Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9607
Peer-review started: April 29, 2021
First decision: July 15, 2021
Revised: July 28, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 6, 2021
Processing time: 183 Days and 3.6 Hours
T-lymphoblastic lymphoma (T-LBL), a neoplasm of immature T-cell precursors or lymphoblasts, is a clinically aggressive disease. In general, patients with T-LBL have a poor prognosis and often have high-risk clinical features, such as mediastinal masses, central nervous system infiltration, or other indications of high tumor burden; however, extensive thrombi are not common.
A 27-year-old woman presented to the Department of General Surgery with cervical lymph node enlargement accompanied by cough, wheezing, and palpitation for 3 mo. A complete blood count showed a white blood cell count of 1.6 × 109/L, a hemoglobin concentration of 135 g/L, and a platelet count of 175 × 109/L. A biopsy sample of the lymph node mass indicated T-cell lymphoblastic lymphoma, and the bone marrow immunophenotype indicated early T-cell precursor acute lymphoblastic leukemia (ETP-ALL). Abdominal and chest enhanced computed tomography showed thrombi in the superior vena cava, inferior vena cava, right hepatic vein, azygos vein, and right atrium. The ultrasonic cardiogram showed a thrombus in the right atrium of 5.23 cm × 4.21 cm. The patient was first treated with low-dose dexamethasone and low-molecular-weight heparin followed by 2 cycles of chemotherapy. Then, the ultrasonic cardiogram showed that thrombus in the right atrium had disappeared and the patient had achieved complete cytological remission. The maintenance therapy of the patient included chidamide 30 mg/wk, and she survived for 6 mo.
The incidence of venous thromboembolism is high in lymphoma; however, extensive thrombi with heart thrombosis is rare. Chemotherapy is the major method of treatment for lymphoma with thrombosis. We successfully treated a patient with T-LBL complicated by extensive thrombi, including a large right atrial thrombus, with combined chemotherapy containing liposomal doxorubicin, and the patient achieved complete remission. Maintenance therapy with chidamide was also effective.
Core Tip: T-lymphoblastic lymphoma (T-LBL), a neoplasm of immature T-cell precursors or lymphoblasts, is a clinically aggressive disease. We present herein, a rare case of T-cell lymphoblastic lymphoma with extensive thrombi and cardiac thrombosis. This case highlights the ultimate importance of monitoring changes in embolus size and whether the embolus falls off during the treatment to avoid potentially serious multi-organ thrombosis complications. In addition, this case also confirmed that pegylated liposomal doxorubicin and chidamide are safe and effective in the treatment of T-LBL/leukemia.
- Citation: Ma YY, Zhang QC, Tan X, Zhang X, Zhang C. T-cell lymphoblastic lymphoma with extensive thrombi and cardiac thrombosis: A case report and review of literature. World J Clin Cases 2021; 9(31): 9607-9616
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9607.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9607
T-lymphoblastic lymphoma (T-LBL) is a rare and aggressive precursor T-cell tumor that can affect the bone marrow (BM) or blood or present as a tissue-based mass involving the thymus, lymph nodes, or extranodal sites. T-LBL mainly occurs in adolescents and young adults[1]. The etiology of T-LBL is still unclear and may be caused by biological, physical, and chemical factors, and changes in molecular genetics may also be related to its occurrence. At present, there is no standard treatment for T-LBL; however, CHOP(adriamycin, cyclophosphamide, vincristine, prednisone), hyper-CVAD(methotrexate, cytarabine, prednisone), or chemotherapy regimens for childhood acute lymphoblastic leukemia are commonly used in the clinic[2-4]. The application of autologous hematopoietic stem cell transplantation (auto-HSCT) or allogeneic HSCT (allo-HSCT) in the treatment of T-LBL patients is still controversial[5,6].
The patient was a 27-year-old female who, 1 mo after giving birth, was admitted to the hospital because of cervical lymph node enlargement accompanied by cough, wheezing, and palpitation for 3 mo.
She was diagnosed with T-LBL at another hospital.
She has no special personal and family history.
The physical examination revealed moderate anemia. A scattered, red maculopapular rash with ulcers visible on the surface was present on the patient’s chest. There were no skin rashes, bleeding spots, or ecchymosis on the skin of the rest of the body. Bilateral enlarged lymph nodes were palpable in the neck area, the largest one located on the right, approximately 3 cm × 5 cm in size, with tenderness to touch and clear boundaries in relation to the surrounding tissue but without fusion. The other superficial lymph nodes were not palpable. There was no tenderness in the sternum, and the rest of the physical examination was unremarkable.
Right cervical lymph node biopsy: Non-Hodgkin's lymphoma, immunohistochemistry as follows: TDT+, BCL-2+, CD79a+, CD5 part+, CD7+, CD99+, CD3-, CD20-, CD10-, CD23-, CD34 (endothelium+), CD1a-, CD2-, CD4-, CD8-, PAX-5(-), and Ki67(70+).
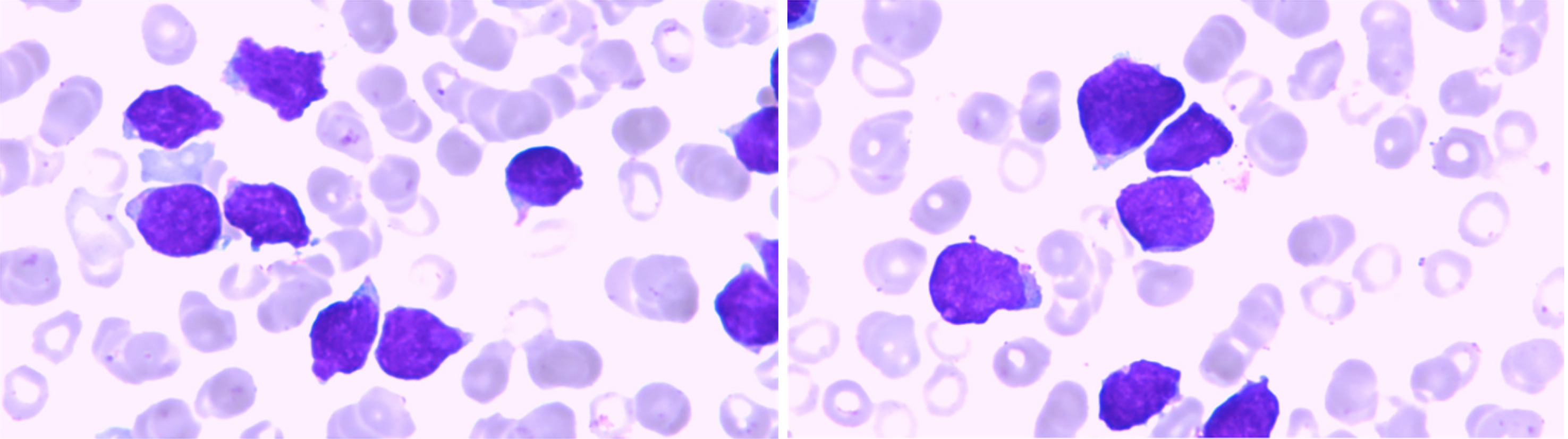
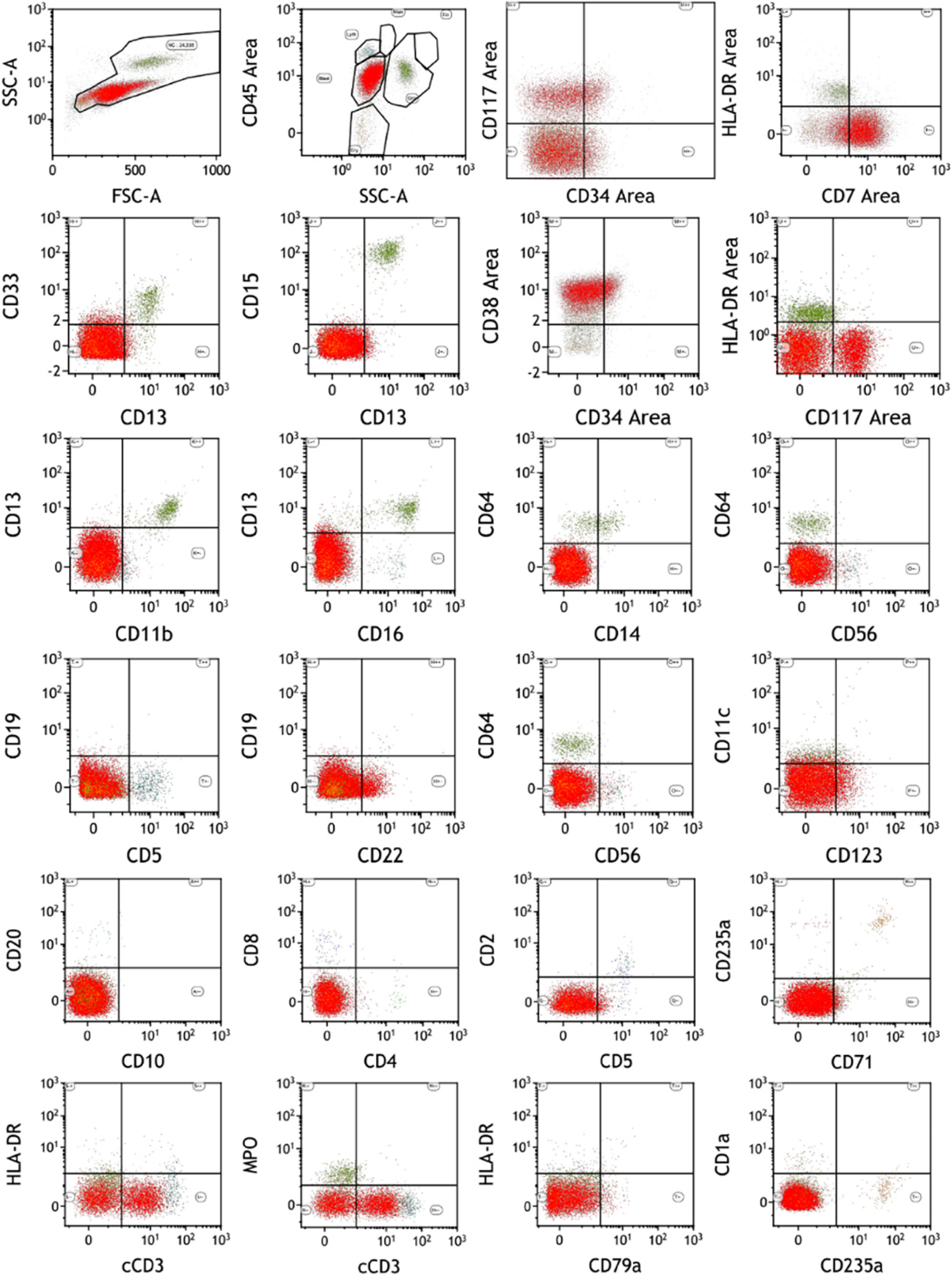
BM cytology and flow cytometry: A large number of abnormal lymphocytes were found, and the immunophenotype was CD34-, CD117p+, CD38+, HLA-DR-, CD13dim, CD33-, CD123 slightly positive, CD22p+, Ccd3+, CD3-, CD5- , CD7+, CD8-, CD4-, CD2-, MPO+, which indicated ETP-ALL (Figure 1 and 2).
Mutation detection: No abnormalities were found regarding mutations of thrombophilia or in the next-generation sequencing (NGS) for T-cell lymphoma. Whole-genome exon sequencing showed that, among the sequences analyzed, 51% had PLA2G7 mutations, 49% had NOTCH2 mutations, 45% had TTN mutations, 43% had PIK3CA mutations, 46% had CCND3 mutations, and 50% had NF1 mutations.
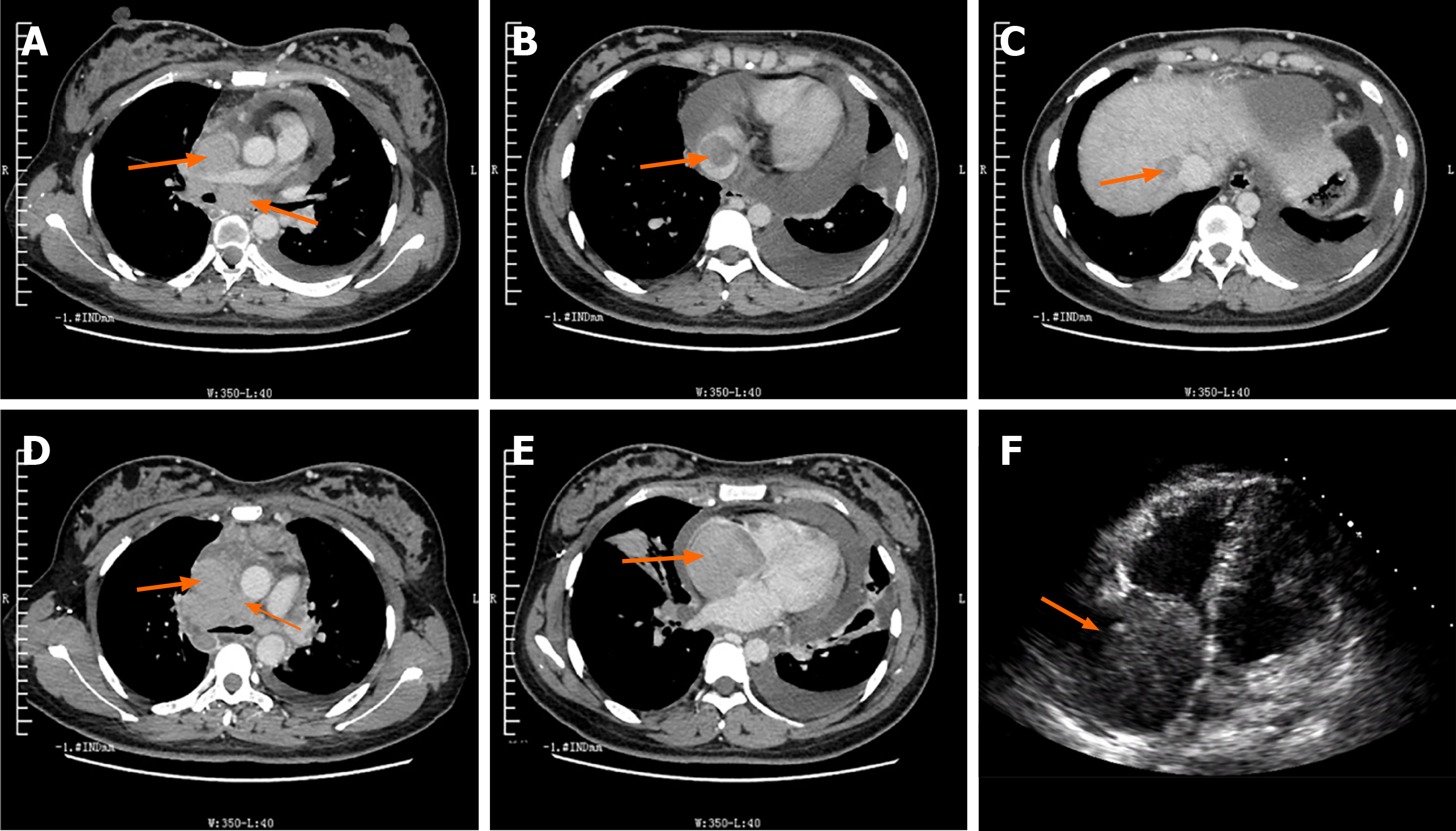
Chest enhanced computed tomography (CT) and ultrasonic cardiogram showed extensive thrombi and heart thrombosis (Figure 3).
A multidisciplinary team (MDT) was assembled immediately after admission, and the suggestion of the consultation was as follows: First, extensive thrombi and cardiac thrombosis indicated cancer thrombosis, but the possibility of thrombus shedding was relatively small. It was suggested that anticoagulation and thrombolysis should be carried out on the basis of active treatment of the primary disease, and the coagulation function and hemogram should be closely monitored. Second, at that time, the patient had no indication for operation, such as circulatory disturbance or tricuspid complete obstruction, but emergency surgery could be performed at any time if the condition changed, or surgical treatment can be decided according to the patient's specific conditions after the control of the primary disease.
The final diagnosis of the presented case is T-cell lymphoblastic lymphoma with extensive thrombosis and cardiac thrombosis caused by lymphoma.
The patient was treated with low-dose dexamethasone and low-molecular-weight heparin in the first 3 d (July 16, 2018 to July 19, 2018), and then we went through the first circle of chemotherapy in sequence, including pegaspargase 3750 IU × 1 d, cyclophosphamide 1.2 g × 1 d, pegylated liposomal doxorubicin (PLD) 20 mg × 3 d, vindesine 4 mg × 1 d, and dexamethasone 10 mg × 7 d. A detailed treatment schedule is shown in Table 1. During the treatment, we closely monitored the patient's vital signs, routine blood test results, coagulation function, and cardiac ultrasound.
| Date | Low-molecular-weight heparin | Pegaspargase | Cyclophosphamide | Pegylated liposomal doxorubicin | Vindesine | Dexamethasone | Methotrexate | Chidamide |
| July 16-July 19 | 5000 U/d | 5 mg/d | ||||||
| July 20-July 26 | 3750 IU × 1 d | 1.2 g × 1 d | 20 mg/d × 3 d | 4 mg × 1 d | 10 mg/d × 7 d | |||
| August 23-August 29 | 3750 IU × 1 d | 1.0 g × 1 d | 20 mg/d × 3 d | 4 mg × 1 d | 10 mg/d × 7 d | 2 g × 1 d | ||
| September 20 | 30 mg 2/wk (follow up for half year) | |||||||
After chemotherapy, the lowest neutrophil count was 0.04 × 109/L, and agranulocytosis with recurrent fever occurred. The body temperature returned to normal after the combination of cefoperazone, sulbactam, and vancomycin. On the 11th day of treatment (July 27, 2018), cardiac ultrasound showed that the embolus in the right atrium had reduced in size to 42 mm × 38 mm, and on the 30th day (August 15, 2018), it had reduced in size to 25 mm × 13 mm. On the 20th day after the first cycle chemotherapy, BM cytology showed that immature lymphocytes accounted for 4%, the cell shape was irregular, and pseudopodia were easily seen. The examination of minimal residual disease (MRD) showed that CD45dim, TDT-, CD99+, CD10+, Ccd3+, CD5-, and CD7+ cells occupied 5.21% of nuclear cells and were abnormal T lymphocytes, which was significantly lower than that before chemotherapy.
Then, we gave the patient the second cycle of chemotherapy, including pegaspargase 3750 IU × 1 d, cyclophosphamide 1.2 g × 1 d, PLD 20 mg × 3 d, vindesine 4 mg × 1 d, dexamethasone 10 mg × 7 d, and methotrexate 2 g × 1 d. On the 45th day (August 30, 2018) after therapy, cardiac ultrasound showed that the embolus in the right atrium had reduced in size to 18 mm × 15 mm. All results of the accessory examination are shown in Table 2.
| Date | Echocardiography (cardiac thrombus in the right atrium) | Bone marrow cytology | Flow cytometry | CTA of head | CT of head |
| July 16 | 52.3 mm × 42.1 mm | The abnormal lymphocytes accounted for 79% | Abnormal T lymphoblasts accounted for 85.5% | ||
| July 20 | 50 mm × 42 mm | ||||
| July 27 | 42 mm × 38 mm | ||||
| August 3 | 35 mm × 28 mm | ||||
| August 9 | 28 mm × 22 mm | ||||
| August 15 | 25 mm × 13 mm | ||||
| August 22 | 22.8 mm × 15 mm | The abnormal lymphocytes accounted for 4% | Abnormal T lymphoblasts accounted for 5.21% | ||
| August 30 | 18 mm × 15 mm | ||||
| September 3 | None | A large area of low-density shadow in the right parietal lobe, which was considered a cerebral infarction | |||
| September 6 | On the right frontal and parietal lobes, there were low-density patches with slightly higher density. On contrast-enhanced scans, slight enhancement could be seen, indicating the possibility of cerebral infarction with a small amount of hemorrhage |
Unfortunately, the patient developed left limb weakness with nausea and progressive aggravation after the activity on September 3, 2018. Physical examination of the patient revealed paralysis of the left upper and lower limbs and decreased muscle tension of the left upper and lower limbs, weak tendon reflex, grade 0 muscle strength of the left upper limb, grade II muscle strength of the left lower limb, and negative pathological findings. The original right atrium mass was not found in an emergency cardiac ultrasound. CT angiography of cephalic and cervical tissue revealed a large area of low-density shadow in the right parietal lobe, which was considered a cerebral infarction. Combined with the patient's medical history, clinical symptoms, and the results of the abovementioned auxiliary examinations, it was considered that the original right atrial embolus had dislodged and had led to cerebral infarction. Subsequently, thrombolytic therapy and neurotrophic therapy were performed. Repeated epileptic seizures occurred on September 4, 2018 and were treated with sedation and antiepileptics. A cranial CT on September 6, 2018 found low-density patches with slightly higher density on the right frontal and parietal lobes. On contrast-enhanced scans, slight enhancement could be seen, indicating the possibility of cerebral infarction with a small amount of hemorrhage. After nutritional nerve treatment and supportive treatment, such as hemostasis, the limb function of the patient improved. Subsequently, the patient was transferred to the rehabilitation department to continue the rehabilitative treatment for limb function. Finally, the patient's limb function basically recovered, and she could take care of herself.
Our follow-up treatment plan for this patient was high-dose chemotherapy and stem cell transplantation, but the patient refused. Later, the patient insisted on oral chidamide 30 mg 2/wk for maintenance treatment, and she survived over 6 mo.
Lymphoblastic lymphoma (LBL) is a rare disease accounting for approximately 8% of all lymphoid malignancies[7]. In recent years, gene expression profiling, NGS, and whole exome sequencing (WES) studies have revealed other differences between T-ALL and T-LBL. Several studies, including the cooperative GRAALL study group, reported that in T-LBL adult patients, the prognosis of these patients was associated with the NOTCH1, FBXW7, N/K-RAS, and PTEN genes[8-10].
In this patient, we performed chromosome karyotyping and NGS of T-cell lymphoma and thrombophilia, but there was no positive finding. Surprisingly, WES found several abnormal gene mutations, including PLA2G7, NOTCH2, TTN, PIK3CA, CCND3 and NF1, although these mutations are not directly related to T-LBL in published reports. The platelet activating factor acetylhydrolase (PLA2G7) gene encodes lipoprotein-associated phospholipase A2 (Lp-PLA2) and is a potent pro- and anti-inflammatory molecule that has been implicated in multiple inflammatory disease processes[11]. Lp-PLA2 represents a potential cardiovascular risk marker, given its correlations with coronary disease and stroke[12]. Using Ingenuity Pathway Analysis software for pathway enrichment analysis, it was found that the PLA2G7 gene may participate in thrombosis through the hepatic fibrosis signaling pathway, the PPAR (peroxisome proliferator-activated receptor)signaling pathway, the AMPK(AMP-activated protein kinase) signaling pathway, or the nuclear factor-kappa beta signaling pathway, and the specific mechanism needs further study. Although the detected sites (PLA2G7: NM_005084:exon10:c.T896A:p.M299K) were not previously reported in COSMIC and related literature, we still believe that the presence of this mutation was associated with the clinical manifestations of massive venous thrombosis and atrial thrombosis in this patient. Notch2 is expressed in many cell types of most lineages in the hematolymphoid compartment and has specific roles in the differentiation and function of various immune cells[13]. In 2015, Neumann M provided a comprehensive mutation study of 81 adult T-ALL patients to identify new targets to improve the understanding of treatment objectives[14]. In this study, the NOTCH pathway was affected in approximately 60% of all T-ALL patients, including mutations in Notch receptor 2 (NOTCH2). In 2017, Doerrenberg et al[15] performed exome sequencing of three infant cases. One of the three infant patients had a heterozygous NOTCH2 mutation, which was predicted as deleterious as it causes an amino acid change from phenylalanine to valine in the extracellular EGF-like domain in the NOTCH2 protein, which is needed for Ca2+-dependent ligand binding. Therefore, it is reasonable to believe that the NOTCH2 mutation is related to the clinical prognosis of this patient. PIK3CA has been found to be oncogenic, and it has been implicated that gene amplification of PIK3CA contributes to the pathogenesis of DLBCL and mantle cell lymphoma[16]. Although no previous articles have reported that this gene is associated with T-LBL, we believe that the discovery of this mutation by whole-exome sequencing may provide a new research direction for the phenotype and prognostic indicators of gene mutations in subsequent T-LBL patients. D-type cyclins form complexes (CCND3) that have been reported to promote cell cycle progression. Although cyclin D functions appear largely tissue-specific, it has been demonstrated that cyclin D3 has unique functions in lymphocyte development and cannot be replaced by cyclin D2[17]. Recently, Liu et al[18] used integrated genomic analysis of 264 T-ALL cases, and their results showed that 83.7% of the cases had mutations in genes encoding cell cycle progression regulators and/or tumor suppressors. The targets of repeated mutations were CDKN2A/CDKN2B (78.4%), CDKN1B (12.9%), RB1 (9.5%) and CCND3 (6.1%). This means that CCND3 can be used as a potential prognostic indicator of T-ALL/T-LBL and can be widely used in clinical practice, but the specific relationship with the prognosis of T-ALL/T-LBL is not yet clear. Neurofibromin 1 (NF1) is a tumor suppressor gene encoding a Ras GTPase that negatively regulates Ras signaling pathways, and the codeletion of NF1 and p120 RasGAP in T cells results in the development of T-cell acute lymphoblastic leukemia[19]. Recent studies have also shown that normal NF1 expression impairs CD1d-mediated NKT-cell activation and antitumor activity against T-cell lymphoma[20]. Therefore, it is reasonable to believe that the NF1 gene mutation detected in this patient is closely related to the occurrence and development of the disease. A TTN mutation, which has not been reported to be associated with T-cell lymphoma or T-ALL, was also detected in this patient.
Unlike other patients with T-LBL, this patient had extensive thrombi and cardiac thrombosis, which, as we mentioned earlier, may be associated with the PLA2G7 mutation. In the choice of clinical treatment, we treated the patient with low-molecular-weight heparin for thrombolytic therapy and low-dose dexamethasone for lightening the tumor load in the first 3 d, and then we administered the first cycle of chemotherapy.
Cardiac involvement by malignant lymphoma is a very rare condition; therefore, since the patient's right atrial thrombosis may have endangered her life at any time, was it necessary to carry out surgical intervention in a timely manner? A MDT was assembled immediately after admission, and cardiac surgery experts decided that the patient had no indication for surgery and suggested that thrombolytic therapy should be carried out first. Combining the results of immunohistochemistry and flow immunotyping, we chose the modified Peg + CHOP regimen for chemotherapy. After the above chemotherapy regimen, the thrombus in the right atrium of the patient was progressively reduced. Although the patient had a temporary cerebral infarction and limb hemiplegia after treatment, the patient recovered well after active thrombolytic therapy.
It is worth mentioning that we used an unconventional chemotherapy regimen containing PLD in this patient and achieved satisfactory results. As early as 2007, Professor Pulini reported the results of a prospective phase II clinical trial of PLD in advanced/refractory primary cutaneous T-cell lymphoma[21] and obtained encou
According to our treatment plan, we suggested that patient should undergo HSCT to consolidate the efficacy and improve the long-term survival rate, but she refused to receive chemotherapy after two cycles of combined chemotherapy. Chidamide, a class I histone deacetylase subtype benzamide inhibitor, exerts effects in T-cell tumors through various mechanisms[26,27]. Chidamide monotherapy for refractory/relapsed PTCL has demonstrated efficacy and tolerable side effects[28,29]. In addition, according to the research results of Wei Guan's team, all six ETP-LBL/ALL patients showed clinical response to chemotherapy, including chidamide, indicating a promising salvage treatment option for refractory or relapsed ETP-LBL/ALL[30], and the regimens containing chidamide were active and well tolerated in refractory and relapsed T-LBL/ALL. Therefore, we suggested that the patient take chidamide 30 mg 2 times/wk orally for maintenance treatment, and she survived over 6 mo. Unfortunately, the patient was lost to follow-up after 6 mo, and we failed to obtain a follow-up efficacy evaluation. However, the successful treatment of this patient still suggests that clinicians can consider chemotherapy including PLD and chidamide in T-LBL patients with high-risk cardiotoxicity.
PLD and chidamide are safe and effective in the treatment of T-LBL/leukemia. The benefit of improving the CR(complete remission), ORR(objective response rate), or PFS(progression-free survival) needs to be further confirmed by prospective clinical trials, and future studies incorporating baseline cardiac risk assessments, long-term follow-up data, and biospecimen collection for correlative science should be undertaken. In the treatment of lymphoma patients with high-risk thrombosis complications, we must pay attention to the results of next-generation and whole-exome sequencing to check for the presence of thrombus-related gene mutations and to the prevention and treatment of thrombus shedding and other complications during treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Raiter A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yu HG
| 1. | You MJ, Medeiros LJ, Hsi ED. T-lymphoblastic leukemia/Lymphoma. Am J Clin Pathol. 2015;144:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Thomas DA, O'Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, Ferrajoli A, Koller C, Beran M, Pierce S, Ha CS, Cabanillas F, Keating MJ, Kantarjian H. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, Rowe J, Webb D. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Ribera JM, Oriol A, Sanz MA, Tormo M, Fernández-Abellán P, del Potro E, Abella E, Bueno J, Parody R, Bastida P, Grande C, Heras I, Bethencourt C, Feliu E, Ortega JJ. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Levine JE, Harris RE, Loberiza FR Jr, Armitage JO, Vose JM, Van Besien K, Lazarus HM, Horowitz MM, Bashey A, Bolwell BJ, Burns LJ, Cairo MS, Champlin RE, Freytes CO, Gibson J, Goldstein SC, Laughlin MJ, Lister J, Marks DI, Maziarz RT, Miller AM, Milone GA, Pavlovsky S, Pecora AL, Rizzo JD, Schiller G, Schouten HC, Zhang MJ; Lymphoma Study Writing Committee, International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood. 2003;101:2476-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Lazarevic VLj, Remberger M, Hägglund H, Hallböök H, Juliusson G, Kimby E, Malm C, Wahlin A, Omar H, Johansson JE. Myeloablative allogeneic stem cell transplantation for lymphoblastic lymphoma in Sweden: a retrospective study. Am J Hematol. 2011;86:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Cortelazzo S, Ferreri A, Hoelzer D, Ponzoni M. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2017;113:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Lepretre S, Touzart A, Vermeulin T, Picquenot JM, Tanguy-Schmidt A, Salles G, Lamy T, Béné MC, Raffoux E, Huguet F, Chevallier P, Bologna S, Bouabdallah R, Benichou J, Brière J, Moreau A, Tallon-Simon V, Seris S, Graux C, Asnafi V, Ifrah N, Macintyre E, Dombret H. Pediatric-Like Acute Lymphoblastic Leukemia Therapy in Adults With Lymphoblastic Lymphoma: The GRAALL-LYSA LL03 Study. J Clin Oncol. 2016;34:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Bonn BR, Rohde M, Zimmermann M, Krieger D, Oschlies I, Niggli F, Wrobel G, Attarbaschi A, Escherich G, Klapper W, Reiter A, Burkhardt B. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121:3153-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Callens C, Baleydier F, Lengline E, Ben Abdelali R, Petit A, Villarese P, Cieslak A, Minard-Colin V, Rullier A, Moreau A, Baruchel A, Schmitt C, Asnafi V, Bertrand Y, Macintyre E. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30:1966-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Sutton BS, Crosslin DR, Shah SH, Nelson SC, Bassil A, Hale AB, Haynes C, Goldschmidt-Clermont PJ, Vance JM, Seo D, Kraus WE, Gregory SG, Hauser ER. Comprehensive genetic analysis of the platelet activating factor acetylhydrolase (PLA2G7) gene and cardiovascular disease in case-control and family datasets. Hum Mol Genet. 2008;17:1318-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Silva IT, Mello AP, Damasceno NR. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A₂ (Lp-PLA₂): a review. Lipids Health Dis. 2011;10:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Sakata-Yanagimoto M, Chiba S. Notch2 and immune function. Curr Top Microbiol Immunol. 2012;360:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Neumann M, Vosberg S, Schlee C, Heesch S, Schwartz S, Gökbuget N, Hoelzer D, Graf A, Krebs S, Bartram I, Blum H, Brüggemann M, Hecht J, Bohlander SK, Greif PA, Baldus CD. Mutational spectrum of adult T-ALL. Oncotarget. 2015;6:2754-2766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Doerrenberg M, Kloetgen A, Hezaveh K, Wössmann W, Bleckmann K, Stanulla M, Schrappe M, McHardy AC, Borkhardt A, Hoell JI. T-cell acute lymphoblastic leukemia in infants has distinct genetic and epigenetic features compared to childhood cases. Genes Chromosomes Cancer. 2017;56:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Cui W, Ma M, Zheng S, Ma Z, Su L, Zhang W. PIK3CA amplification and PTEN loss in diffused large B-cell lymphoma. Oncotarget. 2017;8:66237-66247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Sawai CM, Freund J, Oh P, Ndiaye-Lobry D, Bretz JC, Strikoudis A, Genesca L, Trimarchi T, Kelliher MA, Clark M, Soulier J, Chen-Kiang S, Aifantis I. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell. 2012;22:452-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, McCastlain K, Edmonson M, Pounds SB, Shi L, Zhou X, Ma X, Sioson E, Li Y, Rusch M, Gupta P, Pei D, Cheng C, Smith MA, Auvil JG, Gerhard DS, Relling MV, Winick NJ, Carroll AJ, Heerema NA, Raetz E, Devidas M, Willman CL, Harvey RC, Carroll WL, Dunsmore KP, Winter SS, Wood BL, Sorrentino BP, Downing JR, Loh ML, Hunger SP, Zhang J, Mullighan CG. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 745] [Cited by in RCA: 694] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 19. | Lubeck BA, Lapinski PE, Oliver JA, Ksionda O, Parada LF, Zhu Y, Maillard I, Chiang M, Roose J, King PD. Cutting Edge: Codeletion of the Ras GTPase-Activating Proteins (RasGAPs) Neurofibromin 1 and p120 RasGAP in T Cells Results in the Development of T Cell Acute Lymphoblastic Leukemia. J Immunol. 2015;195:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Liu J, Gallo RM, Khan MA, Renukaradhya GJ, Brutkiewicz RR. Neurofibromin 1 Impairs Natural Killer T-Cell-Dependent Antitumor Immunity against a T-Cell Lymphoma. Front Immunol. 2017;8:1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Pulini S, Rupoli S, Goteri G, Pimpinelli N, Alterini R, Tassetti A, Scortechini AR, Offidani M, Mulattieri S, Stronati A, Brandozzi G, Giacchetti A, Mozzicafreddo G, Ricotti G, Filosa G, Bettacchi A, Simonacci M, Novelli N, Leoni P. Pegylated liposomal doxorubicin in the treatment of primary cutaneous T-cell lymphomas. Haematologica. 2007;92:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Armand P, Bello CM, Benitez CM, Bierman PJ, Chen R, Dabaja B, Dean R, Forero A, Gordon LI, Hernandez-Ilizaliturri FJ, Hochberg EP, Huang J, Johnston PB, Kaminski MS, Kenkre VP, Khan N, Maddocks K, Maloney DG, Metzger M, Moore JO, Morgan D, Moskowitz CH, Mulroney C, Rabinovitch R, Seropian S, Tao R, Winter JN, Yahalom J, Burns JL, Ogba N. NCCN Guidelines Insights: Hodgkin Lymphoma, Version 1.2018. J Natl Compr Canc Netw. 2018;16:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Yildirim Y, Gultekin E, Avci ME, Inal MM, Yunus S, Tinar S. Cardiac safety profile of pegylated liposomal doxorubicin reaching or exceeding lifetime cumulative doses of 550 mg/m2 in patients with recurrent ovarian and peritoneal cancer. Int J Gynecol Cancer. 2008;18:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Oki Y, Ewer MS, Lenihan DJ, Fisch MJ, Hagemeister FB, Fanale M, Romaguera J, Pro B, Fowler N, Younes A, Astrow AB, Huang X, Kwak LW, Samaniego F, McLaughlin P, Neelapu SS, Wang M, Fayad LE, Durand JB, Rodriguez MA. Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: an open label, single arm, phase II trial. Clin Lymphoma Myeloma Leuk. 2015;15:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Zhou D, Li L, Bao C, Zhu J, Zhu L, Yang X, Zheng Y, Zhou M, Luo X, Xie W, Ye X. Replacement of conventional doxorubicin by pegylated liposomal doxorubicin in standard RCHOP chemotherapy for elderly diffuse large B-Cell lymphoma: a retrospective study in China. Int J Clin Exp Med. 2015;8:22497-22502. [PubMed] |
| 26. | Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. 2017;58:1306-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, Zhao W, Zhang H, Sun X, Yang H, Zhang X, Jin J, Jin Z, Li Z, Qiu L, Dong M, Huang X, Luo Y, Wang X, Wu J, Xu J, Yi P, Zhou J, He H, Liu L, Shen J, Tang X, Wang J, Yang J, Zeng Q, Zhang Z, Cai Z, Chen X, Ding K, Hou M, Huang H, Li X, Liang R, Liu Q, Song Y, Su H, Gao Y, Luo J, Su L, Sun Z, Tan H, Wang H, Wang S, Zhou D, Bai O, Wu G, Zhang L, Zhang Y. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 29. | Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 30. | Guan W, Jing Y, Dou L, Wang M, Xiao Y, Yu L. Chidamide in combination with chemotherapy in refractory and relapsed T lymphoblastic lymphoma/Leukemia. Leuk Lymphoma. 2020;61:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |