Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9236
Peer-review started: May 21, 2021
First decision: June 15, 2021
Revised: June 28, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: October 26, 2021
Processing time: 152 Days and 17.2 Hours
Primary pulmonary enteric adenocarcinoma (PEAC) is a very rare subtype of invasive adenocarcinoma, and there have been no large studies on PEAC to date. Therefore, it is necessary to obtain much more information about the clinical and pathological features, diagnosis, differential diagnosis, and treatment of PEAC.
All clinical data of six patients with confirmed PEAC from 2013 to 2018 were collected, and data on diagnosis, differential diagnosis, and treatment of PEAC are discussed combined with all the associated literature. The mean age of six patients was 64.0 ± 5.6 (59-73) years old. Their clinical manifestations were heterogeneous, and during their disease course, there were no gastrointestinal symptoms. There was no evidence from colonoscopy or imaging studies to suggest digestive tract tumors or new metastases. The most commonly mutated gene was KRAS (50.0%), and the pathological features of the six cases were similar to those of colorectal cancer. CDX2 (83.3%) and CK7 (66.7%) had the highest positive rates upon immunohistochemical examination. In the associated litera
Positive results for CDX2 and CK7 play an important role in the diagnosis and differential diagnosis of PEAC, and immunotherapy or targeted therapy focused on KRAS needs to be further studied for the treatment of PEAC.
Core Tip: Primary pulmonary enteric adenocarcinoma (PEAC) is a very rare subtype of invasive adenocarcinoma, and there have been no large studies on PEAC to date. All clinical data of six patients with confirmed PEAC from 2013 to 2018 were collected in this study, and data on the diagnosis, differential diagnosis, and treatment of PEAC are discussed combined with all the associated literature. Our findings highlight that positive results for CDX2 and CK7 play an important role in the diagnosis and differential diagnosis of PEAC, and immunotherapy or targeted therapy focused on KRAS needs to be further studied for the treatment of PEAC.
- Citation: Tu LF, Sheng LY, Zhou JY, Wang XF, Wang YH, Shen Q, Shen YH. Diagnosis and treatment of primary pulmonary enteric adenocarcinoma: Report of Six cases. World J Clin Cases 2021; 9(30): 9236-9243
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9236.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9236
Primary pulmonary enteric adenocarcinoma (PEAC) is a very rare subtype of invasive adenocarcinoma. Its morphological and immunohistochemical findings are similar to those of colorectal cancer, but there is no evidence of any primary colorectal cancer[1].
Pulmonary enteric adenocarcinoma was first reported by Tsao and Fraser[2] in 1991. They reported a case of lung tumor with typical features of a differentiated intestinal epithelium, but after 4 years of follow-up, no primary tumors were found other than the lung tumor, which was considered to be a rare new subtype of pulmonary invasive adenocarcinoma, mainly seen in elderly patients[3].
The diagnosis of PEAC relies mainly on pathological and immunohistochemical results. When a primary pulmonary adenocarcinoma is mainly comprised of tissue with intestinal differentiation (> 50%), and the immunohistochemical results of the tumor cells are positive for at least one colorectal cancer-related immunohistochemical marker (CK20, CDX2, MUC2, villin, etc.), under the premise of the exclusion of gastrointestinal-derived tumors, the patient can finally be diagnosed with PEAC[4,5].
At present, reports related to PEAC are gradually increasing, especially studies on the diagnosis of PEAC and its differential diagnosis from lung metastases of colorectal cancer, but mostly these reports involve individual cases, and there are no large samples to date. Therefore, we collected six cases with PEAC diagnosed at the First Affiliated Hospital, Zhejiang University from 2013 to 2018 for retrospective analysis, and we analyzed the diagnosis, differential diagnosis, and treatment in combination with all associated literature to improve clinicians’ understanding of this disease to identify more effective treatment.
All clinical data of six patients with confirmed PEAC from 2013 to 2018 were collected in this study. The ratio of males to females was 1:5, and their mean age was 64.0 ± 5.6 (59-73) years old. The chief complaints are shown in Table 1, including weakness of limb, cough, and so on.
| Case | Gender | Age (yr) | Smoking history | Chief complaints | Lesion location | Mass size (cm) | Metastatic lymph node | Metastatic locations | Tumor stage | OS (mo) |
| 1 | Male | 61 | - | Weakness of left limb, numbness of left face | Posterior segment of RLL | 3.6 × 2.8 | Hilar and mediastinal | Intracranialregion | T2N2M1 | Lost to follow-up |
| 2 | Female | 73 | - | A lung mass found by imaging studies with slightly cough | Posterior segment of LLL | 2.8 × 1.5 | - | - | T2N0M0 | Lost to follow-up |
| 3 | Female | 59 | - | A lungmass found by imaging studies | LLL | 1.3 × 0.6 | - | - | T1N0M0 | > 58 |
| 4 | Female | 64 | - | Pain of right chest and back | RUL | 2.1 × 2.0 | Mediastinal | Right pleura | T1N2M1 | Lost to follow-up |
| 5 | Female | 59 | - | Cough with fever | Bilateral | 2.7 × 1.5 | - | Intra-pulmonary | T4N0M1 | > 9 |
| 6 | Female | 68 | - | Cough,expect-ration, pain of left lower limb with difficult walking | RLL | 6.7 × 5.4 | Mediastinum | Intra-pulmonary + intracranialregion | T4N2M1 | > 7 |
None of the patients had a smoking history. The clinical manifestations were heterogeneous, and during the course of the disease, there were no gastrointestinal sym
As listed in Table 1, case 1 had a history of tuberculosis and abdominal aortic stent implantation; case 2 suffered from hypertension, and she was allergic to iodine preparations. There was nothing apparent in the past history of case 3, and case 4 had a 10-year history of diabetes mellitus. Case 5 had been ill with hepatolithiasis for almost 40 years and progressed to liver cirrhosis for half a month, and she underwent cholecy
In terms of personal and family history, there was nothing of note for case 5, and the other five patients’ parents were all deceased for unknown reasons.
Case 2’s breath sounds were rough, and case 4’s were lower than normal. There was nothing wrong in any other aspects on the physical examination among six cases.
All six patients had an abnormal increase in serum tumor markers (CEA, CA199, and CA125). The increase in CEA and CA199 was much more obvious than that of CA125, and the highest increase was 509 ng/mL and 1449.9 U/mL, respectively (Table 2). The other relevant serum tumor markers (neuron-specific enolase (NSE), serum cyto
| Case | CEA (ng/mL) | CA199 (U/mL) | CA125 (U/mL) |
| 1 | 33.5 | 40.8 | 33.5 |
| 2 | 2.4 | 5.8 | 7.4 |
| 3 | 1.7 | 2.6 | 9.3 |
| 4 | 509 | 132.6 | 217.8 |
| 5 | 2.7 | 243.6 | 13.7 |
| 6 | 1.1 | 1449.9 | 17 |
The immunohistochemistry examination mainly included specific antibodies against lung tumors and gastrointestinal tumors. The six cases were all tested for CDX2, CK7, and TTF-1. The positive rate of CDX2 was 83.3% (5/6), CK7 was 66.7% (4/6), and TTF-1 was 0 (Table 3).
| Case | CDX2 | CK20 | CK7 | TTF-1 | Napsin A | ALK-lung | Others |
| 1 | + | + | - | - | - | Not tested | Not tested |
| 2 | - | - | + | - | - | Not tested | SPA (-) |
| 3 | + | Not tested | - | - | Not tested | Not tested | CK19 (+), SPA (-) |
| 4 | + | +/- | + | - | - | - | p63 (-), CK5/6 (-), PAX8 (-) |
| 5 | + | Not tested | + | - | - | - | CD20 (-), MUC2 (-) |
| 6 | + | - | + | - | - | - | Ki-67 (low) |
In our study, four patients underwent genetic testing, and two had KRAS mutations (2/4, 50.0%); one had a KRAS missense mutation (20.11%), and the other had a BRAC1 nonsense mutation (2.11%) and a KRAS missense mutation (47.22%). The tumor mutation burden of four cases was low or medium, and the average was 9.1 ± 3.5/Mb (Table 4).
| Case | ALK | BRAF | BRCA1 | BRCA2 | EGFR | ERBB2 | KRAS | ROS1 | TMB (/Mb) |
| 3 | - | - | - | - | - | - | + | - | 6.3 |
| 4 | - | - | - | - | - | - | - | - | 6.3 |
| 5 | - | - | + | - | - | - | + | - | 10.3 |
| 6 | - | - | - | - | - | + | - | - | 13.5 |
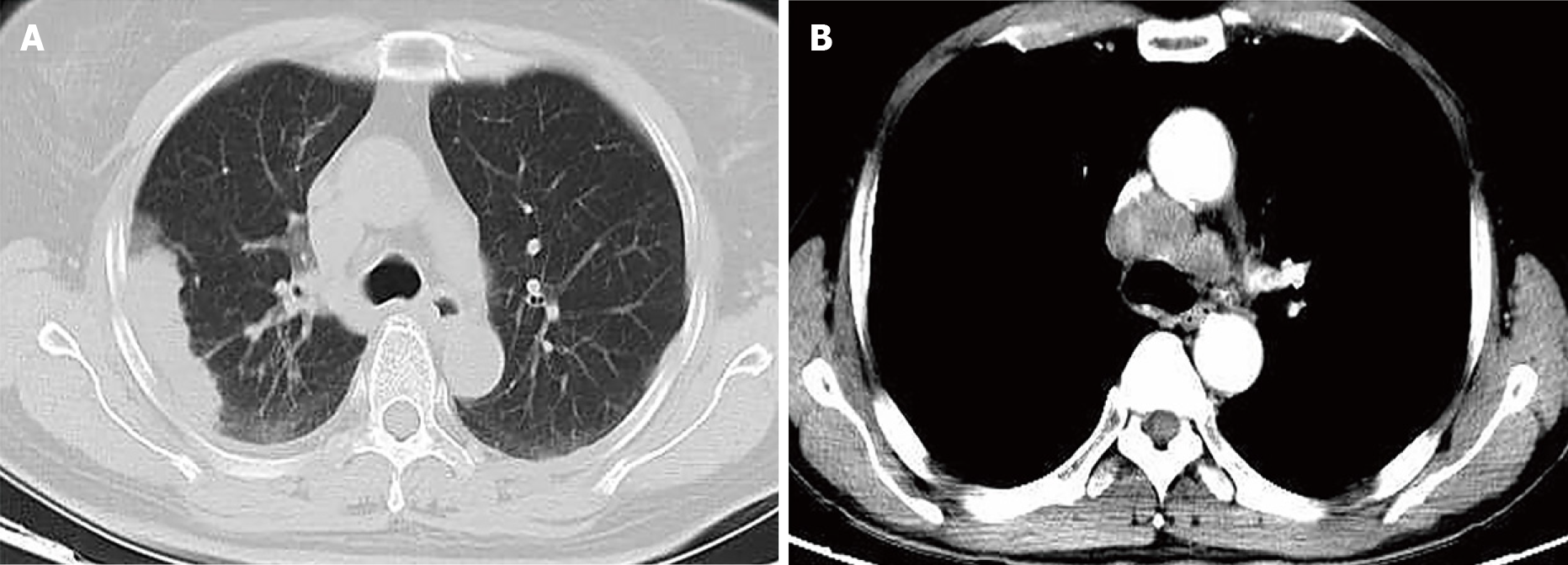
There was no evidence to suggest digestive tract tumors in any patient on colonoscopy and imaging studies. The six patients all showed lung masses in different regions on chest computed tomography (Figure 1, Table 1), with a minimum of 1.3 cm × 0.6 cm and a maximum of 6.7 cm × 5.4 cm, two of which were associated with mediastinal lymph node metastasis (Figure 1B).
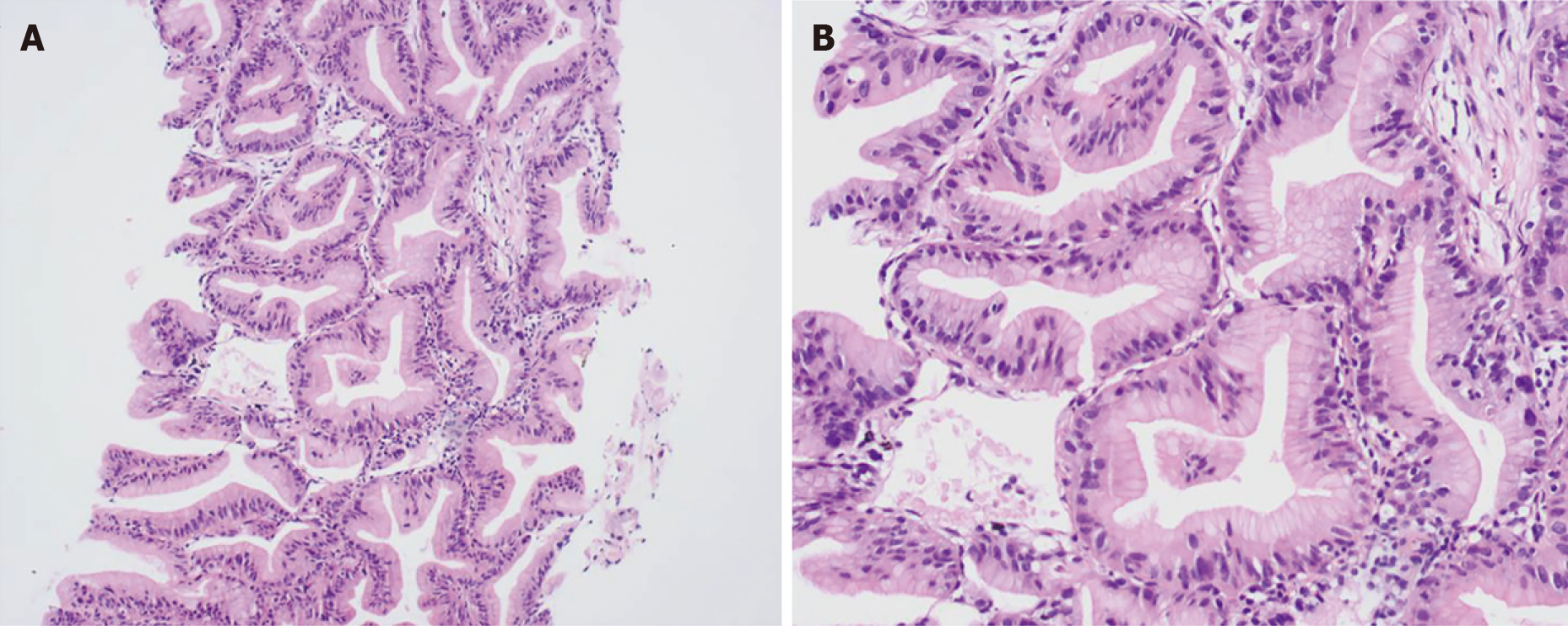
All pathological findings were consistent with pulmonary adenocarcinoma, and there were more than 50% of tissues with intestinal differentiation in each specimen. Taking case 6 as an example, typically, the tumor tissue was arranged in an irregular large glandular tubular shape, and dusty necrosis and obvious nuclear fragmentation were visible in the glandular cavity. The cancer cells were highly columnar in shape and arranged in a pseudostratified layer, and the cytoplasm was red-stained. The brush border could also be seen under high magnification. The nucleus was deeply stained and arranged in a palisade (Figure 2).
Based on the above pathological and immunohistochemical results, the six patients were all diagnosed with PEAC with the exclusion of any gastrointestinal-derived primary tumors.
Among the six patients, two did not undergo any treatment, and the others mainly received surgical resection, radiotherapy, systemic chemotherapy, and so on, and no patient was treated with immunotherapy or targeted therapy (Table 5).
| Case | Lesion location | Treatment |
| 1 | Posterior segment of RLL | Gamma knife for intracranial metastases, with 4 times of pemetrexed + cisplatin, 3 courses of ENDOSTAR, 30 times of radiotherapy, and tumor evaluation was PR; gamma knife again for new intracranial metastases on November 19, 2013 |
| 2 | Posterior segment of LLL | No treatment |
| 3 | LLL | Surgical resection first, reoperation of the resection region because of relapse in September 2015, and no recurrence evidence |
| 4 | RUL | No treatment |
| 5 | Bilateral | TC chemotherapy and bevacizumab, tumor evaluation was SD |
| 6 | RLL | Gamma knife + chemotherapy (pemetrexed + carboplatin), tumor evaluation was SD |
The follow-up time of these patients was August 2019; three were lost to follow-up and the others were still alive. The longest overall survival (OS) was more than 58 mo, and the other two were 7 mo and 9 mo (Table 1). In addition, there was no evidence to suggest digestive tract tumors or any new metastases on colonoscopy and imaging studies at the end of follow-up.
The six patients enrolled in this study were all diagnosed with PEAC. Classical pulmonary adenocarcinoma occurs in nonsmokers[6], especially women. These six patients had no smoking history, and five were female, suggesting that the characteristics of the populations with PEAC and classic pulmonary adenocarcinoma may be similar. In addition, the pathologic results of patients in this study were also consistent with the typical features of PEAC[7].
Common serum tumor markers for lung cancer include CEA, CYFRA 21-1, NSE, CA199, CA125, and so on[8], but their specificity is not high. Among them, CEA is not specific for most tumors, and CA199 is specifically expressed in digestive tract tumors (such as colorectal cancer and pancreatic cancer). CEA and CA199 have been used as tumor markers for colorectal cancer in Japan[9,10]. When CEA > 10 ng/mL and CA199 > 1000 U/mL, the probability of malignancy is high[11]. In this study, CEA and CA199 were significantly elevated in six patients (two with CEA > 10 ng/mL and one with CA199 > 100 U/mL), suggesting that PEAC may have some features in common with colorectal cancer in terms of serum tumor markers.
Because intestinal differentiated tissue accounts for the majority of PEAC, lung cancer markers (CK7, Napsin A, and TTF-1) and colorectal cancer markers (CK20, CDX2, villin, and MUC2) can be expressed simultaneously[12,13]. Previous studies have shown that almost all pulmonary adenocarcinomas express CK7, and most of them also express TTF-1, while MUC2 and CDX2 expression is low or absent. CK7 and CK20 are considered to be reliable markers that can identify PEAC and lung metastases of colorectal cancer[12,14]. With the analyses of these six patients and all the associated literature, CDX2 and CK7 had a higher positive rate on immunohistochemical staining than CK20 and TTF-1, so positive results for CDX2 and CK7 play an important role in the differential diagnosis of PEAC.
Specifically, one case showed no immunohistochemical markers related to colorectal cancer (only for the markers used here), and CK7 was not expressed in any pulmonary enteric adenocarcinomas. This does not seem to be consistent with the theoretical immuno
The sample size of previous studies related to the genetic testing of PEAC is small, and there is no uniform conclusion. Nottegar et al[17] found that KRAS is the most common mutation in PEAC (> 60%), rarely affecting the EGFR, BRAF, and ALK genes. Another study by the same team also showed that KRAS is a common mutated gene expressed in PEAC, and PIK3CA mutations and ALK rearrangements could also be seen, while NRAS mutations were very rare[18]. Feng et al[19] found no correlation between the EGFR gene status and the median survival time in patients with PEAC. For colorectal cancer, KRAS, PIK3CA, BRAF, and NRAS are common mutated genes, among which KRAS is the most common, accounting for 40% of colorectal cancer patients, PIK3CA accounts for 15%, BRAF accounts for 5%, and NRAS accounts for 3%[20]. This study indicates that KRAS is the most common genetic mutation in colorectal cancer, and this result needs to be confirmed in future research.
Details of the previously reported studies associated with PEAC are shown in the Supplementary Material (which illustrates all cases of PEAC until August 2019). The number of cases was 252, and the average age in most cases (n = 107) was 63.9 ± 11.5 (24-88) years old. It is obvious that CK7 (169/197, 85.8%) and CDX2 (155/227, 68.3%) had higher positive rates than CK20 (100/219, 45.7%) and TTF-1 (76/207, 36.7%) in the immunohistochemical results. For genetic testing, the positive rates of EGFR and KRAS were 16.0% (27/169) and 42.9% (60/140), respectively, and there were also several cases with gene mutations of ERBB2, TP53, and so on, but the number of these cases was quite small.
In addition, it is necessary to consider the possible targeted therapy for PEAC, including the corresponding targets for lung cancer (ALK, EGFR, ROS1, etc.) and colorectal cancer (vascular endothelial growth factor, EGFR, etc.), and the possibility of immunotherapy should not be excluded, but the specific targeted therapy and immunotherapy for PEAC is still inconclusive. Based on the findings of this study, immunotherapy or targeted therapy focusing on KARS can be further studied as a treatment for PEAC.
Positive results of CDX2 and CK7 play an important role in the differential diagnosis of PEAC, and immunotherapy or targeted therapy of KRAS can be further explored for the treatment of PEAC. This study promotes an understanding of this rare type of lung adenocarcinoma and provides new ideas about its differential diagnosis and treatment, but a larger sample size of lung enteric adenocarcinoma needs additional study in the future to improve patient prognosis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen SY S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Lin LI, Xu CW, Zhang BO, Liu RR, Ge FJ, Zhao CH, Jia RU, Qin QH, Stojsic J, Wang Y, Xu JM. Clinicopathological observation of primary lung enteric adenocarcinoma and its response to chemotherapy: A case report and review of the literature. Exp Ther Med. 2016;11:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. 1991;68:1754-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Ou SH, Kawaguchi T, Soo RA, Kitaichi M. Rare subtypes of adenocarcinoma of the lung. Expert Rev Anticancer Ther. 2011;11:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | László T, Lacza A, Tóth D, Molnár TF, Kálmán E. Pulmonary enteric adenocarcinoma indistinguishable morphologically and immunohistologically from metastatic colorectal carcinoma. Histopathology. 2014;65:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Wang CX, Liu B, Wang YF, Zhang RS, Yu B, Lu ZF, Shi QL, Zhou XJ. Pulmonary enteric adenocarcinoma: a study of the clinicopathologic and molecular status of nine cases. Int J Clin Exp Pathol. 2014;7:1266-1274. [PubMed] |
| 6. | Yin Z, Zhou B, He Q, Li M, Guan P, Li X, Cui Z, Xue X, Su M, Ma R, Bai W, Xia S, Jiang Y, Xu S, Lv Y. Association between polymorphisms in DNA repair genes and survival of non-smoking female patients with lung adenocarcinoma. BMC Cancer. 2009;9:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Matsushima J, Yazawa T, Suzuki M, Takahashi Y, Ota S, Nakajima T, Yoshino I, Yokose T, Inoue T, Kawahara K, Nakatani Y. Clinicopathological, immunohistochemical, and mutational analyses of pulmonary enteric adenocarcinoma: usefulness of SATB2 and β-catenin immunostaining for differentiation from metastatic colorectal carcinoma. Hum Pathol. 2017;64:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Sato Y, Fujimoto D, Uehara K, Shimizu R, Ito J, Kogo M, Teraoka S, Kato R, Nagata K, Nakagawa A, Otsuka K, Hamakawa H, Takahashi Y, Imai Y, Tomii K. The prognostic value of serum CA 19-9 for patients with advanced lung adenocarcinoma. BMC Cancer. 2016;16:890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Chen M, Liu P, Yan F, Xu S, Jiang Q, Pan J, He M, Shen P. Distinctive features of immunostaining and mutational load in primary pulmonary enteric adenocarcinoma: implications for differential diagnosis and immunotherapy. J Transl Med. 2018;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Kazama S, Watanabe T. [Diagnosis of colorectal cancer by measurement of tumor markers]. Nihon Rinsho. 2014;72:71-76. [PubMed] |
| 11. | Perkins GL, Slater ED, Sanders GK, Prichard JG. Serum tumor markers. Am Fam Physician. 2003;68:1075-1082. [PubMed] |
| 12. | Yousem SA. Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol. 2005;18:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Zhao L, Huang S, Liu J, Zhao J, Li Q, Wang HQ. Clinicopathological, radiographic, and oncogenic features of primary pulmonary enteric adenocarcinoma in comparison with invasive adenocarcinoma in resection specimens. Medicine (Baltimore). 2017;96:e8153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Inamura K, Satoh Y, Okumura S, Nakagawa K, Tsuchiya E, Fukayama M, Ishikawa Y. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. 2005;29:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Hatanaka K, Tsuta K, Watanabe K, Sugino K, Uekusa T. Primary pulmonary adenocarcinoma with enteric differentiation resembling metastatic colorectal carcinoma: a report of the second case negative for cytokeratin 7. Pathol Res Pract. 2011;207:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Miyaoka M, Hatanaka K, Iwazaki M, Nakamura N. CK7/CK20 Double-Negative Pulmonary Enteric Adenocarcinoma With Histopathological Evaluation of Transformation Zone Between Enteric Adenocarcinoma and Conventional Pulmonary Adenocarcinoma. Int J Surg Pathol. 2018;26:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Handa Y, Kai Y, Ikeda T, Mukaida H, Egawa H, Kaneko M. Pulmonary enteric adenocarcinoma. Gen Thorac Cardiovasc Surg. 2016;64:749-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Nottegar A, Tabbò F, Luchini C, Guerrera F, Gaudiano M, Bria E, Brunelli M, Chilosi M, Inghirami G. Pulmonary adenocarcinoma with enteric differentiation: Dissecting oncogenic genes alterations with DNA sequencing and FISH analysis. Exp Mol Pathol. 2017;102:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Feng C, Feng M, Gao Y, Zhao X, Peng C, Yang X, Zhang J. Clinicopathologic Significance of Intestinal-type Molecules' Expression and Different EGFR Gene Status in Pulmonary Adenocarcinoma. Appl Immunohistochem Mol Morphol. 2019;27:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1656] [Article Influence: 110.4] [Reference Citation Analysis (1)] |