Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.10024
Peer-review started: July 2, 2021
First decision: July 26, 2021
Revised: August 10, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 16, 2021
Processing time: 130 Days and 21.7 Hours
Mucosal-associated lymphoid tissue extranodal marginal zone (MALT) lym
A 48-year-old woman was admitted to the hospital with a 1-year history of macroscopic hematuria. Imaging showed a large homogeneous mass with an unclear boundary and an irregular morphology in the bladder. The mass had an abundant blood supply. For further diagnosis, transurethral cystoscopic biopsy and bone marrow biopsy was performed, and the patient was finally diagnosed with primary MALT lymphoma of the bladder. R-CHOP chemotherapy was carried out. After three cycles of chemotherapy, the mass disappeared and the bladder wall thickness was only 4 mm, which indicated excellent therapeutic response to the chemotherapy. To date, the patient remains asymptomatic and she visits our hospital regularly for the completion of the remaining chemotherapy cycles.
Primary MALT lymphoma of the bladder is rare, and there are certain characteristics in the ultrasonographic findings. Imaging findings play an important role in evaluating the therapeutic efficacy and are critical during long-term follow-up after therapy.
Core Tip: Mucosal-associated lymphoid tissue extranodal marginal zone (MALT) lymphoma is a rare tumor that rarely occurs in the urinary bladder. There is no con
- Citation: Jiang ZZ, Zheng YY, Hou CL, Liu XT. Primary mucosal-associated lymphoid tissue extranodal marginal zone lymphoma of the bladder from an imaging perspective: A case report. World J Clin Cases 2021; 9(32): 10024-10032
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/10024.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.10024
Mucosal-associated lymphoid tissue extranodal marginal zone (MALT) lymphoma is a rare tumor that mainly occurs in the gastrointestinal tract[1-3] and has rarely been reported to occur in the urinary bladder, with a low prevalence of 0.2% of the ex
A 48-year-old woman was admitted to the hospital with a 1-year history of macro
In the past year, the patient was found to have recurrent macroscopic hematuria. Her urine was intermittently bright red with blood clots. There were no accompanying symptoms, such as frequent urination, urgent urination, painful urination, fever, or weight loss.
The patient had no significant medical history.
There were no special personal or family illness histories.
The physical examination revealed no abnormities. The patient had no percussion pain in the renal area bilaterally, and no tenderness in the ureteral and bladder areas.
Tumor marker analysis revealed that the serum carbohydrate antigen 125 (CA125) level of the patient was elevated (67.44 U/mL), while other tumor markers were negative. Routine blood tests revealed that the percentage of neutrophils was 77.6%, which was slightly elevated. Routine urinary tests revealed that the patient had a urinary tract infection (the urine white blood cell count was 2510.5/mL, and the urine bacteria count was 27736/mL), accompanied by hematuria and proteinuria (the urine red blood cell count was 37.9/mL, and the urine protein level was 1+). The thrombin profile revealed that the patient had coagulation function abnormalities (the pro
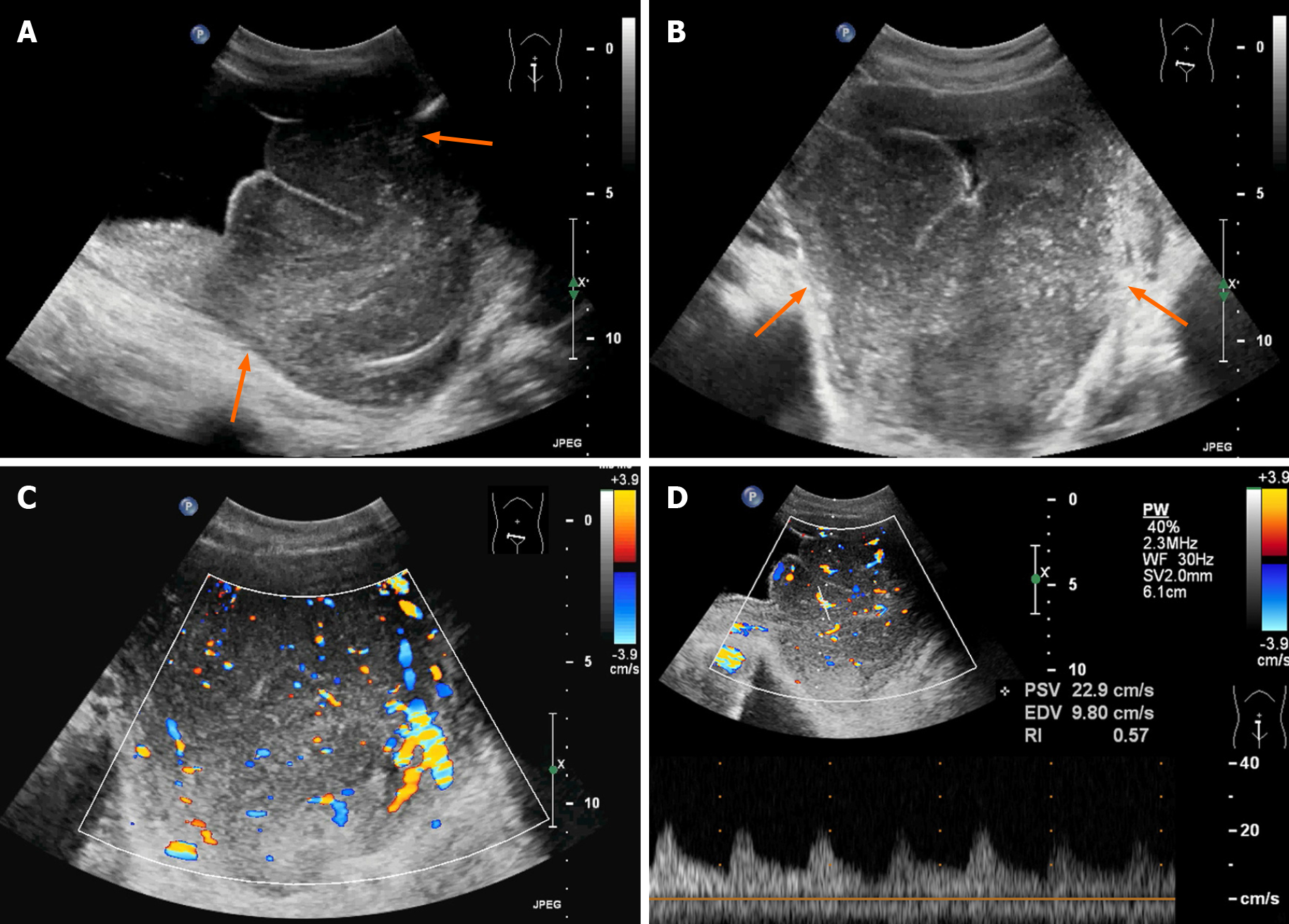
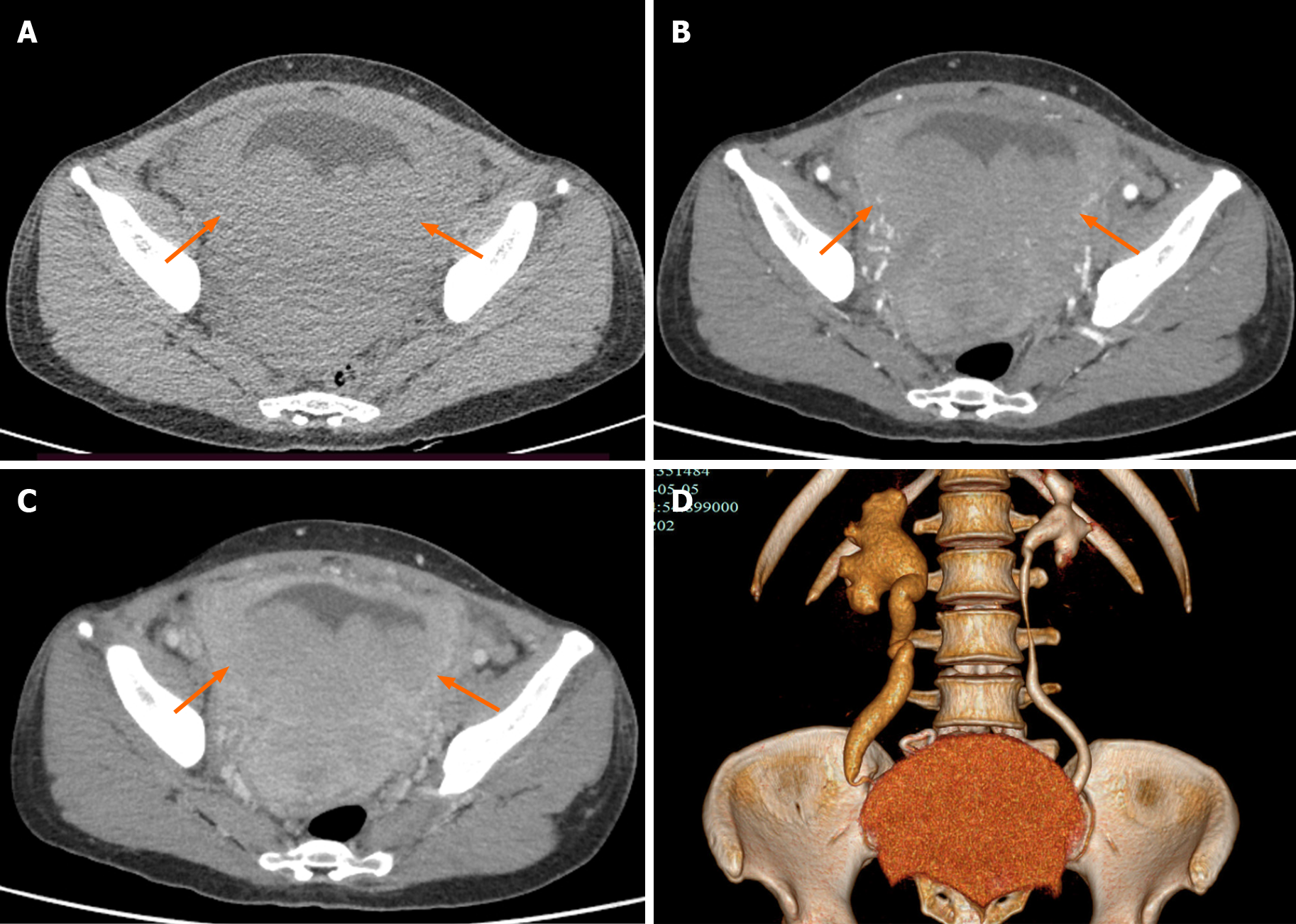
Ultrasound examination revealed a large hypoechoic mass in the bladder, and the size of the mass was 128 mm × 78 mm. Its boundary was not clear, and the shape was irregular. The mass had a homogeneous hypoecho with fine linear echogenic strands distributed inside. No signs of calcification or large patches of necrosis were encountered. Color Doppler flow imaging showed rich blood flow signals in the mass. An artery was detected within the mass, and it had a maximum flow velocity (Vmax) of 22.9 cm/s and a resistance index of 0.57 (Figure 1). The right renal collection system was separated by 13 mm, and the right ureter was fully dilated. A contrast-enhanced computed tomography (CT) scan demonstrated that the bladder wall was thickened, and there were multiple low-density nodules in the bladder with unclear boundaries and irregular morphologies. A continuous enhancement of the nodules was observed. The ureteral and renal pelvis of the right kidney were dilated (Figure 2). The CT images indicated bladder carcinoma, and a biopsy was recommended.
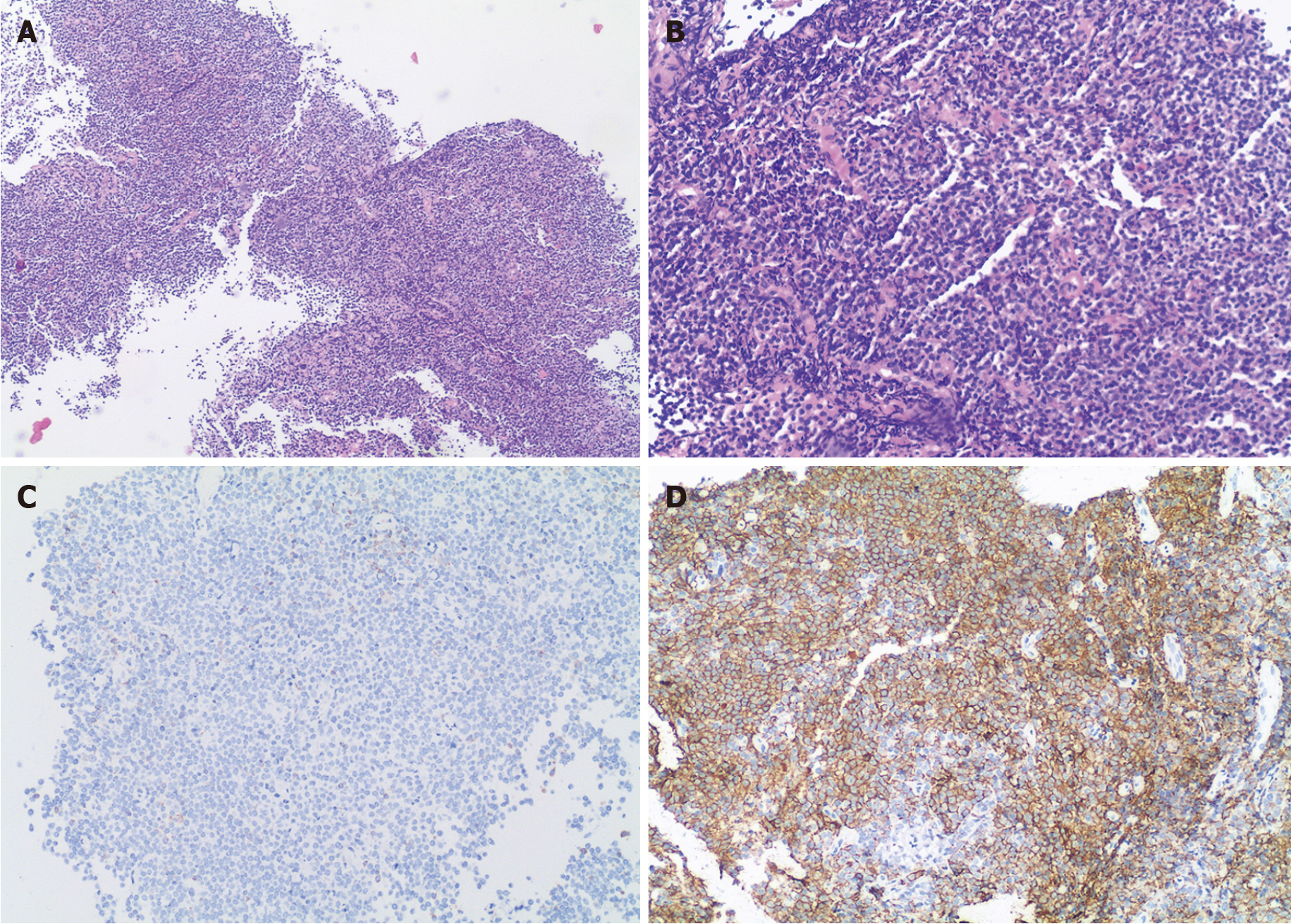
For further diagnosis, a transurethral cystoscopic biopsy was performed. During the operation, multiple bulging bladder masses were found. Tumor tissue samples were collected, and histological findings revealed diffuse infiltration by atypical lymphoid cells. Immunohistochemical staining was positive for CD20, CD21, and CD79a and was negative for CD3, CD5, CD35, CD10, Bcl-6, CD45RO, Mum-1, and Cyclin D1, with a Ki-67 Labeling index of 10% (Figure 3). These data supported the diagnosis of MALT lymphoma. No primary or secondary lymphoma lesions were detected by imaging examinations. Additionally, a bone marrow biopsy revealed no signs of invasion of MALT lymphoma. The patient was diagnosed with primary MALT lymphoma of the bladder.
Laboratory examinations indicated that the patient had a coagulation disorder and was unable to proceed with surgery. Based on the patient’s condition and treatment guidelines, R-CHOP chemotherapy was carried out.
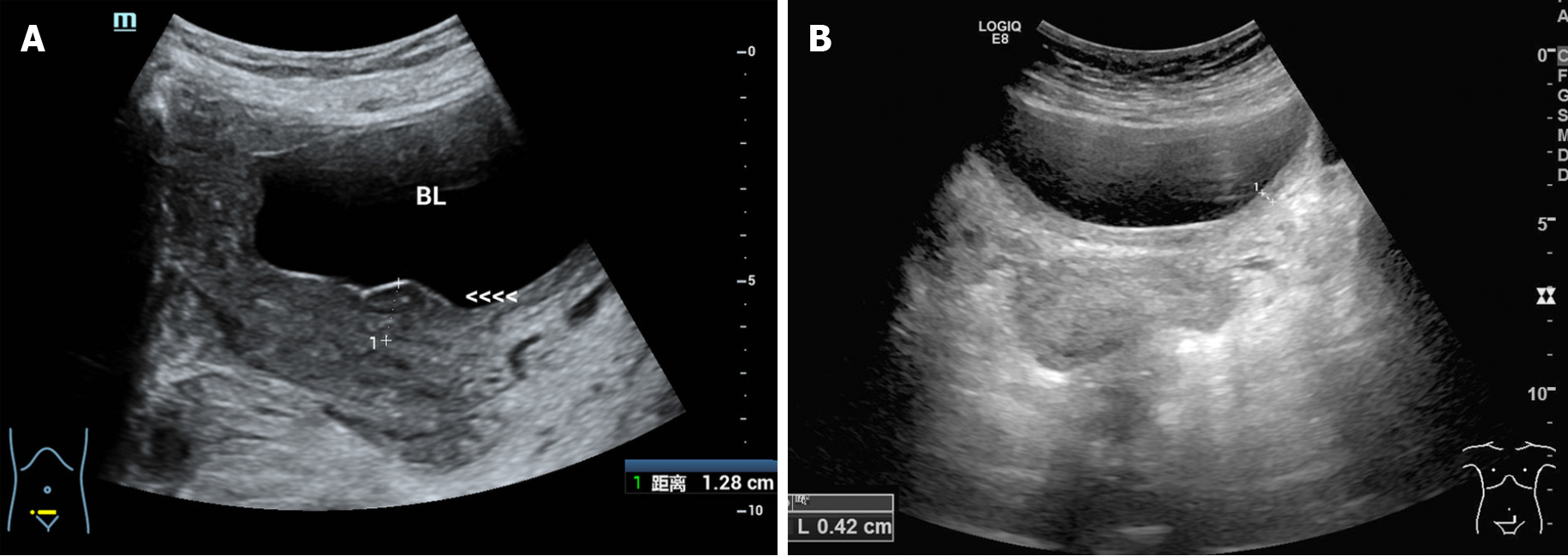
The patient had a favorable outcome. After two-cycles of chemotherapy, ultrasonography showed that the mass had disappeared with localized thickening (13 mm) of the bladder wall. After three-cycles of chemotherapy, the bladder wall thickness was only 4 mm, which indicated an excellent therapeutic response to chemotherapy (Figure 4). To date, the patient remains asymptomatic and she visits our hospital regularly for the completion of the remaining chemotherapy cycles. After finishing all the chemotherapy cycles, CT follow-up will be performed to verify the final thera
MALT lymphoma is a unique subtype of B-cell lymphoma predominantly involving extranodal sites, such as the stomach, orbit, conjunctiva, salivary glands, thyroid and lungs[2,5-7]. The bladder is rarely involved, accounting for only 0.2% of extranodal lymphomas[8]. The risk factors include chronic inflammation, urinary tract infections and autoimmune diseases[9,10]. As no naturally occurring lymphoid tissue exists in the bladder, the pathogenesis of primary bladder MALT lymphoma may be due to the accumulation of extranodal lymphoid tissue resulting from chronic inflammation, which is similar to the mechanism of gastric MALT lymphoma caused by Helicobacter pylori infection[1]. In our case, although the patient did not mention a history of chronic inflammation, her laboratory examinations indicated the existence of a urinary tract infection, which was compatible with the reported risk factors.
The clinical features of bladder MALT lymphoma are nonspecific. It more com
To date, few studies have focused on the imaging findings of primary bladder MALT lymphoma because of its rarity. For the literature review, we summarized the imaging findings of reported cases of primary bladder MALT lymphoma in Table 1, including the present case. We found that most patients (9/13, 69.2%) presented with a solitary mass. The average size of the lesions that had been reported was 7.6 cm (3.4-12.8 cm). It has been reported that primary bladder lymphoma is more commonly seen in the lateral wall of the bladder, with only 10% of cases having a diffuse thickening of the bladder wall[14], which is consistent with our results (15% of cases had diffuse thickened wall). Some of the patients (6/13, 46.2%) had signs of hydronephrosis, which may be due to an obstruction of the urethral orifices caused by large masses. In our case, the patient presented with a large sessile solitary mass with thickening of the bladder wall and right hydronephrosis, which was thought to occur because the mass originated from the lateral wall of the bladder and obstructed the right ureteral outlet, and the hydronephrosis was not likely secondary to tumor invasion.
| No. | Ref. | Solitary /multiple | Size (cm) | Location | Signs of ureteral obstruction | Imaging findings | Follow-up imaging methods | ||
| US | CT | MRI | |||||||
| 1 | Tasu et al[14] | Solitary | NA | Right lateral wall | No | Soft tissue mass | Soft tissue mass | No | NA |
| 2 | Tasu et al[14] | NA | NA | Entire wall | Yes | Circumferential thickening of the bladder wall | Circumferential thickening of the bladder wall | No | CT |
| 3 | Tasu et al[14] | Solitary | NA | Left wall | No | An isolated mass with local thickened bladder wall | An isolated mass with local thickened bladder wall | No | NA |
| 4 | Maninderpal et al[15] | Solitary | 11.2 | Trigone of the bladder | Yes | A hypoechoic soft tissue mass with local thickened bladder wall | A homogeneously enhancing mass with local thickened bladder wall | Homogeneously enhancing mass with hypointensity in T1WI, intermediate intense intensity in T2WI, and hyperintensity in STIR | CT |
| 5 | Szopiński et al[11] | Solitary | 3.6 | Posterior wall | No | Hypoechoic mass | No | No | US, CT |
| 6 | Morita et al[23] | Solitary | NA | NA | NA | No | Soft tissue mass with local thickened bladder wall | No | NA |
| 7 | Bacalja et al[24] | Solitary | 8.5 | Right posterolateral wall | No | Soft tissue mass | Expansive lesion of the bladder wall | No | CT, PET-CT |
| 8 | Matsuda et al[4] | Solitary | NA | Anterior to right side wall | Yes | No | Soft tissue mass with local thickened bladder wall | Soft tissue mass with local thickened bladder wall | US |
| 9 | Simpson et al[25] | Solitary | 6.3 | Anterior lateral | Yes | No | Soft tissue mass | No | PET-CT |
| 10 | Hsu et al[18] | NA | NA | Right wall | Yes | No | Eccentric thickened bladder wall | No | NA |
| 11 | Kadam et al[26] | Solitary | 3.4 | Posterior wall | NA | No | Soft tissue mass | No | NA |
| 12 | Xu et al[8] | Multiple | NA | NA | NA | Roughness in the inner bladder wall | Soft tissue mass | No | CT, PET-CT |
| 13 | Present case | Multiple | 12.8 | Posterior wall | Yes | A mass had a homogeneous hypoecho with fine linear echogenic strands distributed inside | Continuous enhancing mass with thickened wall | No | US |
The general imaging manifestations of MALT lymphoma are solitary or multiple solid homogeneous masses without cysts, necrosis or calcification[15,16]. The ul
Primary bladder MALT lymphoma needs to be differentiated from bladder carci
| US | CT | MRI | |
| Present case | Hypoecho mass with sharp boundaries | Solitary mass with continuously enhancement and thickened bladder wall | No |
| Common findings of cases reported | Hypoecho mass | Solitary mass with homogeneously enhancement | Homogeneously enhancing mass with hypointensity in T1WI, intermediate intense intensity in T2WI, hyperintensity in STIR |
| Common findings of bladder carcinoma | Hypoechoic masses with rough boundaries | Irregular solid mass with significantly heterogeneous enhancement | Early significantly enhancing mass with iso to hyperintensity in T1WI, intermediate intensity in T2WI, hyperintensity in DWI, and decreased ADC value |
| Common findings of glandular cystitis | Diffuse thickening or nodular eminence of the bladder wall | Thickened bladder wall with mild enhancement | Mild enhancing thickened bladder wall with intermediate intensity on T1W, hyperintense on T2W, and slightly hyperintense on DWI |
The most effective therapeutic approach for primary bladder MALT lymphoma remains controversial. A complete resection of the tumor may not be required, as radiotherapy and chemotherapy can be useful and effective[18]. In this case, R-CHOP chemotherapy was used and resulted in remission after taking into consideration the patient’s condition and the therapeutic efficacy of systemic chemotherapy.
In the follow-up of MALT lymphoma, imaging modalities, such as contrast-enhanced CT, are effective in detecting the lesion[19]. The value of positron emission tomography/CT for the staging and response assessment of MALT lymphoma with multiple site involvement has also been reported[20]. However, the optimal follow-up imaging modalities have not been determined due to the limited case number. In our experience, the therapeutic response or the presence of a relapse after the remission of a primary bladder MALT lymphoma could be evaluated by tracking the changes in the bladder wall thickness by ultrasound. In the long-term follow-up of MALT lymphoma in hollow organs, such as the stomach and bladder, ultrasonography can be a valuable tool for the evaluation of wall infiltration by measuring the wall thickness[21,22].
Primary MALT lymphoma of the bladder is rare, and the imaging findings have certain characteristics. The diagnosis of MALT lymphoma should be considered if a large mass has no signs of extravesical spread. Primary bladder MALT lymphoma is a low-grade, localized indolent tumor with a good prognosis, so early diagnosis can reduce unnecessary surgeries. In addition, ultrasound can be used as an imaging method for evaluating the therapeutic efficacy and is critical for long-term follow-up after therapy.
We thank the patient for allowing us to share details of her diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Akbulut S, Elpek GO, Harikrishnan S S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY
| 1. | Matysiak-Budnik T, Jamet P, Ruskoné-Fourmestraux A, de Mascarel A, Velten M, Maynadié M, Woronoff AS, Trétarre B, Marrer E, Delafosse P, Ligier K, Lapôtre Ledoux B, Daubisse L, Bouzid L, Orazio S, Cowppli-Bony A, Monnereau A. Gastric MALT lymphoma in a population-based study in France: clinical features, treatments and survival. Aliment Pharmacol Ther. 2019;50:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, Ricardi U, Salar A, Stamatopoulos K, Thieblemont C, Wotherspoon A, Ladetto M; ESMO Guidelines Committee. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Wei YL, Min CC, Ren LL, Xu S, Chen YQ, Zhang Q, Zhao WJ, Zhang CP, Yin XY. Laterally spreading tumor-like primary rectal mucosa-associated lymphoid tissue lymphoma: A case report. World J Clin Cases. 2021;9:3988-3995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Matsuda I, Zozumi M, Tsuchida YA, Kimura N, Liu NN, Fujimori Y, Okada M, Hashimoto T, Yamamoto S, Hirota S. Primary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type with malakoplakia in the urinary bladder: a case report. Int J Clin Exp Pathol. 2014;7:5280-5284. [PubMed] |
| 5. | Suh C, Huh J, Roh JL. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the extracranial head and neck region: a high rate of dissemination and disease recurrence. Oral Oncol. 2008;44:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Wenzel C, Fiebiger W, Dieckmann K, Formanek M, Chott A, Raderer M. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue of the head and neck area: high rate of disease recurrence following local therapy. Cancer. 2003;97:2236-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, Patterson BJ, Ferreri AJ, Ponzoni M, Devizzi L, Giardini R, Pinotti G, Capella C, Zinzani PL, Pileri S, López-Guillermo A, Campo E, Ambrosetti A, Baldini L, Cavalli F; International Extranodal Lymphoma Study Group. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 2003;101:2489-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Xu H, Chen Z, Shen B, Wei Z. Primary bladder mucosa-associated lymphoid tissue lymphoma: A case report and literature review. Medicine (Baltimore). 2020;99:e20825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 10. | Munari F, Lonardi S, Cassatella MA, Doglioni C, Cangi MG, Amedei A, Facchetti F, Eishi Y, Rugge M, Fassan M, de Bernard M, D'Elios MM, Vermi W. Tumor-associated macrophages as major source of APRIL in gastric MALT lymphoma. Blood. 2011;117:6612-6616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Szopiński TR, Sudoł-Szopińska I, Dzik T, Borówka A, Dembowska-Bagińska B, Perek D. Incidental sonographic detection of mucosa-associated lymphoid tissue lymphoma of the urinary bladder found in a very young woman: report of a case. J Clin Ultrasound. 2011;39:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Zacharos ID, Efstathiou SP, Petreli E, Georgiou G, Tsioulos DI, Mastorantonakis SE, Christakopoulou I, Roussou PP. The prognostic significance of CA 125 in patients with non-Hodgkin's lymphoma. Eur J Haematol. 2002;69:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Benboubker L, Valat C, Linassier C, Cartron G, Delain M, Bout M, Fetissof F, Lefranq T, Lamagnere JP, Colombat P. A new serologic index for low-grade non-Hodgkin's lymphoma based on initial CA125 and LDH serum levels. Ann Oncol. 2000;11:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Tasu JP, Geffroy D, Rocher L, Eschwege P, Strohl D, Benoit G, Paradis V, Bléry M. Primary malignant lymphoma of the urinary bladder: report of three cases and review of the literature. Eur Radiol. 2000;10:1261-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Maninderpal KG, Amir FH, Azad HA, Mun KS. Imaging findings of a primary bladder maltoma. Br J Radiol. 2011;84:e186-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ko KWS, Bhatia KS, Ai QYH, King AD. Imaging of head and neck mucosa-associated lymphoid tissue lymphoma (MALToma). Cancer Imaging. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Jeon EJ, Shon HS, Jung ED. Primary Mucosa-Associated Lymphoid Tissue Lymphoma of Thyroid with the Serial Ultrasound Findings. Case Rep Endocrinol. 2016;2016:5608518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Hsu JS, Lin CC, Chen YT, Lee YC. Primary mucosa-associated lymphoid tissue lymphoma of the urinary bladder. Kaohsiung J Med Sci. 2015;31:388-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kheterpal MK, Dai J, Geller S, Pulitzer M, Ni A, Myskowski PL, Moskowitz A, Kim J, Hong EK, Fong S, Hoppe RT, Kim YH, Horwitz SM. Role of imaging in low-grade cutaneous B-cell lymphoma presenting in the skin. J Am Acad Dermatol. 2019;81:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Vaxman I, Bernstine H, Kleinstern G, Hendin N, Shimony S, Domachevsky L, Gurion R, Groshar D, Raanani P, Gafter-Gvili A. FDG PET/CT as a diagnostic and prognostic tool for the evaluation of marginal zone lymphoma. Hematol Oncol. 2019;37:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Yeh HZ, Chen GH, Chang WD, Poon SK, Yang SS, Lien HC, Chang CS, Chou G. Long-term follow up of gastric low-grade mucosa-associated lymphoid tissue lymphoma by endosonography emphasizing the application of a miniature ultrasound probe. J Gastroenterol Hepatol. 2003;18:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Park BS, Lee SH. Endoscopic features aiding the diagnosis of gastric mucosa-associated lymphoid tissue lymphoma. Yeungnam Univ J Med. 2019;36:85-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Morita K, Nakamura F, Nannya Y, Nomiya A, Arai S, Ichikawa M, Maeda D, Homma Y, Kurokawa M. Primary MALT lymphoma of the urinary bladder in the background of interstitial cystitis. Ann Hematol. 2012;91:1505-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Bacalja J, Ulamec M, Rako D, Bošković L, Trnski D, Vrdoljak E, Krušlin B. Persistence of primary MALT lymphoma of the urinary bladder after rituximab with CHOP chemotherapy and radiotherapy. In Vivo. 2013;27:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Simpson WG, Lopez A, Babbar P, Payne LF. Primary bladder lymphoma, diffuse large B-cell type: Case report and literature review of 26 cases. Urol Ann. 2015;7:268-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Kadam PD, Han HC, Kwok JL. An uncommon case of mucosa-associated lymphoid tissue (MALT) tumor of the bladder. Int Urogynecol J. 2019;30:1017-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |