Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9549
Peer-review started: February 22, 2021
First decision: July 15, 2021
Revised: July 27, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 6, 2021
Processing time: 249 Days and 5.8 Hours
Malignant adenomyoepithelioma (AME) of the breast is a rare tumor in which malignancy can arise from either epithelial or myoepithelial components, or from both cell types. The incidence and prognosis of malignant AME of the breast are difficult to assess due to its rarity. Therefore, the optimal treatment for this disease is still controversial.
We present two middle-aged women (48 and 56 years old) with malignant AME of the breast. Core needle biopsy was performed before surgery. However, breast adenoma and malignant tumors were observed. The preoperative diagnosis of malignant AME of the breast is still challenging for pathologists and clinicians. Both patients underwent mastectomy and sentinel lymph node biopsy, both of which were negative, followed by adjuvant chemotherapy.
The follow-up duration of the two patients was two years and four months, respectively. No signs of relapse or metastasis have been observed thus far.
Core Tip: This report describes malignant adenomyoepithelioma (AME) of the breast, a rare tumor, which is difficult to assess and prone to local relapse and metastasis. No mature clinical experiences are available for reference, and it is thus significant to report unusual cases to help scholars worldwide establish a systematic treatment regimen. We present the treatment process and disease characteristics of two patients in different departments, as well as a brief literature review to investigate the clinicopathological features, diagnosis, treatment, and prognosis of malignant breast AME.
- Citation: Zhai DY, Zhen TT, Zhang XL, Luo J, Shi HJ, Shi YW, Shao N. Malignant adenomyoepithelioma of the breast: Two case reports and review of the literature. World J Clin Cases 2021; 9(31): 9549-9556
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9549.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9549
Breast adenomyoepithelioma (AME) is a rare disorder characterized by a bicellular pattern of ductal and myoepithelial cells[1]. The World Health Organization (WHO) categorized AME into benign forms with a good prognosis, and the malignant form, which manifests as the malignant transformation of epithelial and/or myoepithelial components[2]. Malignant AME is challenging to distinguish from benign diseases, such as intraductal papilloma, tubular adenoma, and sclerosing adenosis[3]. In addition, malignant AME has shown great potential for local recurrence and metastasis to distant sites, including the lung, thyroid, bone, and brain[4-6].
As the morphological features of AME that are capable of predicting malignant potential have not been elucidated, tumors that seem benign have the possibility of becoming malignant at the onset of swelling or after a long period of stability. Less than 80 cases of malignant AME have been documented thus far, occurring over a wide range of ages (39–93 years). It is imperative to accurately diagnose malignant AME, which could have therapeutic and prognostic implications. This report describes two cases of malignant AME of the breast in our center with a brief literature review to investigate the clinicopathological features, diagnosis, treatment, and prognosis of malignant breast AME.
Case 1: A 46-year-old woman had a left breast lump and frequent bloody nipple discharge for two months.
Case 2: A 58-year-old woman had a 4 cm lump on her left breast for one month.
Case 1: Two months previously, the patient found a lump in the left breast with bloody nipple discharge. No tenderness, skin redness, or nipple retraction was described. The lump did not increase significantly, and she experienced frequent symptoms of nipple discharge in the two days prior to presentation.
Case 2: The patient found a round lump near the nipple on her left breast one month previously. She did not pay any attention until she felt slight pain in the left breast one week prior to presentation.
Both patients had an unremarkable medical history.
The patients’ families had no history of breast cancer or other tumors.
Case 1: Physical examination indicated a hard and fused lump 2.0 cm × 2.0 cm in size at the nine o’clock position in her left breast. No lumps were palpable in the axilla bilaterally.
Case 2: A 4.0 cm hard mass with ambiguous borders was palpable in the upper internal quadrant of the left breast. No enlarged lymph nodes were palpable in the axilla bilaterally.
Laboratory examinations in both patients were normal.
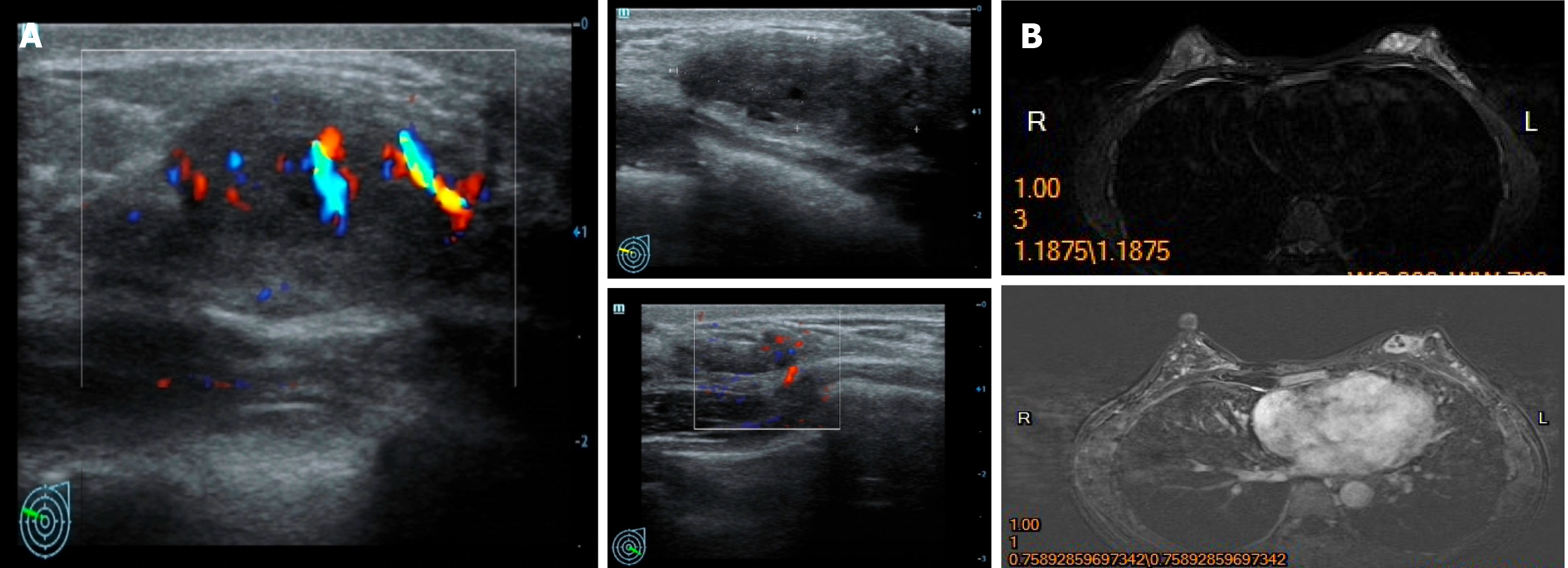
Case 1: Mammary ultrasound revealed a 2.6 cm × 0.9 cm hypoechoic mass in her left breast, and no abnormal lymph nodes were observed in the bilateral axilla (Figure 1A). Similarly, an irregular nodule with lobulated margins measuring 2.3 cm × 1.1 cm was observed in the middle quadrant of the left breast by magnetic resonance imaging (MRI). No invasion of the adjacent skin or nipple was observed (Figure 1B).
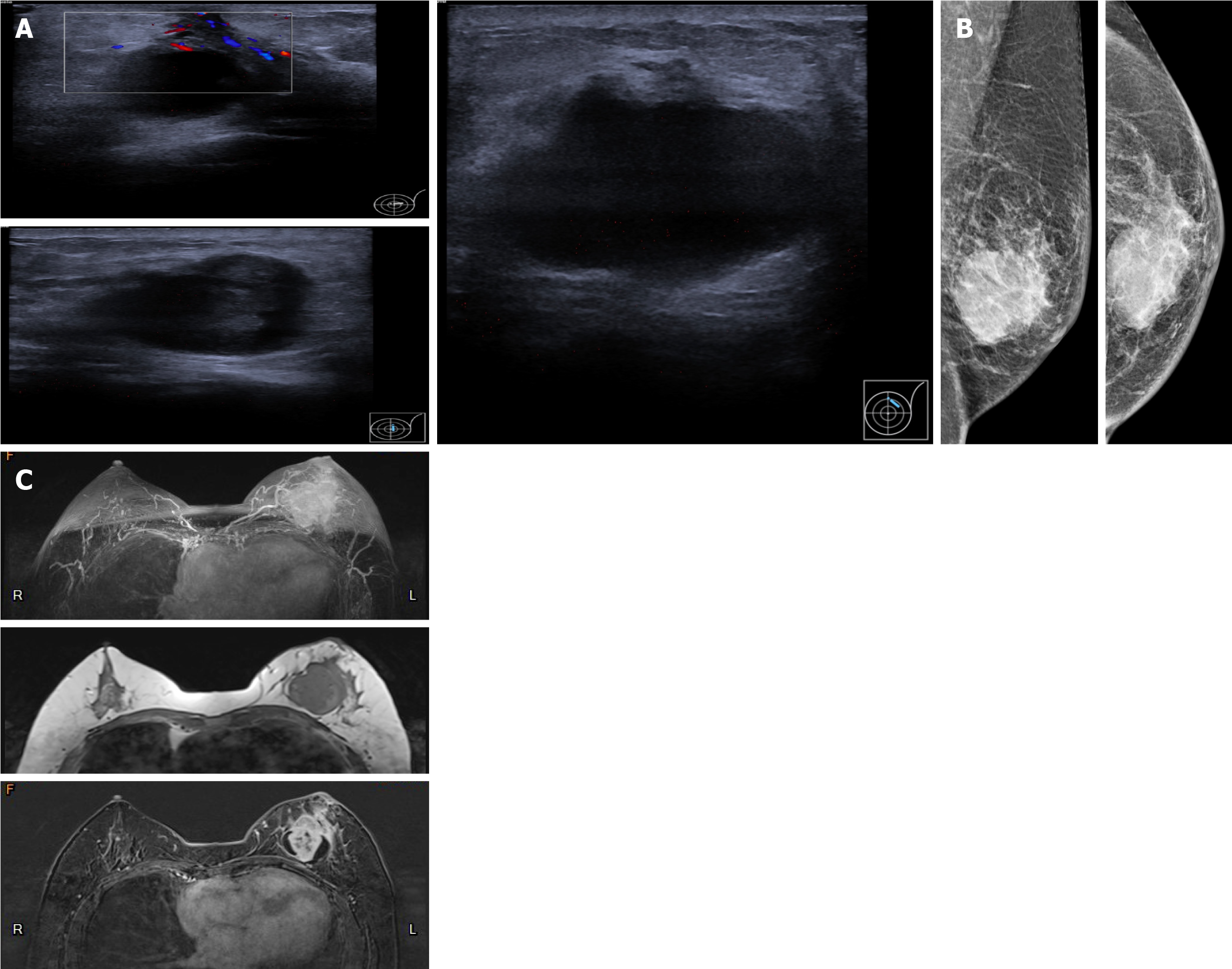
Case 2: Ultrasound revealed a mixed echogenic mass in the left breast with ambiguous borders that measured 4.0 cm × 2.6 cm × 4.5 cm (Figure 2A). Thickened peripheral skin and suspensory ligaments were revealed by mammography (Figure 2B). MRI also showed a cystic lesion exhibiting irregular necrosis, which potentially correlated with a diagnosis of breast cancer with intraductal components (Figure 2C).
Case 1: A biopsy led to the diagnosis of breast adenoma composed of proliferating glandular ducts and a small amount of fibrous tissue. Ductal epithelial cells were negative for estrogen receptor (ER) and progestogen receptor (PR), and peripheral myoepithelial cells were observed. The patient then underwent a lumpectomy based on the primary diagnosis of intraductal papilloma determined by frozen pathological assessment. However, the final histological analysis revealed a malignant AME. A left mastectomy and sentinel lymph node biopsy were performed two months later. No metastases were discovered in the three resected sentinel lymph nodes (0/3).
The first operation revealed a tumor measuring 3.0 cm × 2.6 cm × 1.2 cm that was deemed intraductal papilloma and AME with substantial heterogeneity and mitotic activity. Immunohistochemical studies confirmed its malignant features by revealing that part of the AME was negative for ER, PR, androgen receptor (AR), and CerbB2, and the Ki-67 labeling index was 20%. P63 and calponin marked hyperplastic myoepithelial cells.
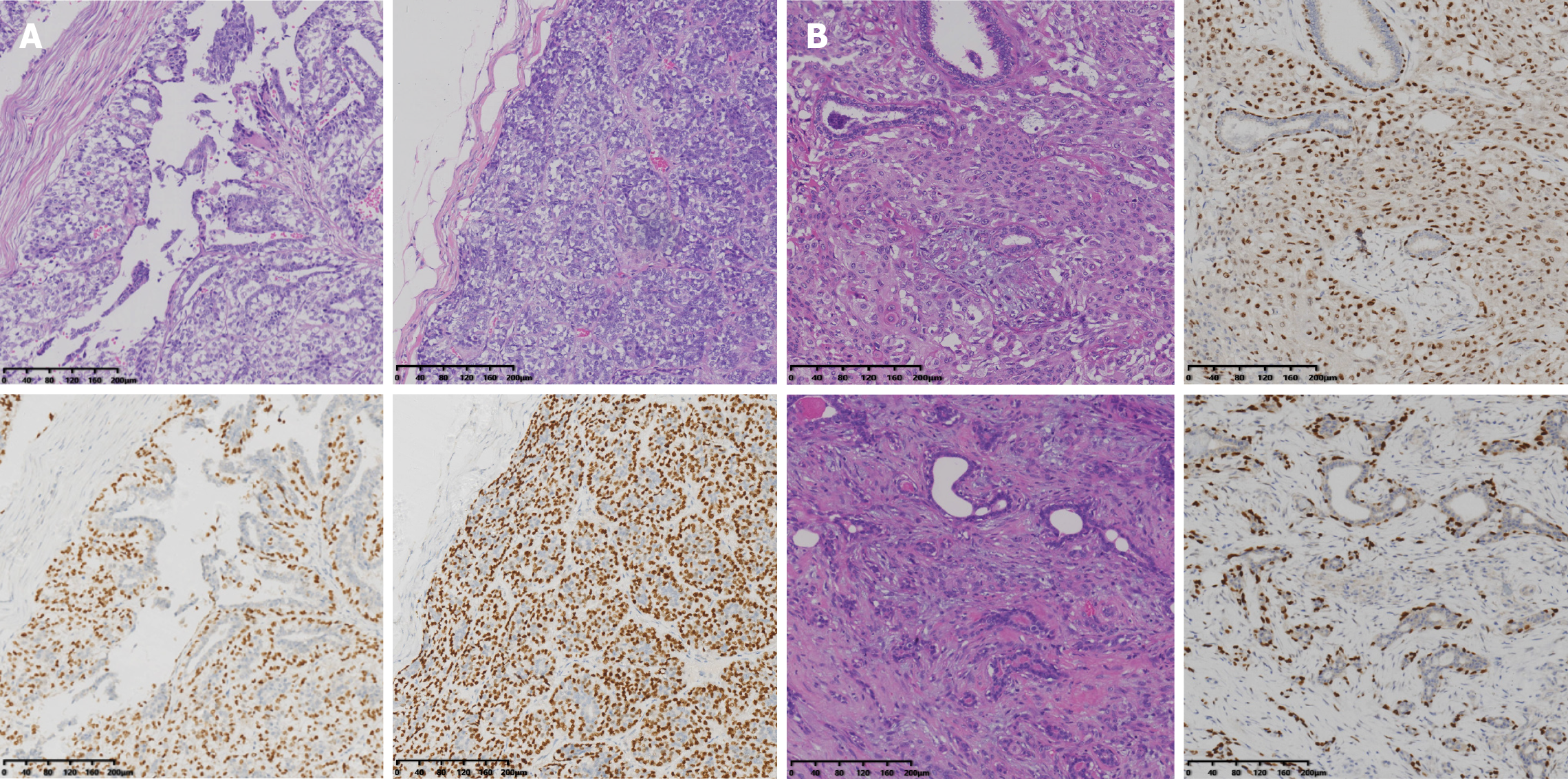
Pathological examination of the final specimen verified that the lesion was malignant AME; immunohistochemical analysis performed in combination showed that both epithelial cells were negative for ER and PR, and the Ki-67 index was 30%. Myoepithelial cells were positive for P63, calponin, CD10, and actin. Moreover, ductal epithelial cells were positive for CK5/6 (Figure 3A).
Case 2: Core needle biopsy suggested that breast carcinoma exhibited two patterns. One was dilated ducts with numerous heterogeneous epithelial cells in the cytoplasm, and the other was cellular nests with hyperplasia in the shape of streaks or glands. Ductal carcinoma in situ with foci of infiltration was considered. Hence, a left breast mastectomy and sentinel lymph node biopsy were performed. Margins of the specimen were negative, as were the lymph nodes (0/5).
Postoperative pathology showed hyperplastic glandular ducts and spindle cell clusters that exhibited heterogenic and miotic features. Apparent squamous metaplasia was observed in approximately 30% of the tissue area. Immunohistochemistry analysis revealed that the tumor was negative for ER, PR, AR, and CerbB2, but positive for P63, P40, CK5/6, E-cadherin, and P120. The P53 index was 30%, and the KI-67 index was approximately 25%. Nonsquamous areas were positive for both actin and calponin (Figure 3B). The lesion exhibited morphological characteristics of both malignant AME and metaplastic carcinoma, as determined by immunohistochemistry and histomorphology analyses. A definitive diagnosis of malignant AME was made after a consultation with several sophisticated experts.
Both patients were diagnosed with malignant AME.
A left mastectomy and sentinel lymph node biopsy were performed two months later. No metastases were discovered in the three resected sentinel lymph nodes (0/3). After surgery, she received adjuvant chemotherapy with epirubicin and cyclophosphamide for four cycles and sequential taxotere for four cycles.
A left breast mastectomy and sentinel lymph node biopsy were performed. At present, the patient has completed four cycles of postoperative adjuvant chemotherapy with epirubicin plus cyclophosphamide and has exhibited no sign of relapse for four months.
The patient has remained disease-free for 24 mo according to ultrasound and MRI assessments.
The patient has exhibited no sign of relapse for four months.
Breast AME is a rare disease characterized by the bicellular proliferation of ductal epithelial and myoepithelial cells. It was initially described by Hamperl in 1970[7]. Tavassoli reported the malignant potential of this tumor type in 1991 and divided it into benign and malignant lesions; in the latter, one or both components can have malignant features[1]. The WHO officially categorized AME as having either benign or malignant features in 2002[2], with the epithelial and/or myoepithelial component exhibiting malignant behavior in the latter.
Fewer than 80 cases of malignant AME of the breast have been reported in approximately 40 papers thus far. It mainly occurs in middle-aged females and occasionally occurs in males[1,8-11] (our patients were 46 and 58 years old at diagnosis). Clinically, the disorder usually manifests as palpable, firm masses measuring 1–7 cm in size[12,13], which are generally described as small and well-defined hypoechoic nodules on imaging examination[14], consistent with our cases of 2.6 cm and 4 cm. The tumors exhibit no specific features upon mammographic and sonographic imaging, but these techniques are essential for the assessment of tumor growth and for guiding subsequent treatment[9]. Malignant AME may indicate the transformation of a previous benign or low-grade AME or emerging morbidity. Regardless of the tumor's pathological type, complete or extensive excision is the primary treatment[1]. It has been reported that narrow margins and incomplete surgery may be attributed to local recurrence[4]. Solid tumors may exhibit clear boundaries with myxoid or cystic changes. In addition, hemorrhage, necrosis, and calcifications can be seen in some circumstances[15-17].
According to the published literature, more than 50% of malignant AMEs invade or infiltrate the periphery, and mitotic figures are prevalent, occurring in up to 10 of 62 high power fields[18]. Approximately twenty-three cases exhibited metastases in the lymph nodes[9], lung[5], liver[6], brain[19], bone[20], thyroid[21], kidney[22], skin[23], and thoracic wall[24,25]. Seventeen cases showed local recurrence signs, but there are thus far no unified opinions on whether hematogenous or lymphatic spread predominated[26,27]. There were no identifiable histopathologic features distinguishing the metastatic and primary tumors, and malignant manifestations such as pleomorphism and necrosis were observed in more than fifty of these cases.
Malignant transformations were derived from myoepithelial components in approximately thirty-two cases, from epithelial components in twenty-one cases, and from both cell types in twenty-six cases. The malignant features included prominent cytological atypia, a high mitotic index, necrosis, and infiltrative proliferation[8]. Fine or core needle biopsy was used in only a few dozen cases, and even fewer samples exhibited malignant features upon cytological examination.
Immunohistochemistry is an effective method for diagnosing malignant AME based on the revelation of biphasic growth patterns. Myoepithelial cells are typically positive for high-molecular weight cytokeratin and myoepithelial markers, including CK5/6, p63, SMA, and S100. Conversely, epithelial cells are positive for low molecular-weight cytokeratins, such as CK7, CAM 5.2, and EMA. Notably, the staining for estrogen and progesterone receptors was negative or weakly positive in a patchy pattern. Her2/neu is broadly reported to be negative in all published studies[11,26]. Therefore, extensive indicators of myoepithelial markers should be utilized if AME is being considered.
The differential diagnosis of AME should include a broad spectrum of entities, such as intraductal papilloma with myoepithelial hyperplasia, sclerosing adenosis, fibroadenoma, adenoma, tubular carcinoma, adenoid cystic carcinoma, low-grade adenosquamous carcinoma, metaplastic carcinoma, malignant myoepithelioma, and papillary carcinoma. When considering a differential diagnosis, it is crucial to interpret the immunohistochemistry results carefully, especially in regard to myoepithelial markers. For instance, intense staining for p63 was observed in both cases, and we believe that immunostaining for both p63 and actin/calponin is significant for highlighting myoepithelial cells.
There is thus far no systematic strategy for treating or managing malignant AMEs except for extensive local tumor resection at an early stage[28]. Baum et al[29] believed that complete excision was the only way to reduce local recurrence and distant metastases, which are distinguishing features of the disease. AME tends to combine with infiltrating ductal carcinoma and other lesions, which cannot be found by imaging examinations. Significantly, distant metastases are much more common than axillary lymph node metastases in malignant AMEs, unlike other breast cancers, according to results from close follow-ups of published cases. Therefore, a simple mastectomy with sentinel lymph node biopsy is recommended if a definite pathological conclusion can be made based on core needle biopsy.
More than one-third of patients suffer from distant metastases and local recurrence, which tend to occur four months to 2 or 3 years after the first diagnosis; thus, adjuvant chemotherapy, which has been used in fewer than ten cases, is currently preferred by many scholars[28]. Lee et al[30] reported that eribulin had a beneficial effect on malignant AME of the breast with multiple hepatic, pleural, and abdominal wall metastases. However, the validity of chemotherapy needs to be verified in more cases in the future.
Both of the patients presented herein were diagnosed and treated at an early stage, which was followed by prompt adjuvant chemotherapy after complete surgery; this may be the only strategy that definitively leads to a favorable outcome. The patients were generally in satisfactory condition at close follow-ups of 24 and 4 mo.
Malignant AME of the breast is a rare tumor composed of ductal epithelial and myoepithelial cells and may undergo malignant transformation of one or both components. This tumor is prone to local relapse and metastasis via a hematogenous route rather than lymphatic routes. We present two cases of malignant AME, illustrating the difficulty of making a definitive diagnosis upon core needle biopsy. No mature clinical experiences are available for reference, and it is thus significant to report unusual cases to help scholars worldwide establish a systematic treatment regimen. Currently, mastectomy combined with sentinel lymph node biopsy is suggested according to its aggressive morphology and the limited number of reported cases.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Preda SD S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991;15:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 204] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Frank GA, Danilova NV, Andreeva IuIu, Nefedova NA. [WHO classification of tumors of the breast, 2012]. Arkh Patol. 2013;75:53-63. [PubMed] |
| 3. | Loose JH, Patchefsky AS, Hollander IJ, Lavin LS, Cooper HS, Katz SM. Adenomyoepithelioma of the breast. A spectrum of biologic behavior. Am J Surg Pathol. 1992;16:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 102] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Maiorano E, Macorano E, Mastropasqua MG. Adenomyoepithelioma. In: Breast Pathology. Elsevier Medicine, 2020: 16-22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kihara M, Yokomise H, Irie A, Kobayashi S, Kushida Y, Yamauchi A. Malignant adenomyoepithelioma of the breast with lung metastases: report of a case. Surg Today. 2001;31:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Jones C, Tooze R, Lakhani SR. Malignant adenomyoepithelioma of the breast metastasizing to the liver. Virchows Arch. 2003;442:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161-220. [PubMed] |
| 8. | Bhalla V, Joshi K, Vohra H, Singh G, Ganguly NK. Effect of growth factors on proliferation of normal, borderline, and malignant breast epithelial cells. Exp Mol Pathol. 2000;68:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hayes MM. Adenomyoepithelioma of the breast: a review stressing its propensity for malignant transformation. J Clin Pathol. 2011;64:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Nadelman CM, Leslie KO, Fishbein MC. "Benign," metastasizing adenomyoepithelioma of the breast: a report of 2 cases. Arch Pathol Lab Med. 2006;130:1349-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Tamura G, Monma N, Suzuki Y, Satodate R, Abe H. Adenomyoepithelioma (myoepithelioma) of the breast in a male. Hum Pathol. 1993;24:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Gafton B, Scripcariu V, Prutianu I, Alexa-Stratulat T, Terinte C, Nicolau A, Moisiuc D, Radu I. Challenges in management of male breast adenomioepithelioma with malignant behavior: Case report. Medicine (Baltimore). 2019;98:e17587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | McLaren BK, Smith J, Schuyler PA, Dupont WD, Page DL. Adenomyoepithelioma: clinical, histologic, and immunohistologic evaluation of a series of related lesions. Am J Surg Pathol. 2005;29:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Zhu J, Ni G, Wang D, He Q, Li P. Lobulated adenomyoepithelioma: a case report showing immunohistochemical profiles. Int J Clin Exp Pathol. 2015;8:15407-15411. [PubMed] |
| 15. | Petrozza V, Pasciuti G, Pacchiarotti A, Tomao F, Zoratto F, Rossi L, Fontana A, Censi F, Sardella B, Di Cristofano C, Porta N, Della Rocca C. Breast adenomyoepithelioma: a case report with malignant proliferation of epithelial and myoepithelial elements. World J Surg Oncol. 2013;11:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yoon JY, Chitale D. Adenomyoepithelioma of the breast: a brief diagnostic review. Arch Pathol Lab Med. 2013;137:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Yang Y, Wang Y, He J, Pan G, Tuo X, Jiang A, Bian L. Malignant adenomyoepithelioma combined with adenoid cystic carcinoma of the breast: a case report and literature review. Diagn Pathol. 2014;9:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Papaevangelou A, Pougouras I, Liapi G, Pierrakakis S, Tibishrani M, Setakis N. Cystic adenomyoepithelioma of the breast. Breast. 2004;13:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Hegyi L, Thway K, Newton R, Osin P, Nerurkar A, Hayes AJ, Fisher C. Malignant myoepithelioma arising in adenomyoepithelioma of the breast and coincident multiple gastrointestinal stromal tumours in a patient with neurofibromatosis type 1. J Clin Pathol. 2009;62:653-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Simpson RH, Cope N, Skálová A, Michal M. Malignant adenomyoepithelioma of the breast with mixed osteogenic, spindle cell, and carcinomatous differentiation. Am J Surg Pathol. 1998;22:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Bult P, Verwiel JM, Wobbes T, Kooy-Smits MM, Biert J, Holland R. Malignant adenomyoepithelioma of the breast with metastasis in the thyroid gland 12 years after excision of the primary tumor. Case report and review of the literature. Virchows Arch. 2000;436:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Takahashi I I, Tashiro H, Wakasugi K, Onohara T, Nishizaki T, Ishikawa T, Matsusaka T, Kume K, Yamamoto I I. Malignant Adenomyoepithelioma of the Breast:A Case with Distant Metastases. Breast Cancer. 1999;6:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Moritz AW, Wiedenhoefer JF, Profit AP, Jagirdar J. Breast adenomyoepithelioma and adenomyoepithelioma with carcinoma (malignant adenomyoepithelioma) with associated breast malignancies: A case series emphasizing histologic, radiologic, and clinical correlation. Breast. 2016;29:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Chen PC, Chen CK, Nicastri AD, Wait RB. Myoepithelial carcinoma of the breast with distant metastasis and accompanied by adenomyoepitheliomas. Histopathology. 1994;24:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Ahmed AA, Heller DS. Malignant adenomyoepithelioma of the breast with malignant proliferation of epithelial and myoepithelial elements: a case report and review of the literature. Arch Pathol Lab Med. 2000;124:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Wiens N, Hoffman DI, Huang CY, Nayak A, Tchou J. Clinical characteristics and outcomes of benign, atypical, and malignant breast adenomyoepithelioma: a single institution's experience. Am J Surg. 2020;219:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kiaer H, Nielsen B, Paulsen S, Sørensen IM, Dyreborg U, Blichert-Toft M. Adenomyoepithelial adenosis and low-grade malignant adenomyoepithelioma of the breast. Virchows Arch A Pathol Anat Histopathol. 1984;405:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Yuan Z, Qu X, Zhang ZT, Jiang WG. Lessons From Managing the Breast Malignant Adenomyoepithelioma and the Discussion on Treatment Strategy. World J Oncol. 2017;8:126-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
| 29. | Baum JE, Sung KJ, Tran H, Song W, Ginter PS. Mammary Epithelial-Myoepithelial Carcinoma: Report of a Case With HRAS and PIK3CA Mutations by Next-Generation Sequencing. Int J Surg Pathol. 2019;27:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 30. | Lee S, Oh SY, Kim SH, Lee JH, Kim DC, Cho SH, Lee M, Kim HJ. Malignant Adenomyoepithelioma of the Breast and Responsiveness to Eribulin. J Breast Cancer. 2015;18:400-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |