Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9255
Peer-review started: May 27, 2021
First decision: June 24, 2021
Revised: July 6, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: October 26, 2021
Processing time: 147 Days and 2.8 Hours
Graft-versus-host disease (GVHD) following liver transplantation (LT) is an unpredictable complication with poor outcome. However, consensus regarding the diagnosis and therapeutic regimen for the disease is yet lacking. The present study summarized the clinical experience on the diagnosis and treatment of acute GVHD (aGVHD) following LT and reviewed the pertinent literature.
Between January 1st, 2000 and December 31st, 2020, a total of 1053 LT were performed in the First Affiliated Hospital of Xi’an Jiaotong University. Six recipients developed aGVHD with clinical symptoms of fever, rash, diarrhea, and pancytopenia. The incidence of aGVHD was 0.57%. The median time from LT to the clinical presentation of aGVHD was 22.17 d. The median time from the beginning of the clinical symptom to histopathological diagnosis was 7.5 d. All six cases underwent treatment of immunosuppressant adjustment, corticosteroids, human normal immunoglobulin, and antithymocyte globulin/IL-2 antagonists. Despite intensive treatment strategies, 4 patients were deceased due to sepsis, multiple organ failure, and cerebral hemorrhage. The remaining two cases were discharged as treatment successfully. However, one died because of tuberculosis infection on the 6th month of follow-up, the other one was alive healthy during 30 mo of follow-up.
The rapid diagnosis of aGVHD is mainly based on the time from the first symptom, histopathological features, and the donor T-lymphocyte chimerism. Our cases report highlights massive corticosteroid therapy and age difference between donors and recipients could accelerate to aGVHD. Moreover, gut microbial interventions and donor-targeted serotherapy may provide novel therapeutics.
Core Tip: Acute graft-versus-host disease (aGVHD) is rare after liver transplantation (LT) with an incidence rate of 0.57% and the high mortality (up to 83.33%), which poses a critical challenge to our hospital. The diagnosis of aGVHD mainly depends on clinical symptoms, laboratory examinations especially donor T-lymphocytes chimerism analysis and histopathological. The larger the age distribution and the earlier the first symptoms, it is herald that the more rapid the progression in post-LT aGVHD. The most appear steroid-resistant in post-LT aGVHD. Thus, lower the dose of the corticosteroids, improved antibiotic stewardship, Particularly, gut microbial interventions and donor-targeted serotherapy may provide novel therapeutics.
- Citation: Tian M, Lyu Y, Wang B, Liu C, Yu L, Shi JH, Liu XM, Zhang XG, Guo K, Li Y, Hu LS. Diagnosis and treatment of acute graft-versus-host disease after liver transplantation: Report of six cases. World J Clin Cases 2021; 9(30): 9255-9268
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9255.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9255
Graft-versus-host disease (GVHD) is defined as the recognition of the recipient tissue antigens as foreign and antagonistic reactions by donor lymphocytes. GVHD is a rare but frequently lethal complication after solid organ transplantation (SOT). Acute GVHD (aGVHD) is rare after liver transplantation (LT) than hematopoietic stem cell transplantation (HSCT) with an estimated incidence rate of 0.5%–2%; the high mortality associated with this complication (up to 85%) poses a critical challenge[1-3]. aGVHD after LT could present between 1 and 8 weeks post-transplantation. Chronic GVHD (cGVHD) which is rare poorly understood presents a delayed onset (typically > 100 d after LT)[4]. The variable incidence when comparing single-center data could be attributed to the differences in diagnostic criteria, the disease, and early symptoms, which are often non-specific. Thus, consensus regarding GVHD diagnosis and therapeutic regimen is lacking.
Organs which are commonly affected by GVHD after LT are skin, gastrointestinal (GI) tract, and bone marrow. Hence, the frequently encountered clinical signs and symptoms including fever, rash, and diarrhea. On the other hand, pancytopenia is observed in advanced stages of the disease[1]. Pancytopenia, diarrhea, the age difference between donor and recipient, and time from the occurrence of the first symptom to diagnosis and treatment are known as the risk factors that affecting GVHD-related mortality[5]. The most common causes of death in patients with GVHD include sepsis, multiorgan failure, and GI bleeding[3]. The diagnosis of GVHD is challenging, owing to the overlap in the clinical presentation of drug reactions and viral infection. Therefore, early diagnosis is difficult and requires a high index of clinical suspicion. In this study, we aimed to share our experience of six patients who developed aGVHD after LT, in order to emphasize the importance of early diagnosis and treatment of the disease.
Between January 1st, 2000 and December 31st, 2020, a total of 1053 orthotopic LT (OLT) procedures were performed in the Liver Transplantation Institute in the First Affiliated Hospital of Xi’an Jiao Tong University, Shaanxi, China. Six patients presented (all males) signs and symptoms compatible with aGVHD were enrolled. Demographic and some characteristics of six LT patients with aGVHD are shown in Table 1. The chief complaints of the patients are shown in Table 2. Five of the six patients (case 1, 3, 4, 5, 6) had the initial symptom of fever, the highest temperature was 38.5-40℃, the other one (case 2) had initial symptom of pancytopenia. Two patients (case 5, 6) were discharged from hospital because of condition improved on 13 d and 17 d post-LT, whereas readmission to the hospital because of severe fever and rash. Four patients (case 1, 2, 3, 4) developed aGVHD shortly post-LT.
| Patients | Recipient age | Recipient sex | Etiology | LT type | Date of OLT | Recipient blood type | Donor blood type | Donor age | Age disparity donor-recipient |
| Case 1 | 64 | Male | HCC, liver cirrhosis | OLT | 2003 | B | B | 43 | 21 |
| Case 2 | 59 | Male | HBV, liver cirrhosis | OLT | 2006 | A | A | 50 | 9 |
| Case 3 | 53 | Male | HCC, HBV, liver cirrhosis | OLT | 2016 | A | A | 51 | 2 |
| Case 4 | 63 | Male | HCC, HBV, OLT | OLT | 2018 | O | O | 31 | 32 |
| Case 5 | 40 | Male | HBV, liver cirrhosis | OLT | 2018 | B | B | 46 | 6 |
| Case 6 | 64 | Male | HBV, liver cirrhosis | OLT | 2019 | B | B | 52 | 12 |
| Patients | Initial symptom | Involved systems | From LT to GVHD (d) | Diagnostic tools | From LT to biopsy(d) | From GVHD to diagnosis(d) | Skin biopsy | GVHD related death | Ferritin level (ng/mL) | Survival day | Mortality cause |
| Case 1 | Fever | Skin, GI, BM | 15 | Skin biopsy, bone marrow smear | 24 | 9 | Grade II | Yes | - | 31 | Cerebral hemorrhage |
| Case 2 | Pancytopenia | Skin, BM | 28 | Skin biopsy, bone marrow smear | 32 | 4 | Grade II | No | - | 180 | Tuberculosis |
| Case 3 | Fever | Skin, GI, BM | 24 | Skin biopsy, bone marrow smear | 34 | 10 | Grade II | Yes | - | 51 | Sepsis |
| Case 4 | Fever | Skin, BM, CNS | 17 | Skin biopsy | 19 | 2 | Grade III | Yes | 31527 | 21 | Sepsis |
| Case 5 | Fever | Skin, GI, BM | 31 | Skin biopsy, bone marrow smear | 38 | 7 | Grade II | No | - | Alive | - |
| Case 6 | Fever | Skin, GI, BM | 18 | Skin biopsy | 31 | 13 | Grade III | Yes | - | 45 | Sepsis |
The etiology for OLT of six patients were as follows: hepatitis B virus (HBV) + hepatocellular carcinoma (HCC) (case 3), HCC + liver cirrhosis (case 1), OLT+HBV+HCC (case 4), and HBV + liver cirrhosis (case 2, 5, 6). The common symptoms of aGVHD post-LT including fever, skin rash, diarrhea, and pancytopenia. Fever and skin rash were common initial symptoms in aGVHD patients (Table 2). The skin rash in GVHD is non-specific[6]. It is erythematous, maculopapular, and can involve any part of the body, including palms, soles, face, neck, limbs, and the volar surfaces of extremities and trunk. Finally, necrosis and denudation of the epidermis are observed. Oral mucosal ulcerations formed at the same time in all patients. Herein, four patients (case 1, 3, 5, 6) showed involvement of skin, GI tract, and bone marrow (Table 2). The other two patients (case 2, 4) showed skin and bone marrow involvement, and one of them (case 4) also showed an involvement of the central nervous system (CNS). Case 4 initially presented fever (38.5℃) and skin rash on day 17 post-LT, but then progressed rapidly to loss of consciousness and pancytopenia that might be related to this disease. Before readmission to our department, one patient (cases 6) was misdiagnosed with drug reactions and viral infection.
There was no history of essential hypertension, diabetes mellitus, or relevant cerebrovascular disease in the 6 patients. One patient (case 1) suffered from HCC combined with liver cirrhosis, and he received transcatheter arterial chemoembolization before OLT. One patient (case 4) underwent OLT due to HBV combined with HCC 13 years before, and a retransplantation was performed due to HCC recurrence. One patient (case 3) suffered from hepatocellular cancer with HBV before OLT. Three cases (case 2, 5, 6) suffered from hepatitis B cirrhosis before OLT.
Four patients had smoking history. Family members of the patients had no history of confirmed malignant tumours and viral hepatitis.
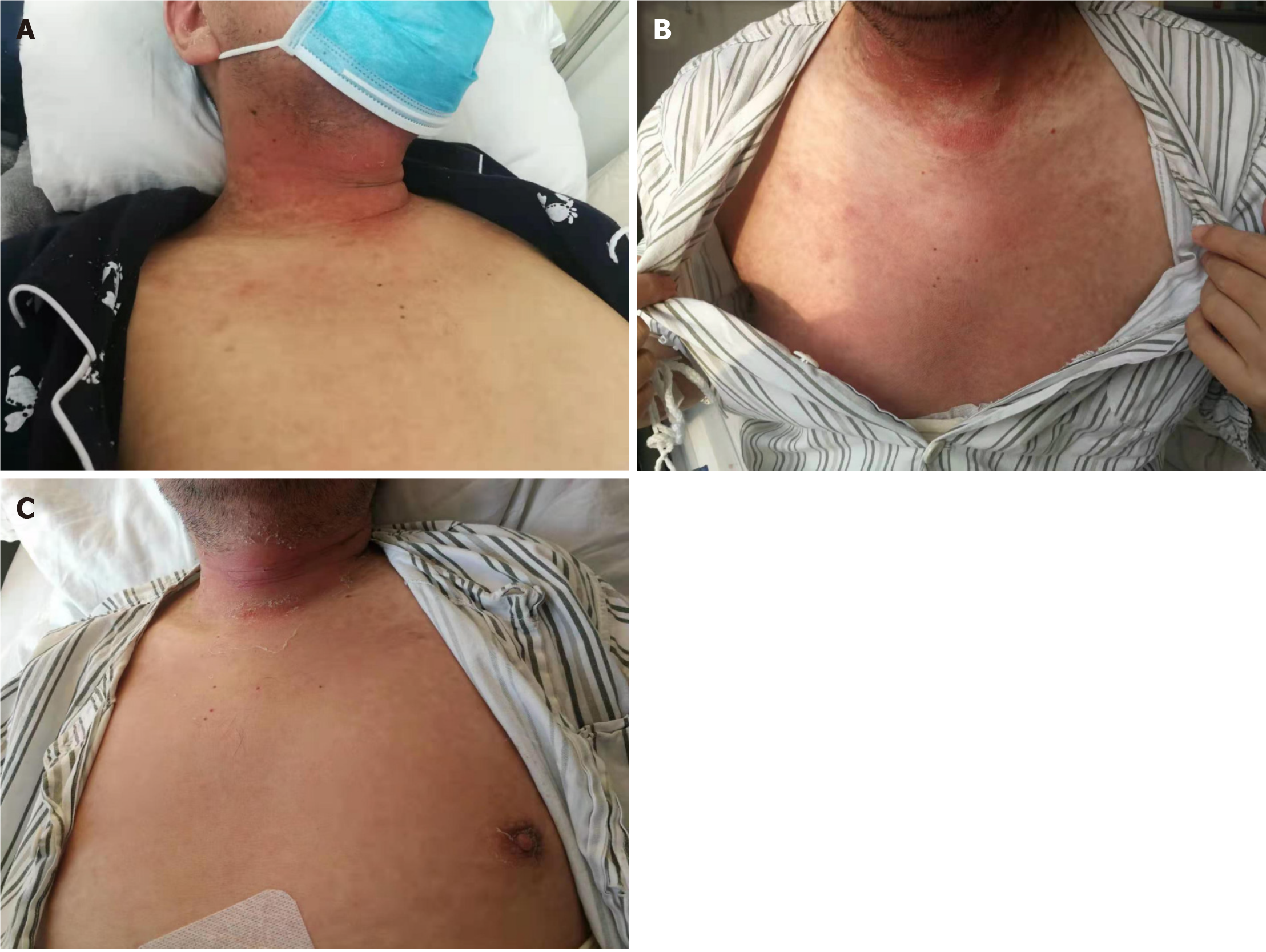
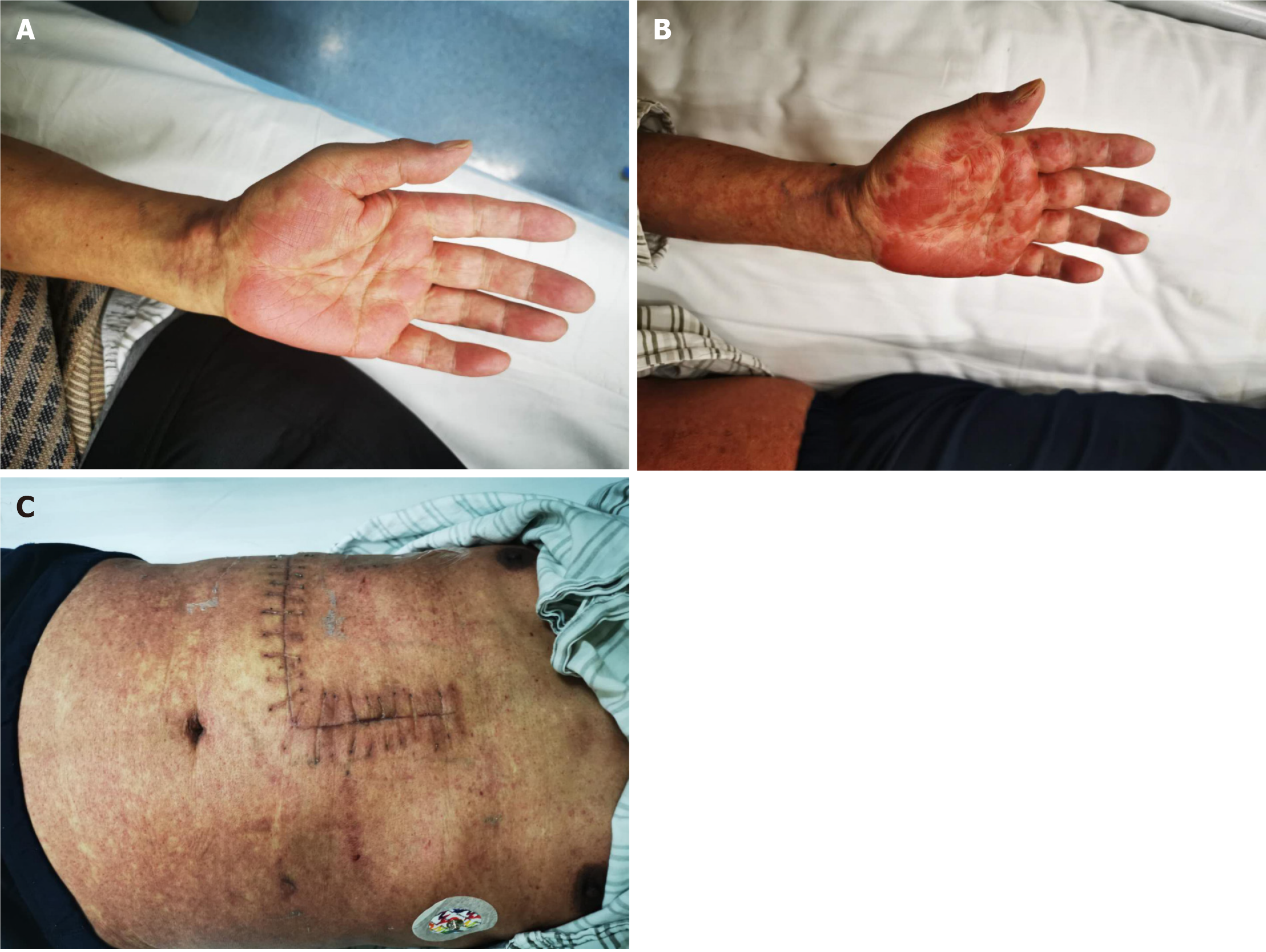
Blood pressure, respiratory rate, and heart rate were all in the normal range on began to come on except the body temperature was 38.5-40℃. Oral mucosal ulcerations were found in all patients. There was no other obvious physical sign except skin rash in all patients, such as the skin rash in case 5 (Figure 1), and case 6 (Figure 2). It is erythe
Laboratory examinations revealed progressive pancytopenia with a significant decrease of white blood cell (WBC), hemoglobin and platelets (PLT), especially the WBC decreased most significantly, the lowest down to 0.05 × 109/L (normal range, 3.5-9.5 × 109/L), PLT decreased to 12 × 109/L (normal range, 125-350 × 109/L). While liver function was in the normal range in all patients. The serum cytomegalovirus (CMV) or Epstein-Barr virus (EB) were negative. Special strains and cultures for fungi, bacteria, and viruses were negative.
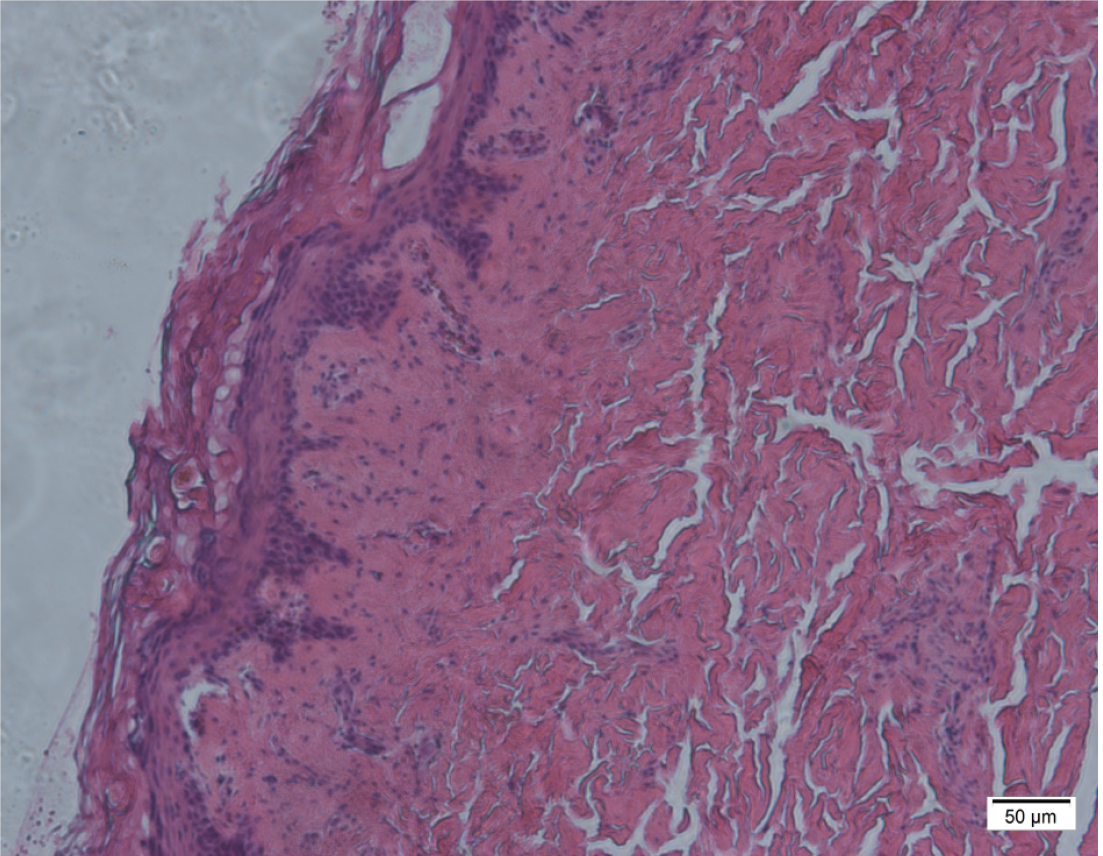
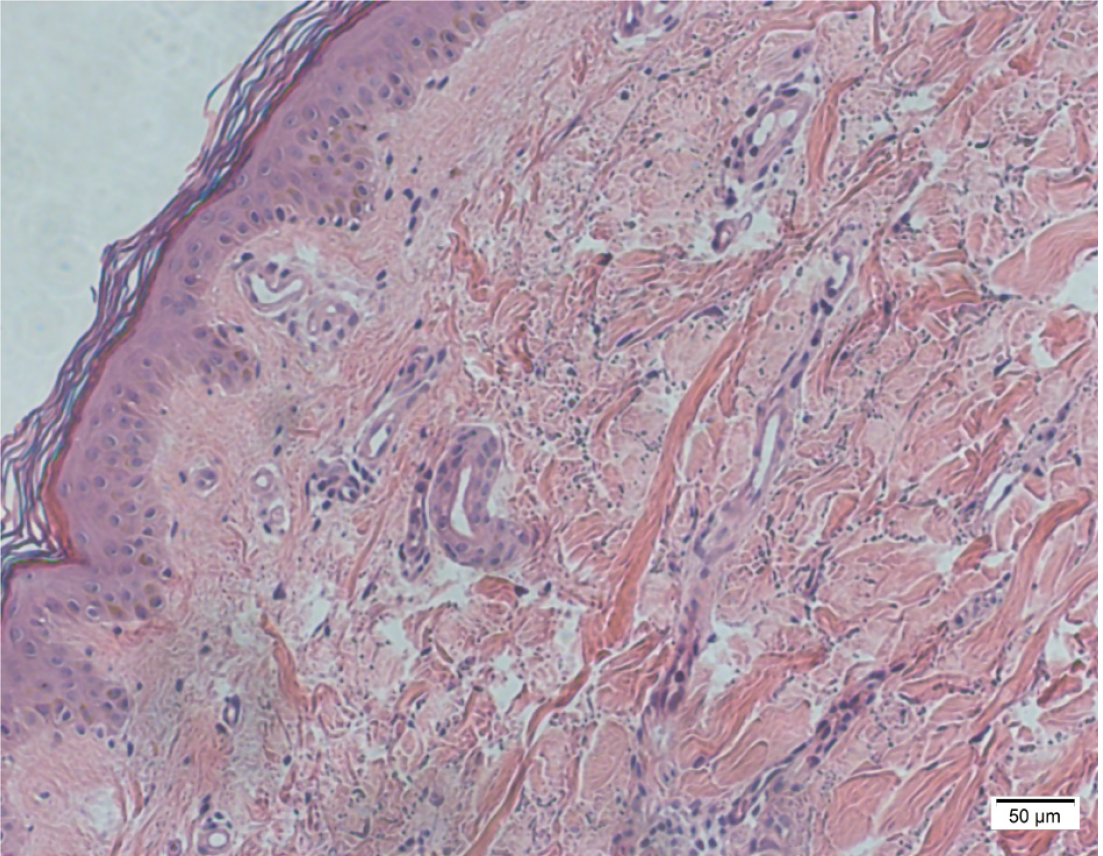
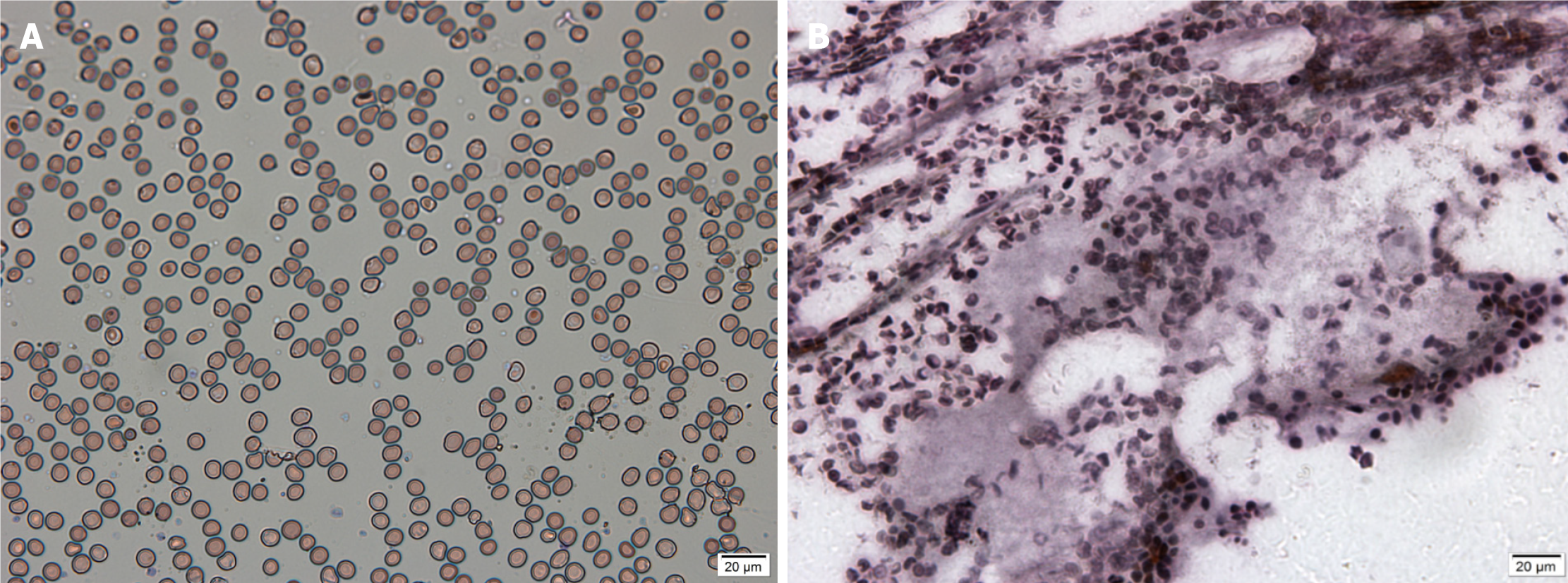
The chest computed tomography/X-ray examination showed no inflammation present. Skin biopsy and bone marrow biopsy were performed as soon as aGVHD was suspected, on pathological evaluation most of epidermal vacuolization and lymphocytic infiltration in case 5 and case 6 (Figure 3 and Figure 4), and bone marrow smear and biopsy revealed complete aplasia in patients in case 3 (Figure 5).
Multidisciplinary expert consultation was conducted for all the patient who was suspected of aGVHD post-LT in our LT center, because aGVHD following LT is a rare complication with poor outcome. Multidisciplinary experts from departments including hematology, dermatology, infection, kidney transplantation, rheumatology participate in the consultation. Experts mainly suggested that considering the time of onset, appearance of skin lesions and distribution, aGVHD is highly suspected, and skin biopsy and bone marrow biopsy should be performed immediately. Patients with impaired consciousness need to take cerebrospinal fluid testing. Massive corticosteroid therapy was suggested to be the main treatment and anti-infection treatment should be strengthened. Myelosuppression should be improved as soon as possible combined with blood purification to reduce immune response.
Currently, there is no widely accepted clinical or laboratory diagnostic test for GVHD. A high index of suspicion for diagnosis would be beneficial for the early diagnosis and treatment. Any patient presenting fever, skin rash, diarrhea, and pancytopenia after LT was suspected of aGVHD. If the biopsies revealed GVHD, treatment was started immediately.
The histopathological grading of skin involvement was as follows[3]: vacuolar alteration of the basal layer (grade I), keratinocyte apoptosis, and lymphocytic infiltration of the dermo-epidermal layer (grade II), dermo-epidermal separation and vesicle formation (grade III), and necrosis and denudation of epidermis (grade IV). Histopathologically, severe GVHD was defined as grade III and IV, while mild GVHD was defined as grade I and II. aGVHD was confirmed by skin biopsy and bone marrow smear in all 6 patients (Table 2). Severe GVHD was observed in 2 patients (case 4, 6), and mild GVHD was detected in 4 patients (case 1, 2, 3, 5).
Moreover, we detected the classification and analysis of peripheral blood T lymphocytes in recipients and found that the proportion of CD3+CD8+ T cell increased gradually (from 81.06% to 85.9%), while the CD4/CD8 ratio was decreased (from 0.19 to 0.13) in case 4, indicating progressive deterioration of patient’s GVHD. A similar situation was observed in case 6, and the proportion of CD3+CD8+ T cells in peripheral blood lymphocytes was 89.21%; the CD4/CD8 ratio was 0.05. The aGVHD progressed rapidly in those cases, and both patients were deceased However, aGVHD was detected and cured in case 5, the proportion of CD3+CD8+ T cells of peripheral blood lymphocytes was 49.7%, and the CD4/CD8 ratio was 0.37. This suggested that a high proportion of CD3+CD8+ T cells in peripheral blood T-lymphocytes of recipients persistently increases after LT with compatible clinical and histological features of GVHD and is associated with the eventual development of aGVHD after LT.
The first methylprednisolone (10 mg/kg) dose was administered immediately before the portal vein opening during graft implantation. This dose was tapered from 100 mg/d to 20 mg/d during the postoperative period. The administration was stopped at 1 mo postoperatively, except in patients with autoimmune hepatitis, primary sclerosing cholangitis, or primary biliary cirrhosis. Postoperative steroid-free therapy was used for HCC patients. Tacrolimus/cyclosporin and mycophenolate mofetil were initiated on days 1–3 postoperatively. The primary goal was to maintain the valley concentration in tacrolimus or the peak concentration in cyclosporin at 5–8 ng/mL and 600–800 ng/mL in 1–3 mo, respectively.
All patients were isolated and transferred to the surgical intensive care unit in the critical condition of aGVHD. Special nurses were arranged to carry out respiratory tract and digestive tract protection treatments.
Immunosuppressant adjustment: Administration of tacrolimus remained unchanged (case 1, 5, 6) or tacrolimus was decreased (case 4) and cyclosporin were decreased (case 2, 3) (Table 3).
| Patients | Methylprednisolone | Immunosuppressant | IVIG | CRRT | G-CSF/GM-CSF | ||||
| Tacrolimus | Cyclosporin | Basiliximab/Zenapax | TNF-α inhibitor | ATG | |||||
| Case 1 | 10 mg/kg/d | + | - | Zenapax (2 doses) | - | - | + | - | + |
| Case 2 | 10 mg/kg/d | - | ↓ | - | - | - | + | - | + |
| Case 3 | 8 mg/kg/d | - | ↓ | - | - | 50 mg/d, 2 d | + | - | + |
| Case 4 | 10 mg/kg/d | ↓ | - | - | Infliximab, 300 mg/d, 1 d | - | + | + | + |
| Case 5 | 7 mg/kg/d | + | - | - | - | - | + | - | + |
| Case 6 | 8 mg/kg/d | + | - | Basiliximab (2 doses) | - | - | + | - | + |
Corticosteroid regimen: As soon as aGVHD was diagnosed or highly suspected, methylprednisolone was administered, and the initial dose was approximately 7–10 mg/kg/d for 2-3 d, and tapered to 20 mg within a week.
Human normal immunoglobulin: As soon as aGVHD was diagnosed or highly suspected, human normal immunoglobulin was administered for 3–5 d, and the dose was approximately 0.15–0.2 g/kg/d;
Other joint programs: A dost of 20 mg basiliximab/Zenapax was administered intravenously every 4 d in cases 1 and case 6, a total of 2 agent. While antithymocyte globulin (ATG) 50 mg/d for two days in case 3 and tumor necrosis factor-alpha (TNF-α) inhibitor (Infliximab) 300 mg/d for one day in case 4.
Improve myelosuppression: Granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and thrombopoietin were applied in all cases until myelosuppression was improved.
Antibiotics: Patients with pancytopenia related to aGVHD after LT and corticosteroids regimen were at an elevated risk for opportunistic infections, sepsis, and death. The broad-spectrum antibacterial, antiviral, and antifungal prophylaxis were administered early during the disease.
Nutrition support therapy: Severe protein-calorie malnutrition as a result of GVHD led to protein-losing enteropathy and malabsorption, which are common and should be treated with 1.5 g/kg/d protein in addition to vitamin, micronutrient, and essential trace element replacement therapy.
Other treatment: One patient (case 4) developed renal insufficiency and no urine during treatment and was treated with continuous renal replacement therapy.
The prevalence of GVHD in our institute was 0.57%. The median recipient and donor ages were 57.17 ± 9.41 years and 45.50 ± 7.87years, respectively. The median disparity between the recipient and donor age was 13.67± 11.04 years. The ABO blood group matching was identical in all patients (Table 1 and Table 2). The age disparity of the donor-recipient was > 20 years in two cases: 21- and 32-years for case 1 and case 4, respectively. The initial symptoms of aGVHD occurred in these patients post-LT earlier than others at 15 d and 17 d, respectively. One of the critical findings was that the greater the age disparity, the faster the progression and the higher the mortality in aGVHD after LT.
The median time from LT until the clinical presentation of GVHD was 22.17 ± 6.49 d (Table 2). The median time from the beginning of the clinical symptom to histopathological diagnosis was 7.50 ± 4.04 d. Three patients (case 2, 4, 5) were diagnosed as GVHD by histopathology within 1 wk after clinical manifestations. The remaining three patients (case 1, 3, 6) were confirmed on days 9, 10, and 13, respectively.
Despite intensive treatment strategies, 4/6 (66.67%) patients (case 1, 3, 4, and 6) died due to sepsis, multiple organ failure, and cerebral hemorrhage. One of them (case 4) had several risk factors contributing to the development of aGVHD include donor-recipient age disparity of > 30 years, retransplantation, and HCC recurrence, and the aGVHD progressed rapidly and developed symptoms of the CNS. Despite the aggressive treatment with methylprednisolone and TNF-α inhibitor, the patient died after 21 d. Only two patients (case 2, 5) were discharged due to successful treatment. Case 2 recovered on day 10 after mild bone marrow suppression, and the skin rash subsided gradually. The patient died due to tuberculosis on the 6th month of follow-up[7]. Case 5 patient recovered on day 23 after severe bone marrow suppression, and the skin rash subsided gradually. The patient was alive and healthy without complications during 30 mo of follow-up. Thus, the overall mortality rate was 83.33% (5/6 patients) in aGVHD after LT at our hospital.
GVHD may be divided into two groups, humoral and cellular, according to the underlying immunological mechanism[8]. Humoral-type GVHD occurs in ABO blood group patients with incompatible or nonidentical liver grafts and is mediated by antibody production by donor T lymphocytes against the red cell antigen of the recipient. It is characterized by fever and hemolysis; the symptoms are mild with hemolytic anemia[1,9]. On the other hand, cellular-type GVHD is a rare complication with high mortality. It arises due to a major histocompatibility complex (MHC) mismatch, which affects the activation and clonal expansion of cytotoxic donor T lymphocytes. Some conditions are required for GVHD development following transplantation[10]: (1) presence of immunocompetent T lymphocytes in the transplanted graft; (2) differences in human leukocyte antigen (HLA) between the donor and recipient; and (3) inability of the recipient to eliminate donor-induced lymphocytes.
The GVHD pathogenesis was first described by Billingham[11] in 1966. Subsequently, Burdick et al[12] published their first case report of GVHD development following LT in 1988. In 2004, Taylor et al[13] described a three-phase model for the development of GVHD after LT, which was extrapolated from the experience with GVHD after stem cell transplant. Phase 1 is characterized by a pre-LT immunocompromised, inflammatory state that enhances host antigen-presenting cells (APCs) via upregulated MHC class I and II expression. Phase 2 occurs after LT, with the transfer of immunocompetent donor leukocytes. These passenger lymphocytes are activated upon interaction with the upregulated host APC HLA peptides. In the presence of MHC mismatch after LT, these activated lymphocytes undergo IL-2-dependent clonal expansion, favoring the expression of memory cells and cytotoxic effector cells. Phase 3 is characterized by cell death and tissue dysfunction, which are effectuated by the cytotoxic donor T lymphocytes targeting antigens expressed by the host tissue[9,11,14].
Some risk factors contributing to the development of GVHD after LT include complete HLA matching, HLA class I match, donor-recipient age disparity of > 20 years, retransplantation, rejection before GVHD, autoimmune hepatitis, HCC, and viral infections (CMV and HSV)[3]. The patients in the current study presented one or more risk factors, including age disparity > 20 years (case 1 and 4), retransplantation (case 4), and HCC (case 1, 3, and 4) (Table 2).
The most common target organs of donor-induced lymphocytes in post-LT GVHD are skin, GI mucosa, and bone marrow[15]. Since the liver graft is not one of the target organs, hepatic functions are normal[10]. Skin rash is maculopapular but may develop into bullous formation, followed by desquamation of the whole body over a period[16]. Bone marrow involvement occurs as thrombocytopenia, anemia, leucopenia, or pancytopenia. Diarrhea episodes with different severity may develop secondary to the destruction of GI epithelium[13]. There is accumulating evidence from the literature[17] that early GVHD may be directed also against other tissues. In particular, organs such as kidney, CNS, and lungs may be involved in patients experiencing aGVHD after allogeneic hematopoietic stem cell transplantation. Pahari et al[18] reported the first case of CNS-GVHD following LT. Subsequently, seizures and altered mental status were recorded, and the patient died of sepsis and multiorgan failure. In the current study, the common first symptoms, skin rash (100%) and fever (83.33%), typically occurred on day 22.
The diagnosis of GVHD is challenging, but currently, there is no widely accepted clinical or laboratory diagnostic test for GVHD. Early cultures (including fungal blood cultures and stool for Clostridium difficile), serum CMV or EB PCR, and chest radiography were conducted to exclude competing infections. Skin biopsies (epidermal vacuolization and lymphocytic exocytosis) or colonoscopy/flexible sigmoidoscopy (crypt apoptosis) and bone marrow (marrow hypoplasia/aplasia) must be performed to exclude competing etiologies and provide diagnostic clarity in GVHD[3]. Differential diagnosis is critical for GVHD early treatment initiation. The differential diagnosis of GVHD after LT includes drug reactions, graft rejection, and bacterial (C. difficile) or viral (CMV) infections[15].
Taylor et al[13] investigated the presence of donor lymphocyte chimerism in recipient peripheral blood as a diagnostic aid for GVHD after LT. The donor lymphocyte microchimerism (< 1% donor lymphocyte chimerism) is often seen in LT recipients and is deemed crucial for immune tolerance and graft acceptance by the host. However, macrochimerism (1%–80%), i.e., donor lymphocyte chimerism in recipient tissues (peripheral blood, skin, GI tract, bone marrow, or buccal mucosa), especially with a high proportion of CD8+ T cells, persists 3-4 wk after LT and thus is associated with the eventual development of GVHD. Recently, chimerism analysis assessed the donor DNA using fluorescent in situ hybridization and the short tandem repeat (STR) loci to quantitate the donor’s contribution to any of the nucleated cell populations in the skin, peripheral blood, GI mucosa, and/or bone marrow, and cerebrospinal fluid[18,19].
Whereas, there were some data of other reported different. Zhao et al[20] reported that they detected peripheral blood donor T-lymphocyte chimerism in 55 peripheral blood samples by STR-PCR in post-LT, and found donor T-lymphocytes chimerism in 11 peripheral blood samples. Among these 11 recipients, eight recipients finally developed aGVHD, and the percentages of donor T-lymphocytes chimerism were > 10% (21.0%–98%), and the percentage for the other three recipients were < 10% and had no aGVHD. Noguchi et al[21] reported that a GVHD case in a 59-year-old woman with dermatomyositis-associated interstitial pneumonia, who took immunosuppressants including corticosteroids before receiving right lung transplantation from a 13-year-old brain-dead male donor. She developed systemic erythema with desquamation and pancytopenia by day 20, and she was diagnosed with GVHD following examination of bone marrow aspiration results that 15% of nucleated cells were donor type on day 27. Corticosteroid pulse therapy reduced the symptoms and decreased donor-type cell percentage. However, chimerism analysis of peripheral blood on day 68 revealed that 95% granulocytes were recipient type, but 95% T cells were Donor type. The patient died of multiple organ failure despite treatment. In this case, donor T-lymphocytes chimerism preceded whole blood donor chimerism and the deterioration of GVHD.
Though chimerism is an important investigation, presence of chimerism in absence of clinical symptoms and histological findings is non-specific, making macrochimerism only a diagnostic tool[2,19,22]. Given that whole-blood chimerism analysis can underestimate donor type cell chimerism because of the presence of abundant granulocytes, donor T-lymphocytes chimerism might be useful for the early diagnosis of GVHD. It has been suggested that an increased level of donor CD8+ T/NK cells > 10% is indicative of GVHD. Also the severity and duration of chimerism varies with the evolution of patient and monitoring donor T-lymphocytes chimerism in these target organs, even after treatment and resolution of symptoms, could guide the treatment[23].
Since we do not donor T-lymphocyte chimerism analysis in the six patients with aGVHD in previously, we make a diagnosis and differential diagnosis of aGVHD based on the time of the first symptoms, clinical manifestations, laboratory testing, and microbiological and histopathological data. With the rapid development of science, chimerism analysis assessed to quantitate the donor’s contribution populations to any of the nucleated cell can be an important diagnostic indicator of aGVHD after LT. Dynamic detection of chimerism analysis is more beneficial to early diagnosis and treatment guidance.
In addition, it was found in one patient (case 4) that Ferritin level was markedly elevated (31527 ng/mL, range: 30-400 ng/mL). This phenomenon was consistent with that reported by Murali et al[3], wherein the mean ferritin peak in patients who died of GVHD was 9930 ng/mL (range: 2225–20333), while that in the surviving patient was 733 ng/mL. Ferritin level also can be an important diagnostic indicator of aGVHD after LT.
The evidence guiding therapy for post-LT aGVHD is limited, and without prospective clinical trials for reference, most options are related to several other single-center reported cases with treatment experience and extrapolated experience from aGVHD of HSCT patients. The standard treatment of post-HSCT patients is based on systemic steroids, which might result in a sustained response in 60% of the patients[24,25]. In a seminal study, MacMillan et al[26] showed that first-line therapy of aGVHD with corticosteroids (60 mg daily followed by an 8-wk taper) resulted in response rates of 50% and 1-year survival of 53%. As a result, corticosteroids remain a cornerstone of the initial treatment of aGVHD in post-LT. Strikingly, post-LT aGVHD is less responsive to corticosteroid corticosteroids than post-HSCT aGVHD, and most appear steroid-resistant aGVHD[27]. A therapeutic option for SOT-associated GVHD is to hinder the activation of donor T cells by increasing immunosuppression. However, this might increase the risk of lethal infections. On the other hand, reduction and even complete withdrawal immunosuppression facilitate host T cells to balance out their donor counterparts and lead to either graft rejection or GVHD worsening. Neither of the two conflicting strategies has yielded satisfactory results[3].
Despite several studies in post-HSCT aGVHD, no agents for the treatment of corticosteroids resistant or refractory GVHD have emerged as a gold standard to alleviate the dismal outcome for these patients[28]. Ruxolitinib, a Janus kinase inhibitor, has recently emerged as a promising treatment for steroid-refractory aGVHD in post-HSCT[29,30]. Furthermore, some research indicate that[31,32] the effective and safe use of Tocilizumab as a rescue treatment in a patient with steroid-refractory aGVHD. However, ruxolitinib and tocilizumab for SOT-associated aGVHD are absent experience, which could make it an interesting option for the treatment of this potentially fatal complication. Therefore, treatment of steroid-resistant aGVHD is an unmet clinical need in solid organ transplantation.
Without strong evidence guiding therapy to steroid-resistant aGVHD in solid organ transplantation, and the search for better prophylactic and therapeutic strategies is critical to improve transplant out-comes. Furthermore, the high rate of myelosuppression and infection is a major cause of high mortality in aGVHD after LT. As a result, a broad-spectrum antibacterial, antiviral, and antifungal prophylaxis should be administered early in the disease process[33]. Recently, Zhao et al[20] suggest that aGVHD patients caused by the addition of immunosuppressive agents as "aGVHD induced by immunosuppression". Thus, lower-dose corticosteroids (1.5 mg/kg/d within one week) for aGVHD after LT could to improve transplant out-comes, and try to avoid repeated corticosteroid therapy otherwise it would highly promote the progression of aGVHD.
Strikingly, GVHD after LT is less responsive to corticosteroids than GVHD after HSCT. Therefore, most patients were administered additional immunosuppression, which include sirolimus, abatacept, anti-lymphocyte globulin (rituximab), or ATG, TNF-α inhibitors (both infliximab and etanercept) and IL-2 receptor (CD25) antibodies (basiliximab and daclizumab) in patients[3,34]. However, these agents have been used in a trial in a few patients (including our patients) after LT with variable and inefficient results. Therefore, we also believe that the increase of immunosuppressant could induce the rapid progression of aGVHD after LT. Furthermore, the target organs of aGVHD are injured by cytokines and inflammatory mediators, and blood purification could effectively remove these cytokines and inflammatory mediators, which might play an active role in the treatment of aGVHD.
Recently, several studies found that human pathologies and diseases are linked to the gut microbiota. Animal and human studies have shown that gut microbial populations and diversity are altered after allogeneic transplantations, such as LT and HSCT[35-37]. Moreover, when complications, such as infection, rejection, and GVHD occur, gut microbial populations and diversity present a significant dysbiosis. A better understanding of the correlation between gut microbiota and complications after allogeneic transplantation might promote gut microbiota as a therapeutic target in the future. Furthermore, these interventions might include truncated use of antibiotics in afebrile neutropenic patients[38] or improved antibiotic stewardship with reduced use of antibiotics, such as carbapenems and piperacillin-tazobactam, to preserve gut microbial diversity[39]. Conversely, active measures should be explored in-depth to determine whether fecal microbiota transplants[40] and engineered microbial interventions reduce GVHD risk and successfully treat patients with GVHD.
Zuber et al[41] presented an innovative strategy, donor-targeted therapy would ideally mitigate graft-versus-host reactivity while sparing recipient immune functions. It was termed donor-targeted serotherapy, based on the adoptive transfer of anti-HLA donor-specific antibodies for the treatment of refractory GVHD in HLA-mismatched settings. The two children with end-stage renal disease developed severe steroid-resistant acute GVHD along with full and sustained donor T cell chimerism after isolated kidney transplantation. They used this novel therapeutic approach, wherein aGVHD symptoms were promptly resolved in one child. The study provided a proof-of-concept for a highly targeted novel therapeutic strategy for SOT-associated GVHD.
Presently, GVHD is a rare complication after LT lacking universal treatment guidelines but a high mortality rate (5/6 patients, 83.33%). The diagnosis mainly depends on clinical symptoms and laboratory examinations, especially donor lymphocytes chimerism analysis and histopathological findings. We considered that donor T-lymphocyte chimerism STR is of paramount importance for the diagnosis of aGVHD. Herein, we incorporated STR into the aGVHD diagnostic criteria and dynamic monitoring. Because early diagnosis and treatment are critical, a confirmatory test is not essential. Corticosteroids have been the mainstay of therapy in post-LT aGVHD. However, aGVHD after LT is less responsive to corticosteroids, and most appear steroid-resistant aGVHD. Therefore, treatment strategies to mitigate mortality are paramount, and prospective clinical trials with novel therapeutics are imperative.
We thank doctors and nurses at Department of Surgical Intensive Care Unit, Hepatobiliary Surgery, The First Affiliated Hospital of Xi’an Jiaotong University for treatment and nursing at aGVHD patients. We also thank the Pathology Department for the pathological diagnosis of the patient's skin tissue.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng Y, Sun C S-Editor: Wang JL L-Editor: A P-Editor: Wang LYT
| 1. | Wood A, Eghtesad B, Lindenmeyer CC. Graft-Versus-Host Disease After Liver Transplantation. Clin Liver Dis (Hoboken). 2020;15:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Chaib E, Silva FD, Figueira ER, Lima FR, Andraus W, D'Albuquerque LA. Graft-versus-host disease after liver transplantation. Clinics (Sao Paulo). 2011;66:1115-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Murali AR, Chandra S, Stewart Z, Blazar BR, Farooq U, Ince MN, Dunkelberg J. Graft Versus Host Disease After Liver Transplantation in Adults: A Case series, Review of Literature, and an Approach to Management. Transplantation. 2016;100:2661-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Matsukuma KE, Wei D, Sun K, Ramsamooj R, Chen M. Diagnosis and differential diagnosis of hepatic graft versus host disease (GVHD). J Gastrointest Oncol. 2016;7:S21-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 5. | Schulman JM, Yoon C, Schwarz J, Vagefi PA, Mully TW, Shinkai K. Absence of peripheral blood chimerism in graft-vs-host disease following orthotopic liver transplantation: case report and review of the literature. Int J Dermatol. 2014;53:e492-e498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Kim GY, Schmelkin LA, Davis MDP, El-Azhary RA, Farrell AM, Meves A, Lehman JS. Dermatologic manifestations of solid organ transplantation-associated graft-versus-host disease: A systematic review. J Am Acad Dermatol. 2018;78:1097-1101.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Wang B, Lu Y, Yu L, Liu C, Wu Z, Liu X. Diagnosis and treatment for graft-versus-host disease after liver transplantation: two case reports. Transplant Proc. 2007;39:1696-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kang WH, Hwang S, Song GW, Jung DH, Park GC, Ahn CS, Moon DB, Kim KH, Ha TY, Kim WJ, Kim SH, Cho HD, Kwon JH, Jwa EK, Lee SG. Acute Graft-vs-Host Disease After Liver Transplantation: Experience at a High-volume Liver Transplantation Center in Korea. Transplant Proc. 2016;48:3368-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Triulzi DJ, Nalesnik MA. Microchimerism, GVHD, and tolerance in solid organ transplantation. Transfusion. 2001;41:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: a comprehensive literature review. World J Gastroenterol. 2012;18:5240-5248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 11. | Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 62:21-78. [PubMed] |
| 12. | Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV, Bradley JA, Taylor CJ. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004;77:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Carnevale R, Raparelli V, Nocella C, Bartimoccia S, Novo M, Severino A, De Falco E, Cammisotto V, Pasquale C, Crescioli C, Scavalli AS, Riggio O, Basili S, Violi F. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J Hepatol. 2017;67:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Gonultas F, Akbulut S, Barut B, Kutluturk K, Yilmaz S. Graft-versus-host disease after living donor liver transplantation: an unpredictable troublesome complication for liver transplant centers. Eur J Gastroenterol Hepatol. 2020;32:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kakotrichi A, Hind J. Graft-versus-host disease in paediatric liver transplantation: A review of the literature. S Afr Med J. 2017;107:12133. [PubMed] |
| 17. | Mariotti J, Penack O, Castagna L. Acute Graft-versus-Host-Disease Other Than Typical Targets: Between Myths and Facts. Transplant Cell Ther. 2021;27:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Pahari H, Nagai S, Skorupski S, Salgia R. Graft-versus-host disease of the central nervous system after liver transplantation: A rare complication. Am J Transplant. 2018;18:2591-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005;5:2968-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Zhao XF, Lin DD, Li N, Wu JS, Guo QL, Wang L. Diagnosis and treatment of acute graft-versus-host disease after liver transplantation: A report of 11cases. Transpl Immunol. 2020;62:101307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Noguchi M, Shindo T, Yamada Y, Date H. T-cell chimerism prior to graft-versus-host disease. Eur J Cardiothorac Surg. 2021;60:194-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Hahn AB, Baliga P. Rapid method for the analysis of peripheral chimerism in suspected graft-versus-host disease after liver transplantation. Liver Transpl. 2000;6:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Rai V, Dietz NE, Agrawal DK. Immunological basis for treatment of graft versus host disease after liver transplant. Expert Rev Clin Immunol. 2016;12:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 913] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 25. | Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, Shaughnessy PJ, Wall DA, Carpenter PA. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 479] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 26. | MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, Davies SM, Blazar BR. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Chen XB, Yang J, Xu MQ, Wen TF, Yan LN. Unsuccessful treatment of four patients with acute graft-vs-host disease after liver transplantation. World J Gastroenterol. 2012;18:84-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, Khouri IF, Shpall EJ, Anderlini P, Rondon G, Andersson BS, Champlin R, Couriel DR. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv Hematol. 2011;2011:601953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 158] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Risitano AM, Peffault de Latour R. Ruxolitinib for steroid-resistant acute GVHD. Blood. 2020;135:1721-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | von Bubnoff N, Ihorst G, Grishina O, Röthling N, Bertz H, Duyster J, Finke J, Zeiser R. Ruxolitinib in GvHD (RIG) study: a multicenter, randomized phase 2 trial to determine the response rate of Ruxolitinib and best available treatment (BAT) versus BAT in steroid-refractory acute graft-versus-host disease (aGvHD) (NCT02396628). BMC Cancer. 2018;18:1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Melgarejo-Ortuño A, Escudero-Vilaplana V, Revuelta-Herrero JL, Bailen R, Collado-Borrell R, Gomez-Centurión I, Oarbeascoa G, Kwon M, Herranz-Alonso A, Diez-Martin JL, Sanjurjo-Saez M. Tocilizumab as salvage treatment of refractory pulmonary acute graft-versus-host disease. J Oncol Pharm Pract. 2021;27:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Yucebay F, Matthews C, Puto M, Li J, William B, Jaglowski SM, Penza SL, Vasu S, Benson DM, Andritsos LA, Devine SM, Efebera YA, Roddy JVF. Tocilizumab as first-line therapy for steroid-refractory acute graft-versus-host-disease: analysis of a single-center experience. Leuk Lymphoma. 2019;60:2223-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Miller HK, Braun TM, Stillwell T, Harris AC, Choi S, Connelly J, Couriel D, Goldstein S, Kitko CL, Magenau J, Pawarode A, Reddy P, Riwes M, Yanik GA, Levine JE. Infectious Risk after Allogeneic Hematopoietic Cell Transplantation Complicated by Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Elfeki MA, Genco PV, Pungpapong S, Nakhleh RE, Nguyen JH, Harnois DM. Abatacept use in graft-versus-host disease after orthotopic liver transplantation: a case report. Transplant Proc. 2014;46:2422-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Wang W, Xu S, Ren Z, Jiang J, Zheng S. Gut microbiota and allogeneic transplantation. J Transl Med. 2015;13:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Fredricks DN. The gut microbiota and graft-versus-host disease. J Clin Invest. 2019;129:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Noor F, Kaysen A, Wilmes P, Schneider JG. The Gut Microbiota and Hematopoietic Stem Cell Transplantation: Challenges and Potentials. J Innate Immun. 2019;11:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Aguilar-Guisado M, Espigado I, Martín-Peña A, Gudiol C, Royo-Cebrecos C, Falantes J, Vázquez-López L, Montero MI, Rosso-Fernández C, de la Luz Martino M, Parody R, González-Campos J, Garzón-López S, Calderón-Cabrera C, Barba P, Rodríguez N, Rovira M, Montero-Mateos E, Carratalá J, Pérez-Simón JA, Cisneros JM. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4:e573-e583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 39. | Weber D, Jenq RR, Peled JU, Taur Y, Hiergeist A, Koestler J, Dettmer K, Weber M, Wolff D, Hahn J, Pamer EG, Herr W, Gessner A, Oefner PJ, van den Brink MRM, Holler E. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 40. | DeFilipp Z, Peled JU, Li S, Mahabamunuge J, Dagher Z, Slingerland AE, Del Rio C, Valles B, Kempner ME, Smith M, Brown J, Dey BR, El-Jawahri A, McAfee SL, Spitzer TR, Ballen KK, Sung AD, Dalton TE, Messina JA, Dettmer K, Liebisch G, Oefner P, Taur Y, Pamer EG, Holler E, Mansour MK, van den Brink MRM, Hohmann E, Jenq RR, Chen YB. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 41. | Zuber J, Boyer O, Neven B, Jollet I, Renac V, Berthaud R, Levy R, Lamarthée B, Visentin J, Marchal A, Gouge-Biebuyck N, Godron-Dubrasquet A, Aladjidi N, Rabah MO, Winter S, Léon J, Dussiot M, Rabant M, Krid S, Krug P, Charbit M, Lacaille F, André I, Cavazzana M, Llanas B, Allard L, Pirenne F, Gross S, Djoudi R, Tiberghien P, Taupin JL, Blanche S, Salomon R. Donor-targeted serotherapy as a rescue therapy for steroid-resistant acute GVHD after HLA-mismatched kidney transplantation. Am J Transplant. 2020;20:2243-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |