymphohistiocytosis was considered, which was suspected to be associated with infectious factors.
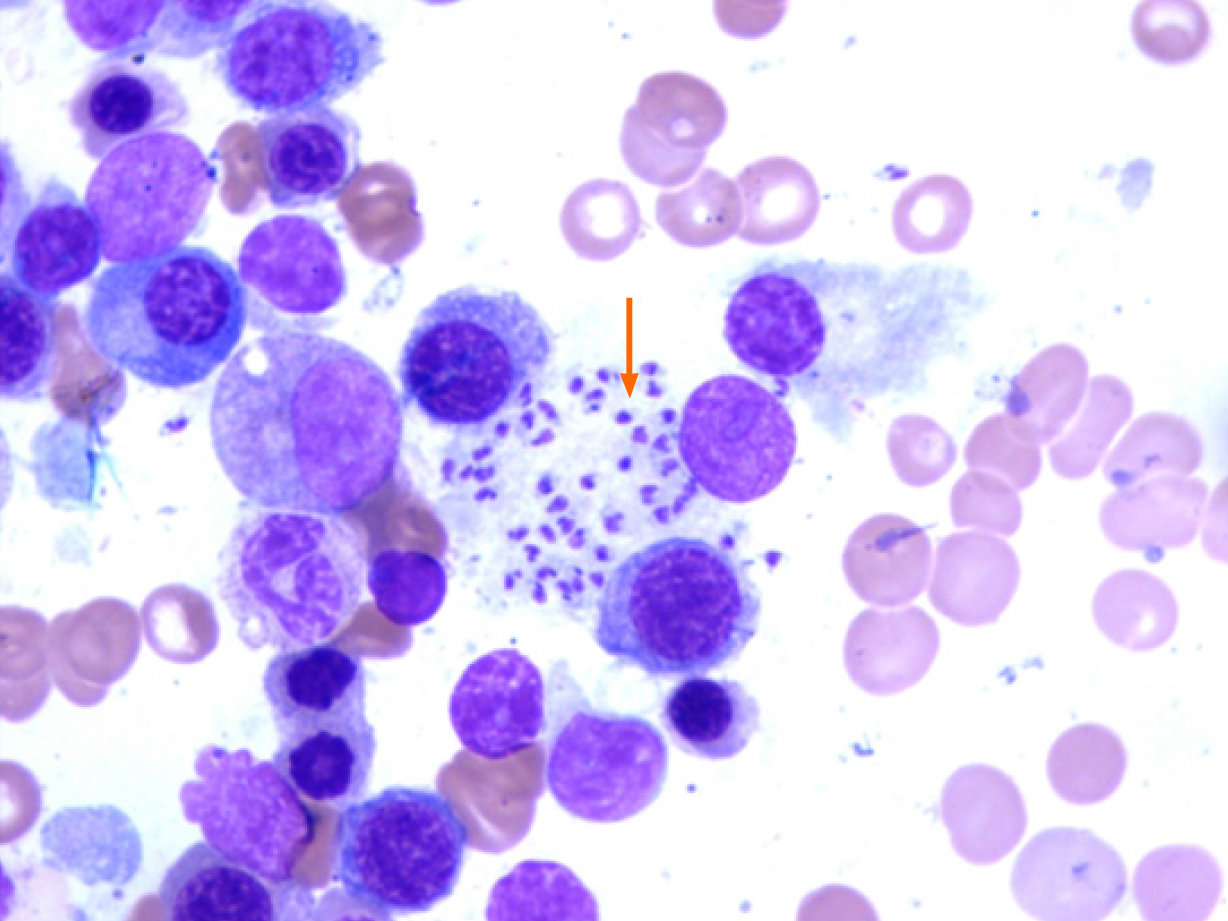
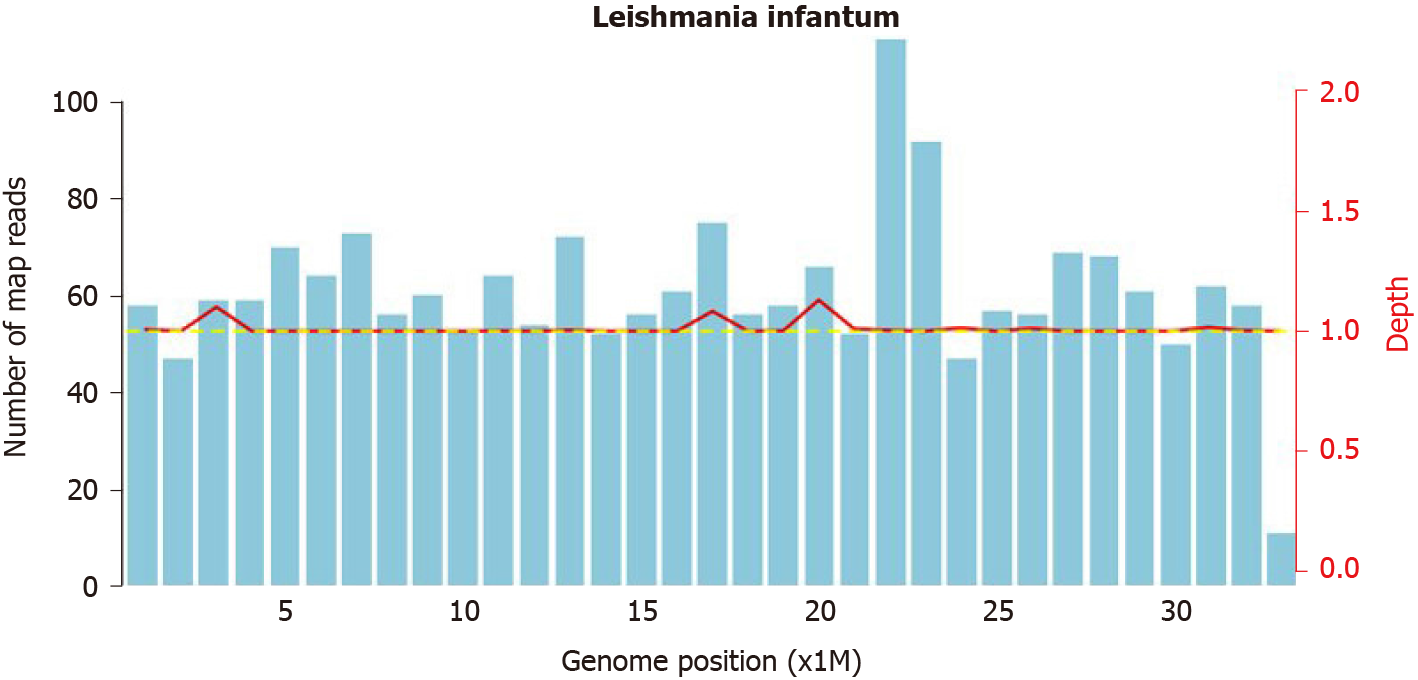
Samples of bone marrow and spleen were sent to Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine in Shanghai, China, for consultation. Leishmania amastigotes were found in monocytes and macrophages of the spleen and bone marrow samples (Figure 2). At the same time, the pathogenic microorganism mNGS data from the patient’s peripheral blood was submitted and verified to contain genomic components of Leishmania ifantum (Figure 3). A total of 23889362 reads were generated from mNGS sequencing with a Q30 of 94.2%, and 102 unique reads of Leishmania ifantum were detected. The sequencing depth of mNGS was 20 X. The number of mapped reads was 50-75 bp using a database covering 9945 species of bacteria, 6760 species of viruses, 1551 species of fungi, and 305 species of parasites. Therefore, the patient was diagnosed as VL, according to the suspicious epidemiological history, clinical manifestations, and laboratory examination results.
The patient was treated with liposomal amphotericin B (LamB) at 30 mg per day for 21 d; the total dose was 630 mg for the anti-infective treatment. Diammonium glycyrrha, dicyclool, and polyene phosphatidylcholine were given for hepatic function protection.
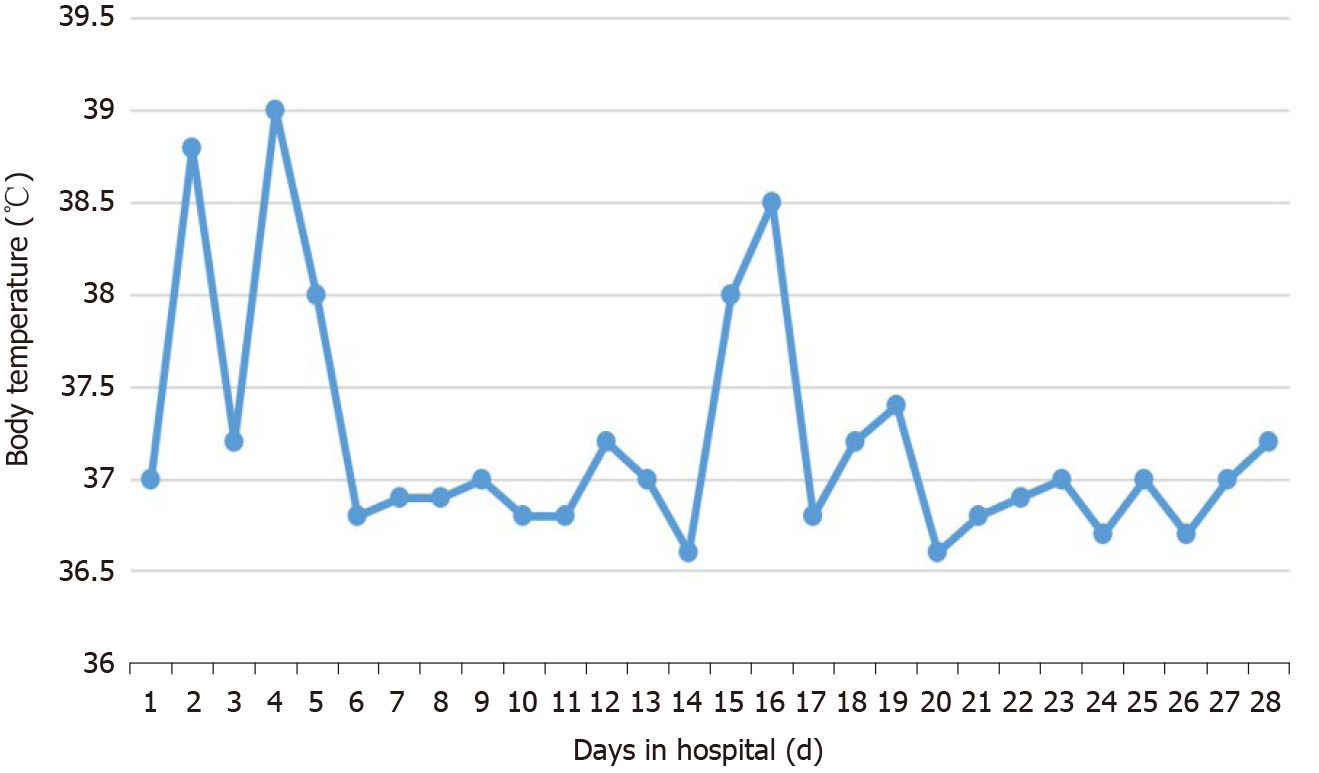
The body temperature returned to normal at 7 d after treatment (Figure 4). The patient’s leukocytes and platelets returned to normal within 1 mo. mNGS re-examination of peripheral blood at 1 mo after the treatment detected no pathogenic microorganism. After 2 mo, the liver returned to its normal size and hemoglobin levels returned to normal. The decrease of globulin was relatively slow. Follow-up at 5 mo after the end of treatment showed globulin to be 43.4 g/L and IgG to be 2790 mg/mL, which was significantly lower than before treatment but still higher than the normal level (Table 1).
| Days of illness | WBC, 109/L | PLT, 109/L | Hb, g/L | LY, % | GLO, g/L |
| Day 1 | 1.69 | 30 | 84 | 49.70 | 77.00 |
| Day 18 | 3.96 | 127 | 98 | 58.30 | 83.00 |
| Day 41 | 7.37 | 49 | 112 | 65.40 | 99.90 |
| Day 66 | 6.34 | 280 | 95 | 62.80 | 103.10 |
| Day 88 | 6.70 | 450 | 109 | 44.90 | 97.00 |
| Day 151 | 8.57 | 377 | 138 | 44.10 | 58.20 |
| Day 186 | 8.57 | 429 | 138 | 45.90 | 49.00 |
| Day 221 | 9.04 | 427 | 148 | 43.80 | 43.40 |
| Day 289 | 9.40 | 372 | 136 | 41.00 | 36.30 |
| Normal range | 3.50-9.50 | 125-350 | 130-175 | 20.00-50.00 | 20.00-40.00 |
The main clinical manifestations of VL are irregular fever, hepatosplenomegaly, emaciation, pancytopenia, and globulin elevation. Its clinical manifestations are complex and atypical, and it is easily misdiagnosed as lymphoma, multiple myeloma, hemophagocytic syndrome, systemic lupus erythematosus, and cirrhosis, among others[3].
VL is a low-prevalence disease in China, with epidemic areas mainly distributed in Xinjiang, Gansu, Sichuan, Shaanxi, Shanxi, and other provinces (autonomous regions)[4]. However, in recent years, owing to the increase in population mobility, imported cases have been increasing. Medical personnel in non-endemic areas lack an understanding of VL, which easily leads to failure of timely diagnosis and treatment of patients. A retrospective study in Brazil assessed the median time from the onset of clinical symptoms to the diagnosis, and determined such to be 25 d and that, on average, patients had to visit seven medical services to obtain an accurate diagnosis[5]. However, in non-endemic areas, like the United Kingdom, the median time was 6 mo[6]. Delayed diagnosis may be life-threatening and cause serious consequences. Therefore, early, rapid and accurate diagnosis and treatment are of vital importance.
The patient described herein was from the endemic area of Longnan, Gansu Province. In line with the epidemiological characteristics of the disease, he showed irregular fever, hepatosplenomegaly, pancytopenia, and increased globulins. However, as the patient was at-present in a non-endemic area and attended the hematology department due to the clinical manifestations of pancytopenia and hepatosplenomegaly, the clinical doctors did not have experience with the disease and failed to pay attention to the epidemiological history; this, combined with the fact that the initial bone marrow examination did not find Leishmania, led to the spleen excision pathology being considered to represent “hemophagocytic syndrome”. When the bone marrow and spleen pathologies were checked by Renji Hospital, however, Leishmania amastigotes were found, and re-examination of the mNGS data confirmed presence of Leishmania infantum genomic components, allowing for the final diagnosis of the patient’s true condition.
At first, this case was only considered as HPS, which encompasses a group of clinical syndromes caused by abnormal activation, proliferation, and secretion of large amounts of inflammatory cytokines by lymphocytes, monocytes, and macrophages, triggered by various factors. HPS can be divided into primary and secondary forms; the former is heredity, and usually occurs in infants, and the latter is often associated with infection, malignant tumors, and autoimmune diseases[7]. Leishmania parasites can stimulate the mononuclear macrophage system, causing significant proliferation and activation, accompanied by phagocytosis of blood cells, and can be considered as an infectious factor causing HPS. HPS and VL, therefore, have similar clinical manifestations, including fever, hepatosplenomegaly, and pancytopenia. This underlies the commonality of misdiagnosis and missed diagnosis. Clinicians should distinguish between primary and secondary HPS, and make a clear etiological diagnosis of secondary HPS. The possibility of VL should be considered when diagnosing HPS[8,9]. Treatment measures for secondary HPS should be established according to the primary disease; following treatment appropriate for primary HPS may lead to adverse outcomes.
Laboratory tests for VL include etiological examination, serum immunological detection, and molecular biological detection. The detection of leishmaniasis in bone marrow or other biological specimens is the gold standard for the diagnosis of VL, and is highly specific. However, the amount of parasites in the sample and the experience of pathologists can affect sensitivity[10-12]. Serum immunological detection aimed at rK39 IgG antibody detection can detect anti-leishmaniasis antibody in human serum. It is a routinely used method to detect VL, but the antibody level does not reflect the parasite load and can remain positive for months or years, precluding its utility as a basis for evaluation of the curative effect; moreover, it is often difficult to carry out this test in non-endemic areas because of a lack of rK39 test strips[10,11]. Molecular biological detection includes polymerase chain reaction (PCR) methodology, which has high sensitivity and can be used to evaluate parasite load, so it can be used as a means of diagnosis and evaluation of curative effect. PCR has been suggested as appropriate for use in the diagnosis of asymptomatic or subclinical infection and to help guide prevention and control of VL[13]. However, this method is currently lacking in standardization and is difficult to carry out clinically.
Unlike traditional detection methods, mNGS involves sequencing nucleic acids directly in a sample and comparing them with the sequence of pathogens in a database. It is a molecular biological detection method that can sensitively identify a variety of different pathogens at the same time, and obtains genus information for suspected pathogenic microorganisms, which can be applied to the diagnosis of pathogens[14,15]. In an mNGS study by Zhang et al[16], all Leishmania cases were correctly diagnosed by this technology. For our case, during the follow-up period, the reduction of mNGS sequencing read abundance was consistent with the clinical recovery of the patient, which showed that mNGS could be used as an auxiliary detection method for leishmaniasis and for direct monitoring of the therapeutic effect. Moreover, after 7 d of treatment with LamB, the patient’s body temperature returned to normal, the parasites became undetectable by routine bone marrow examination and mNGS, and the mNGS technology was verified as an effective tool for assisted diagnosis and early treatment efficacy testing in VL. However, challenges such as low sample content, plenty of host nucleic acid, and high cost still limit the clinical use of NGS. Once these challenges are overcome, NGS can be more widely used in clinical practice[17-19].
To summarize, in general, the clinical manifestations of VL are not typical, and the rates of missed diagnosis or misdiagnosis are high. In clinical practice, medical personnel should raise their awareness of VL, and in the clinical diagnosis of HPS they must consider inclusion of leishmaniasis as a cause of secondary HPS. In addition, mNGS may be used as an auxiliary diagnostic tool and efficacy-monitoring method for suspected cases of VL.
We appreciate all the medical staff for their efforts in treating the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ribeiro IB, Zhang X S-Editor: Chang KL L-Editor: A P-Editor: Liu JH
| 1. | van Griensven J, Diro E. Visceral leishmaniasis. Infect Dis Clin North Am. 2012;26:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Liberopoulos E, Kei A, Apostolou F, Elisaf M. Autoimmune manifestations in patients with visceral leishmaniasis. J Microbiol Immunol Infect. 2013;46:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Evers G, Pohlen M, Berdel WE, Thoennissen NH, Titze U, Köhler G, Weckesser M, Anthoni C, Mesters RM. Visceral leishmaniasis clinically mimicking lymphoma. Ann Hematol. 2014;93:885-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zheng C, Wang L, Li Y, Zhou XN. Visceral leishmaniasis in northwest China from 2004 to 2018: a spatio-temporal analysis. Infect Dis Poverty. 2020;9:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Luz JGG, Carvalho AG, Naves DB, Dias JVL, Fontes CJF. Where, when, and how the diagnosis of human visceral leishmaniasis is defined: answers from the Brazilian control program. Mem Inst Oswaldo Cruz. 2019;114:e190253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Fletcher K, Issa R, Lockwood DN. Visceral leishmaniasis and immunocompromise as a risk factor for the development of visceral leishmaniasis: a changing pattern at the hospital for tropical diseases, london. PLoS One. 2015;10:e0121418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Janka GE, Lehmberg K. Hemophagocytic syndromes--an update. Blood Rev. 2014;28:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 8. | Özdemir N, Koç B, Arslantaş E, Odaman Al I, Kelleci Ç, Uysalol EP, Bayram C, Ayçiçek A. Hemophagocytic Lymphohistiocytosis Associated With Visceral Leishmaniasis. J Pediatr Hematol Oncol. 2018;40:395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Diamantidis MD, Palioura A, Ioannou M, Tsangalas E, Karakousis K Sr. Hemophagocytic Lymphohistiocytosis as a Manifestation of Underlying Visceral Leishmaniasis. Cureus. 2020;12:e11911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | De Brito RCF, Aguiar-Soares RDO, Cardoso JMO, Coura-Vital W, Roatt BM, Reis AB. Recent advances and new strategies in Leishmaniasis diagnosis. Appl Microbiol Biotechnol. 2020;104:8105-8116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, Carvalho E, Ephros M, Jeronimo S, Magill A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2017;96:24-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Chen H, Fan C, Gao H, Yin Y, Wang X, Zhang Y, Wang H. Leishmaniasis Diagnosis via Metagenomic Next-Generation Sequencing. Front Cell Infect Microbiol. 2020;10:528884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Silva LA, Romero HD, Fagundes A, Nehme N, Fernandes O, Rodrigues V, Costa RT, Prata A. Use of the polymerase chain reaction for the diagnosis of asymptomatic Leishmania infection in a visceral leishmaniasis-endemic area. Rev Inst Med Trop Sao Paulo. 2013;55:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Dekker JP. Metagenomics for Clinical Infectious Disease Diagnostics Steps Closer to Reality. J Clin Microbiol. 2018;56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Guo F, Kang L, Xu M. A case of pediatric visceral leishmaniasis-related hemophagocytic lymphohistiocytosis diagnosed by mNGS. Int J Infect Dis. 2020;97:27-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Zhang HC, Zhang QR, Ai JW, Cui P, Wu HL, Zhang WH, Wang T. The role of bone marrow metagenomics next-generation sequencing to differential diagnosis among visceral leishmaniasis, histoplasmosis, and talaromycosis marneffei. Int J Lab Hematol. 2020;42:e52-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Subbiah M, Thirumalapura N, Thompson D, Kuchipudi SV, Jayarao B, Tewari D. Detection of Anaplasma Phagocytophilum in Horses With Suspected Tick-Borne Disease in Northeastern United States by Metagenomic Sequencing. Front Vet Sci. 2021;8:673193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Couto N, Schuele L, Raangs EC, Machado MP, Mendes CI, Jesus TF, Chlebowicz M, Rosema S, Ramirez M, Carriço JA, Autenrieth IB, Friedrich AW, Peter S, Rossen JW. Author Correction: Critical steps in clinical shotgun metagenomics for the concomitant detection and typing of microbial pathogens. Sci Rep. 2019;9:6406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Dulanto Chiang A, Dekker JP. From the Pipeline to the Bedside: Advances and Challenges in Clinical Metagenomics. J Infect Dis. 2020;221:S331-S340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |