Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9783
Peer-review started: June 6, 2021
First decision: July 5, 2021
Revised: August 13, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: November 16, 2021
Processing time: 156 Days and 14.7 Hours
Severe bony Bankart lesions are a difficult challenge in clinical treatment and research. The current treatment methods consist mostly of Latarjet-Bristow surgery and its modified procedures. While good results have been achieved, there are also complications such as coracoid fracture, bone graft displacement, and vascular and nerve injury.
To analyze the techniques and biomechanical properties of transversely fixing a bone block from the scapular spine using bone allograft pins with suture threads to repair bony Bankart lesions.
Fresh human shoulder joint specimens and a cadaver specimen model for scapular bone grafting with allograft pin fixation for repair of bony Bankart lesions were used. When the humeral rotation angles were 0°, 30°, 60° and 90°, and the axial loads were 30 N, 40 N, and 50 N, the humerus displacement was studied by biomechanical experiments.
When the angle of external rotation of the humerus was 0°, 30°, 60°, and 90°, with axial loads of 30 N, 40 N, and 50 N, the data of the normal control group, allograft pin repair group, and titanium alloy hollow screw repair group were compared with each other by the q-test, which showed that there were no statistically differences among the three groups (P > 0.05).
The joints repaired with bone block from the scapular spine transversely fixed with allograft bony pins to repair bony Bankart lesions show good mechanical stability. The bone block has similar properties to normal glenohumeral joints in terms of biomechanical stability.
Core Tip: Severe bony Bankart lesions are a difficult challenge in clinical treatment and research. The feasibility of using scapular spine graft bone allograft pins to repair Bankart lesions of the shoulder joint, and its biomechanical experimental results were preliminarily evaluated. This study found that the joints repaired with bone block from the scapular spine transversely fixed with allograft bony pins to repair bony Bankart lesions show good mechanical stability. This new method has no adverse effects on the bone donor area, avoiding the complications of coracoid extraction.
- Citation: Lu M, Li HP, Liu YJ, Shen XZ, Gao F, Hu B, Liu YF. Scapular bone grafting with allograft pin fixation for repair of bony Bankart lesions: A biomechanical study. World J Clin Cases 2021; 9(32): 9783-9791
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9783.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9783
The shoulder joint is a typical multiaxial ball-and-socket joint. The humeral head is approximately spherical, and the glenoid is small and shallow[1]. This structure gives the shoulder joint greater flexibility. However, this structure also leads to a decrease in joint stability results[1]. Consequently, when the shoulder joint is dislocated, anteroinferior glenohumeral dislocation occurs[1]. Patients with primary traumatic anterior dislocation of the glenohumeral joint often develop redislocation[2,3]. Bony Bankart injury is traumatic avulsion of the glenoid labrum, or it indicates recurrent shoulder instability with a large bone defect due to anterior or anteroinferior shoulder dislocation. In clinical practice, 5.4% to 70% of shoulder instability cases result from traumatic factors[4-8]. The current treatments for bony Bankart injury, especially severe bony Bankart injury, mainly include open and arthroscopic Bristow-Latarjet surgeries[6]. Since the coracoid process serves as the donor area for bone grafting, some important structures such as the coracoacromial arch and pectoralis minor muscle can be damaged, resulting in shoulder joint instability and arthritis[9-12].
We proposed a new method of glenoid reconstruction using an autologous scapular bone graft from the scapular spine, which is trimmed and grafted into the glenoid defect following transfixation with bone allograft pins. In the present study, the feasibility and biomechanical properties of this surgical method were preliminarily evaluated using fresh human shoulder joint specimens.
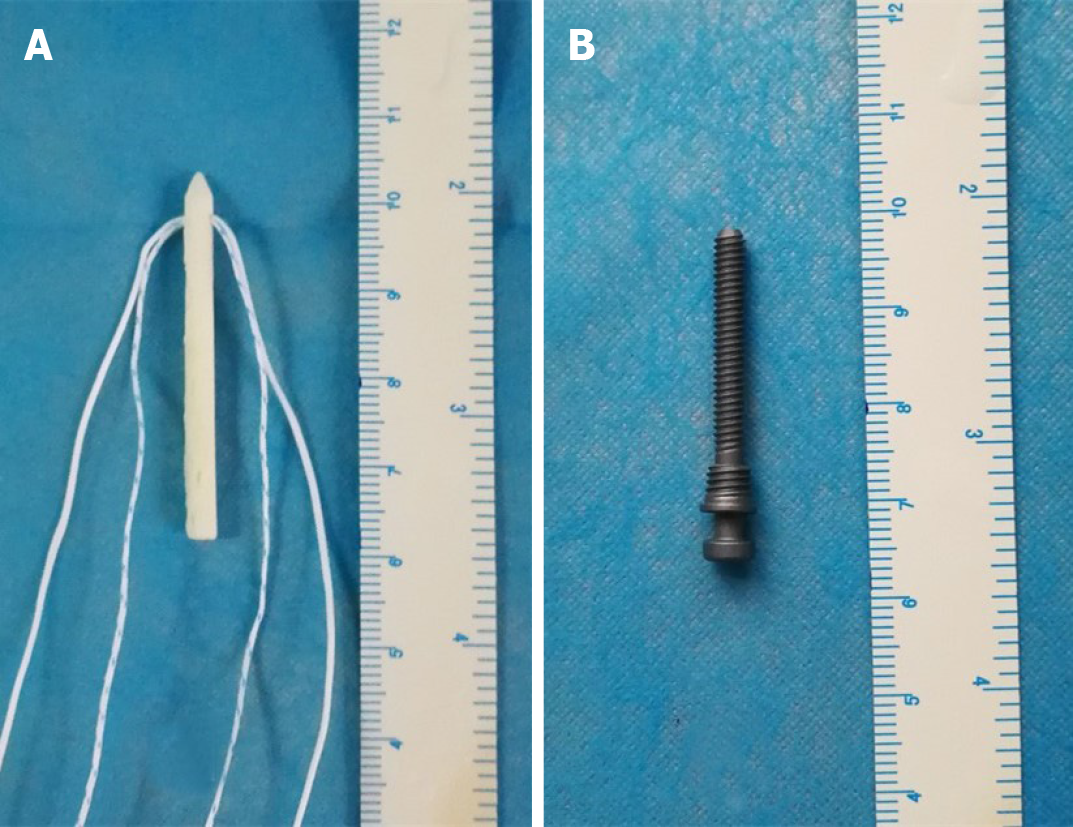
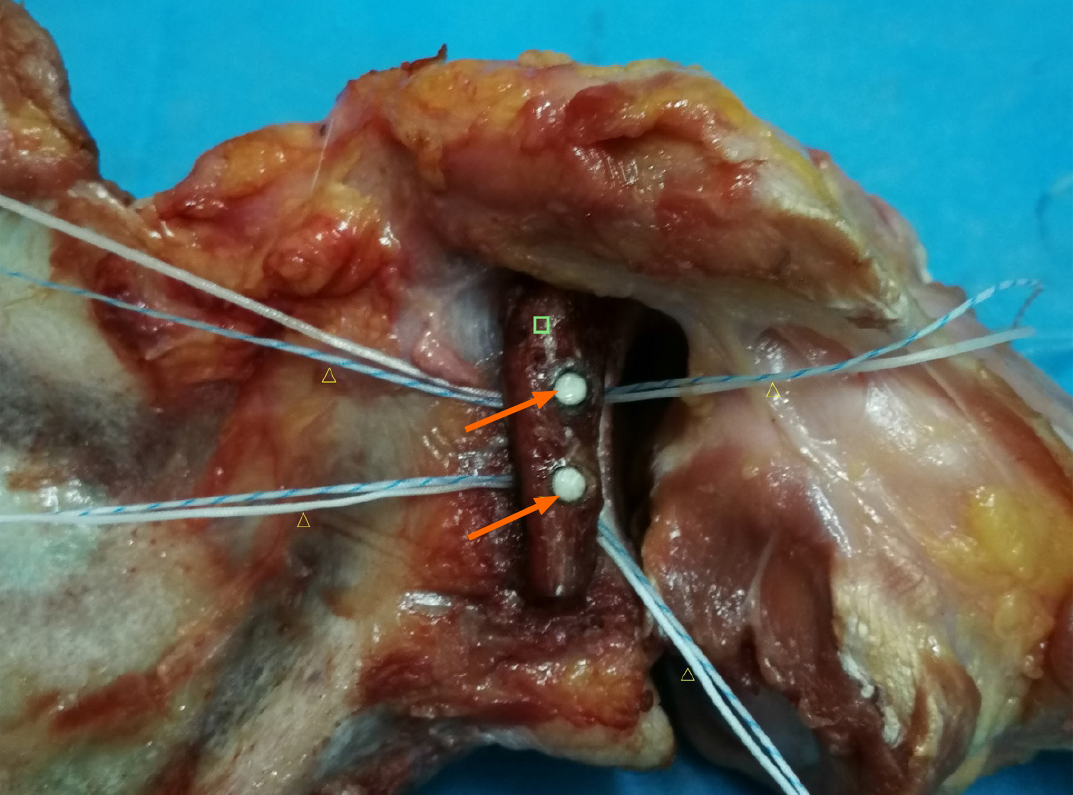
There were 16 titanium alloy hollow screws (Figure 1A) and 16 allograft pins (Figure 1B) with suture threads. Twenty-four fresh human shoulder joint specimens were randomized into a titanium alloy hollow screw repair group (TS group), an allograft pin repair group (AP group), and a normal control groups (NC group), with eight shoulders in each group. The specimens were thawed at room temperature (24 °C) overnight, and the skin and subcutaneous tissue were removed. The muscle tissue around the articular capsule was partially removed, and the intact articular capsule and tendon were preserved. Then, a shoulder joint-bone-ligament muscle specimen was obtained. The humerus was severed at the upper middle part, and the periosteum was exposed. The glenoid cavity of the AP group and TS group was opened, and its transverse diameter was measured. A line 25% longer than the transverse diameter of the glenoid was drawn. The medial glenoid cavity and labrum were removed using a hand saw to create a model of severe Bankart injury in the porcine shoulder joint specimen. All the specimens were visually inspected to ensure that there were no fractures or joint capsule injuries. In the AP group, a bone knife was used to harvest a bone block from the scapular spine, as required, and the harvested bone block was trimmed, with two to three holes drilled. Bone allograft pins were hammered into the autologous bone graft, and the suture thread was inserted into the pinholes at the head. The pins were then hammered below the glenoid cavity (Figure 2), followed by suturing of the soft tissue with the bone graft. Finally, the articular capsule of the shoulder was sutured. In the TS group, a method similar to that in the AP group was used to fix the shoulder joint.
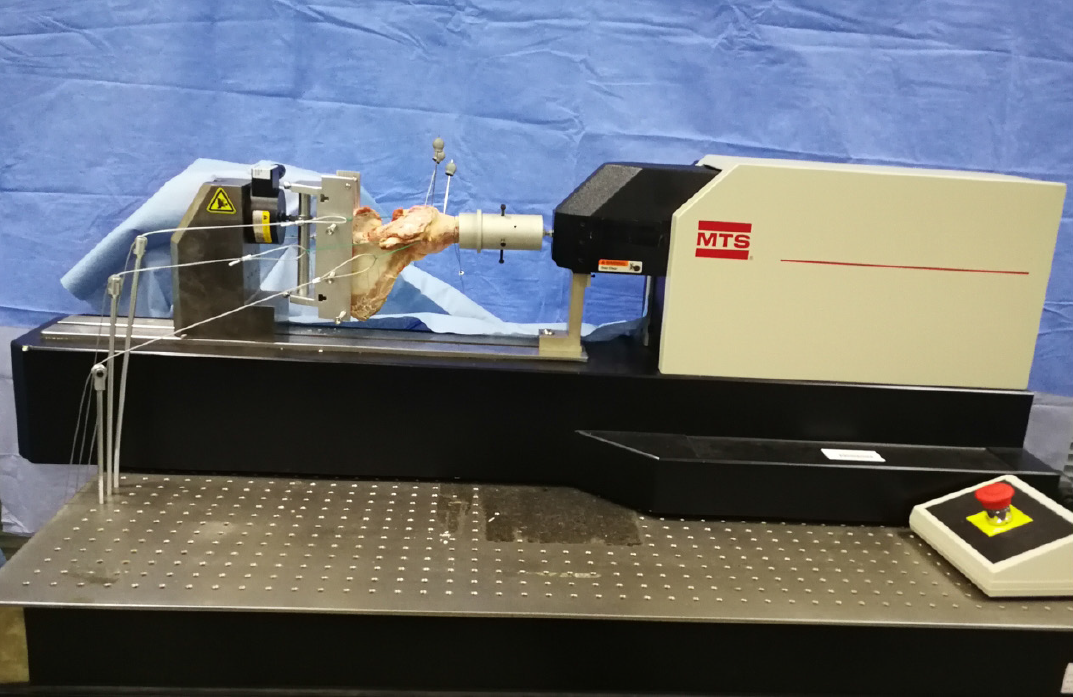
A Kirschner wire was inserted into the greater tubercle, coracoid process, and acromion of the shoulder joint specimen, and a marker was placed for motion capture on the Kirschner wire; when embedding the shoulder joint, we ensured that the scapula had an anterior tilt of approximately 20°. We fixed the embedded joints on the MTS Tytron 250 system. Sutures and thin steel wire ropes were used to connect three 1.02 kg custom weights to the supraspinatus tendon, infraspinatus tendon, and anterior and lower parts of the humerus. Custom weights of 1.53 kg and 0.51 kg were connected to the subscapularis tendon and round tendon, respectively, and the wire rope and weight were guided to the outside of the worktable along the muscle contraction direction. The weight drooped along the edge of the worktable through the pulley, and did not touch the ground to simulate the 10 N preload force on the supraspinatus and infraspinatus muscles, the 15 N preload force on the subscapularis, and the 5 N preload force on the teres minor muscle in the simulated physiological state. At the proximal end of the humerus, there was a 10 N tensile force of 45° forward and downward (using a wire rope to connect a 1.02 kg custom weight) to simulate the anterior and lower forces (Figure 3). After the humeral head was in contact with the scapular glenoid, when the humerus is externally rotated to 0°, 30°, 60°, and 90°, axial loads of 30 N, 40 N, and 50 N were applied to the glenoid, and the humerus was observed relative to displacement of the glenoid.
Statistical analyses were performed using SPSS software (IBM Corp., Armonk, NY, United States), and the paired q-test was used. P < 0.05 was considered statistically significant.
When the external rotation of the humerus was at the same angle, with axial loads of 30 N, 40 N, and 50 N, the displacement of the humerus in each group increased; under the same load, when the angle of external rotation of the humerus increased, the displacement of humerus gradually decreased. Considering that the joint capsule was subjected to rotating torque at this time, the movement of the humerus was limited, so the displacement of the humerus decreased (Table 1).
| ERA, load | NC | AP | TS |
| 0°, 30 N | 2.89 ± 0.13 | 2.64 ± 0.17 | 2.65 ± 0.36 |
| 0°, 40 N | 3.36 ± 0.17 | 2.95 ± 0.20 | 3.19 ± 0.27 |
| 0°, 50 N | 3.96 ± 0.30 | 3.41 ± 0.35 | 3.38 ± 0.18 |
| 30°, 30 N | 2.51 ± 0.28 | 2.35 ± 0.19 | 2.37 ± 0.16 |
| 30°, 40 N | 2.92 ± 0.11 | 2.54 ± 0.47 | 2.75 ± 0.221 |
| 30°, 50 N | 3.22 ± 0.21 | 2.68 ± 0.23 | 2.82 ± 0.36 |
| 60°, 30 N | 2.12 ± 0.13 | 1.96 ± 0.18 | 1.88 ± 0.33 |
| 60°, 40 N | 2.23 ± 0.14 | 2.19 ± 0.31 | 2.13 ± 0.25 |
| 60°, 50 N | 2.67 ± 0.39 | 2.51 ± 0.30 | 2.43 ± 0.31 |
| 90°, 30 N | 1.08 ± 0.18 | 1.02 ± 0.23 | 1.03 ± 0.22 |
| 90°, 40 N | 1.04 ± 0.38 | 1.08 ± 0.12 | 1.16 ± 0.29 |
| 90°, 50 N | 1.12 ± 0.15 | 1.12 ± 0.15 | 1.27 ± 0.31 |
When the angle of external rotation of the humerus is 0° and 30°, with axial loads of 30 N, 40 N, and 50 N, the data of the NC group, AP group, and TS group were compared with each other using the q-test, which showed that the differences in data among the NC group, TS group, and AP group were not statistically significant (P > 0.05). The shoulder joints of the TS group and AP group had the same stability. The shoulder joints repaired with titanium alloy hollow screws and allograft pins had the same stability as the normal shoulder joints. When the humerus was externally rotated at 60° and 90°, and the axial loads were 40 N and 50 N, the same results were obtained (P > 0.05), indicating that the shoulder joints in the TS group and AP group had the same stability. The shoulder joint repaired with the titanium alloy hollow screws and allograft pins had the same stability as the normal shoulder joints (Table 2).
| ERA | AL | Groups | P value |
| 0° | 30 N | NC, AP | P > 0.05 |
| 0° | 30 N | NC, TS | P > 0.05 |
| 0° | 30 N | AP, TS | P > 0.05 |
| 0° | 40 N | NC, AP | P > 0.05 |
| 0° | 40 N | NC, TS | P > 0.05 |
| 0° | 40 N | AP, TS | P > 0.05 |
| 0° | 50 N | NC, AP | P > 0.05 |
| 0° | 50 N | NC, TS | P > 0.05 |
| 0° | 50 N | AP, TS | P > 0.05 |
| 30° | 30 N | NC, AP | P > 0.05 |
| 30° | 30 N | NC, TS | P > 0.05 |
| 30° | 30 N | AP, TS | P > 0.05 |
| 30° | 40 N | NC, AP | P > 0.05 |
| 30° | 40 N | NC, TS | P > 0.05 |
| 30° | 40 N | AP, TS | P > 0.05 |
| 30° | 50 N | NC, AP | P > 0.05 |
| 30° | 50 N | NC, TS | P > 0.05 |
| 30° | 50 N | AP, TS | P > 0.05 |
| 60° | 30 N | NC, AP | P > 0.05 |
| 60° | 30 N | NC, TS | P > 0.05 |
| 60° | 30 N | AP, TS | P < 0.05 |
| 60° | 40 N | NC, AP | P > 0.05 |
| 60° | 40 N | NC, TS | P > 0.05 |
| 60° | 40 N | AP, TS | P > 0.05 |
| 60° | 50 N | NC, AP | P > 0.05 |
| 60° | 50 N | NC, TS | P > 0.05 |
| 60° | 50 N | AP, TS | P > 0.05 |
| 90° | 30 N | NC, AP | P > 0.05 |
| 90° | 30 N | NC, TS | P > 0.05 |
| 90° | 30 N | AP, TS | P < 0.05 |
| 90° | 40 N | NC, AP | P > 0.05 |
| 90° | 40 N | NC, TS | P > 0.05 |
| 90° | 40 N | AP, TS | P > 0.05 |
| 90° | 50 N | NC, AP | P > 0.05 |
| 90° | 50 N | NC, TS | P > 0.05 |
| 90° | 50 N | AP, TS | P > 0.05 |
When the humerus was externally rotated to 60° and 90°, and the axial load was 30 N, the data of the AP group and NC group, TS group, and NC group were tested by the q-test, and the results indicated that, when the shoulder joints repaired with titanium alloy hollow screws and allograft pins were compared with the normal shoulder joint, they had the same degree of stability (P > 0.05). In contrast, the difference in data between the TS group and the AP group was statistically significant (P < 0.05). The joint capsules of the two groups were subjected to torsional force, and when the humerus was externally rotated at 60° and 90°, the axial load force was as small as 30 N, which limited the displacement of the humeral head. This resulted in a small forward and lower component force and limited displacement of the humeral head, leading in turn to a significant difference in the data between the AP group and TS group. As the axial load increased to 40 N and 50 N, there was no significant difference between the AP group and TS group (Table 2).
The stability of the shoulder joint depends on multifactor synergy. Bankart lesions are the most common cause and an important pathological basis of recurrent anterior dislocation of the shoulder[9-12]. In 2014, Kim et al[13] proposed the classification of bony Bankart lesions according to bone fracture size as confirmed by three-dimensional computed tomography images: With mild lesion, the size of the bone block was < 12.5% of the width of the glenoid cavity; with moderate lesion, the size of the bone block was 12.5% to 25% of the width of the glenoid cavity; and with severe lesion, the size of the bone block was > 25% of the width of the glenoid cavity. The current clinical principles for treating these three types of injuries as follows: For mild lesion, arthroscopic repair of the glenoid labrum is recommended to reset the bone block to the greatest extent; for moderate lesion, bone reduction is recommended, and the bone block displacement is restricted to < 2 mm; and for severe lesion, recon
The current treatments for severe bony Bankart injury mainly include open or arthroscopic Bristow-Latarjet surgeries[6,14]. Since the coracoid process serves as the donor area for bone grafting, some important structures attached to the coracoid process such as the coracoacromial arch, biceps tendon, and pectoralis minor muscle will be damaged during bone harvesting, giving rise to shoulder joint dysfunction[15,16]. Structural damage to the coracoacromial ligament is the greatest hidden peril of coracoid process transfer, whereas the coracoacromial ligament is the key structure for maintaining the stability of the shoulder joint[17]. The complication rate after coracoid process transfer is up to 30%, accompanied by a recurrent dislocation rate of 2.9%, recurrent subluxation rate of 5.8%, revision rate of 7%, and average extorsion loss of 13.0°[18]. Griesse et al[19] reported some cases of coracoid and glenoid fractures. Other complications documented include bone block displacement, screw displacement, fracture, infection, and neurovascular injury[19].
To avoid the rupture of normal shoulder joint structures by Latarjet or Bristow surgery, Auffarth et al[20] achieved good results by autologous iliac bone grafting. However, the results of the studies by Younger et al[21] and Summers et al[22] showed that the incidence of pain at the iliac donor site was 2.5% to 49%, and other complications, such as infection, hematoma, cutaneous nerve injury, scar formation, pseudoaneurysm, muscular herniation, arteriovenous fistula, and fracture of the anterosuperior iliac spine at the donor site, also occur frequently, disrupting the postoperative patient’s quality of life[21,22]. Therefore, these complications should be considered by surgeons.
Autologous scapular grafts can eliminate the complications caused by coracoid process transfer and humeral bone removal and maintain the normal anatomical structure of the shoulder joint, which is beneficial to postoperative rehabilitation and recovery of shoulder mobility. As shown by anatomical studies in Chinese people, the width and height of the scapular spine are 7.26-19.58 and 11.48-23.86 mm, respectively, fully meeting the requirements for bone grafting of Bankart lesions[23,24].
Metal consumables for bone fixation have a higher elastic modulus than normal bone tissues. Implantation of metal consumables will produce stress shielding, affecting bone formation and even causing bone absorption, pin channel enlargement, fixation loosening, or even failure[25]. The elastic modulus of carbon fiber is close to that of normal bone tissue, but its biocompatibility is poor. Long-term use of carbon fibers can produce small debris and cause aseptic inflammation[26]. Absorbable biomaterials as consumables mostly consist of polylactic acid, and the degradation products will cause a local acidic environment, which is not conducive to bone formation and causes fixation loosening and failure[25,26]. In addition, the absorption time of absorbable biomaterials often mismatches the time of bone formation, and these materials are prone to becoming invalid prior to bone ingrowth, resulting in fixation failure[25,26].
Bone allograft pins have good histocompatibility, osteoconductivity, and osteoinductivity, making them conducive to the fusion of bone grafts with the glenoid cavity and bone ingrowth[27]. “Creeping substitution”, which was initially described by Axhausen in 1908, is still in use today[10]. This theory reveals that the grafted bone block has osteogenic activity and serves as a scaffold to provide osteogenic environment for new bone formation on its surface[10]. Moreover, the grafted bone block gradually degrades and is completely replaced by new bone tissues[10]. Clinically used allogeneic bones include cancellous and cortical bones, both of which have a natural three-dimensional mesh structure enriched with various growth factors required for osteogenesis and characterized as having both bone conduction and osteoinductive advantages[10]. Overall, bone allograft pins with stitching threads have good biomechanical properties (with an elastic modulus close to normal bone tissues), producing little effect on imaging examinations, degrading completely, and not affecting secondary surgery. These pins have good osteogenic activity, and a stitching thread that not only tightly fixes the bone graft, but also stitches the bone graft with the surrounding soft tissues, further enhancing the fixation strength.
In conclusion, autologous scapular grafting with bone allograft pin fixation is a feasible and effective surgery for repairing bony Bankart lesions, thus increasing shoulder joint stability.
The pathological mechanism of anterior glenohumeral joint dislocation is mostly static stable structural damage. Bony Bankart lesions accounted for 5.4% to 70% of cases of traumatic instability of the glenohumeral joint and 90% of recurrent shoulder dislocations. Bony Bankart lesions lead to scapular glenoid defects, which are often repaired by the Latarjet-Bristow procedure. While good results have been achieved, there are also complications, such as coracoid fracture, bone graft displacement, and vascular and nerve injury.
To avoid the complications caused by the removal of the grafted bone from the coracoid, this study used allograft bone from the scapular spine and the allograft pins to repair and reconstruct bony Bankart lesions of the shoulder joint. To avoid obtaining grafted bone from the coracoid, the complications and medical costs were decreased. In vitro biomechanics experiments were performed.
This study aimed to analyze the technique and biomechanical properties of transversely fixing a bone block from the scapular spine using bone allograft pins with suture threads to repair bony Bankart lesions.
Twenty-four fresh human shoulder joint specimens were randomized into a titanium alloy hollow screw repair group, an allograft pin repair group, and a normal control group, with eight shoulders in each group. Model establishment and repair were performed as required, and biomechanical testing was performed using the MTS Tytron 250 system.
When the angle of external rotation of the humerus was 0°, 30°, 60°, and 90°, with axials loads of 30 N, 40 N, and 50 N, the data of normal control group, allograft pin repair group, and titanium alloy hollow screw repair group, which revealed that there were no statistically significant differences among the three groups (P > 0.05).
The joints repaired with bone block from the scapular spine transversely fixed with allograft bony pins to repair bony Bankart lesions show good mechanical stability.
This new method is conducive to treating injury post-surgery, is feasible, reduces medical costs, and is expected to be popularized and applied in the clinic.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kanematsu R S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Jin AM, Wang HQ. Clinical Anatomy of Orthopedics. Jinan: Shandong Science and Technology Press, 2010: 31-52. |
| 2. | Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38-A:957-977. [PubMed] |
| 3. | Simonet WT, Melton LJ 3rd, Cofield RH, Ilstrup DM. Incidence of anterior shoulder dislocation in Olmsted County, Minnesota. Clin Orthop Relat Res. 1984;186-191. [PubMed] |
| 4. | Bigliani LU, Newton PM, Steinmann SP, Connor PM, Mcllveen SJ. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med. 1998;26:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 358] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 567] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 6. | Jiang CY, Zhu YM, Liu X, Li FL, Lu Y, Wu G. Do reduction and healing of the bony fragment really matter in arthroscopic bony Bankart reconstruction? Am J Sports Med. 2013;41:2617-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Porcellini G, Campi F, Paladini P. Arthroscopic approach to acute bony Bankart lesion. Arthroscopy. 2002;18:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Sugaya H, Moriishi J, Kanisawa I, Tsuchiya A. Arthroscopic osseous Bankart repair for chronic recurrent traumatic anterior glenohumeral instability. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ee GW, Mohamed S, Tan AH. Long term results of arthroscopic Bankart repair for traumatic anterior shoulder instability. J Orthop Surg Res. 2011;6:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Bevevino AJ, Kang DG, Lehman RA Jr, Van Blarcum GS, Wagner SC, Gwinn DE. Systematic review and meta-analysis of minimally invasive transforaminal lumbar interbody fusion rates performed without posterolateral fusion. J Clin Neurosci. 2014;21:1686-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Chen WX, Bao N, Zhao JN. [Advances on treatment of Bankart lesions]. Yixue Yanjiusheng Xuebao. 2016;29:309-313. [DOI] [Full Text] |
| 12. | Longo UG, Loppini M, Rizzello G, Ciuffreda M, Maffulli N, Denaro V. Latarjet, Bristow, and Eden-Hybinette procedures for anterior shoulder dislocation: systematic review and quantitative synthesis of the literature. Arthroscopy. 2014;30:1184-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Kim YK, Cho SH, Son WS, Moon SH. Arthroscopic repair of small and medium-sized bony Bankart lesions. Am J Sports Med. 2014;42:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Huang CM. [Repair of bony Bankart lesions]. Zhonghua Jianzhouwaike Dianzi Zazhi. 2016;4:56-60. [DOI] [Full Text] |
| 15. | He S. Applied anatomical study of the scapula and surgical treatment of scapular fracture. M.Sc. Thesis, Hebei Medical University. 2012. [DOI] [Full Text] |
| 16. | Schaaf H, Lendeckel S, Howaldt HP, Streckbein P. Donor site morbidity after bone harvesting from the anterior iliac crest. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Zhao J, Huangfu X, Yang X, Xie G, Xu C. Arthroscopic glenoid bone grafting with nonrigid fixation for anterior shoulder instability: 52 patients with 2- to 5-year follow-up. Am J Sports Med. 2014;42:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Griesser MJ, Harris JD, McCoy BW, Hussain WM, Jones MH, Bishop JY, Miniaci A. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 451] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 19. | Griesser MJ, Harris JD, McCoy BW, Hussain WM, Jones MH, Bishop JY, Miniaci A. Glenoid fracture after Bristow-Latarjet shoulder stabilization: a case report and review of the literature. J Shoulder Elbow Surg. 2013;22:e17-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Auffarth A, Schauer J, Matis N, Kofler B, Hitzl W, Resch H. The J-bone graft for anatomical glenoid reconstruction in recurrent posttraumatic anterior shoulder dislocation. Am J Sports Med. 2008;36:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1434] [Cited by in RCA: 1309] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 22. | Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 455] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Sun H, Li Z, Wang JH, Liu ZP, Sun ZJ, Xu K, Wang YH. Anatomical study of the scapula. Linchuang He Shiyan Yixue Zazhi. 2015;14:1858-1861. [DOI] [Full Text] |
| 24. | Li W. Clinical Anatomy Study of scapula. M.D. Thesis, Southern Medical University. 2007. [DOI] [Full Text] |
| 25. | Wang X, Chen Y, Chen D, Yuan W, Chen X, Zhou X, Xiao J, Ni B, Jia L. Anterior decompression and interbody fusion with BAK/C for cervical disc degenerative disorders. J Spinal Disord Tech. 2009;22:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Lee JH, Lee JH, Park JW, Lee HS. Fusion rates of a morselized local bone graft in polyetheretherketone cages in posterior lumbar interbody fusion by quantitative analysis using consecutive three-dimensional computed tomography scans. Spine J. 2011;11:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J Am Acad Orthop Surg. 1995;3:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 397] [Article Influence: 13.2] [Reference Citation Analysis (0)] |