Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9144
Peer-review started: March 28, 2021
First decision: July 8, 2021
Revised: July 13, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: October 26, 2021
Processing time: 206 Days and 22.2 Hours
The concurrence of acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL) is rare. Previous reports of such cases have focused mainly on clinical diagnosis and characteristics, so the mechanism remains unclear, and therapy options have been poorly explored.
Here, we report two cases of synchronous AML and CLL. Flow cytometry revealed two distinct abnormal cell populations (myeloblasts and lymphoid cells) according to scatter characteristics. CD5-positive B cell lymphoma with myeloid leukemia invasion was observed on lymph node biopsy. Chemotherapy regimens indicated for both AML and CLL were used in our patients, and our patients achieved complete response after chemotherapy. Next-generation sequencing of 88 genes was performed.
We conclude that early mutation and dysregulation at the hematopoietic stem cell stage and the accumulation of multiple rearrangements may cause the concurrence of CLL and AML. The treatment of infection and combination therapy aimed at the CLL component are significant in the management of patients with concurrent CLL and AML.
Core Tip: The concurrence of acute myeloid leukemia (AML) and chronic lymphocytic leukemia is rare, and patients with both diseases have a poor prognosis. The clinical features, include male predominance and a predilection for older patients, and the AML-M2 subtype is the most frequent subtype. Infection and rapid progression are the most common causes of death in these patients.
- Citation: Chen RR, Zhu LX, Wang LL, Li XY, Sun JN, Xie MX, Zhu JJ, Zhou D, Li JH, Huang X, Xie WZ, Ye XJ. Synchronous diagnosis and treatment of acute myeloid leukemia and chronic lymphocytic leukemia: Two case reports. World J Clin Cases 2021; 9(30): 9144-9150
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9144.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9144
With the application of flow cytometry and next-generation sequencing (NGS), the number of patients diagnosed with concurrent acute myeloid leukemia (AML) and B cell chronic lymphocytic leukemia (CLL) has dramatically increased recently. Patients with CLL have an increased risk of secondary neoplasms, especially cancers of the skin and lung[1]. Generally, the cases reported to date can be categorized into three types: (1) AML cases with treated CLL, in which alkylating agents and purine analogs such as fludarabine can cause treatment-related AML or MDS[2]; (2) AML cases with untreated CLL that remains undetected for a period of time[3]; and (3) Cases with synchronous diagnosis of AML and CLL (of which 21 have been reported since 1973)[4]. Infection and rapid progression are the most common causes of death in patients with both AML and CLL, which may be associated with the immune dysfunction induced by CLL.
The balance of antileukemia and anti-infection therapy remains challenging. Only one patient who received allogeneic hematopoietic stem cell (HSC) transplantation (allo-HSCT) after achieving complete remission after chemotherapy has been reported[5], and only two patients with concurrent de novo AML with inv(16) and CLL had a relatively good prognosis[4,6]. However, few of the reported cases have involved simultaneous treatment of both types of tumors or demethylation therapy. We report two cases with synchronous diagnosis of AML and CLL that were treated with chemotherapy directed at both the CLL and AML components.
Case 1: A 66-year-old man presented to our hospital in July 2020 with progressive enlargement of the right parotid gland and pancytopenia for over five months.
Case 2: A 62-year-old woman was hospitalized with fatigue and abnormal blood counts for one week in April 2020.
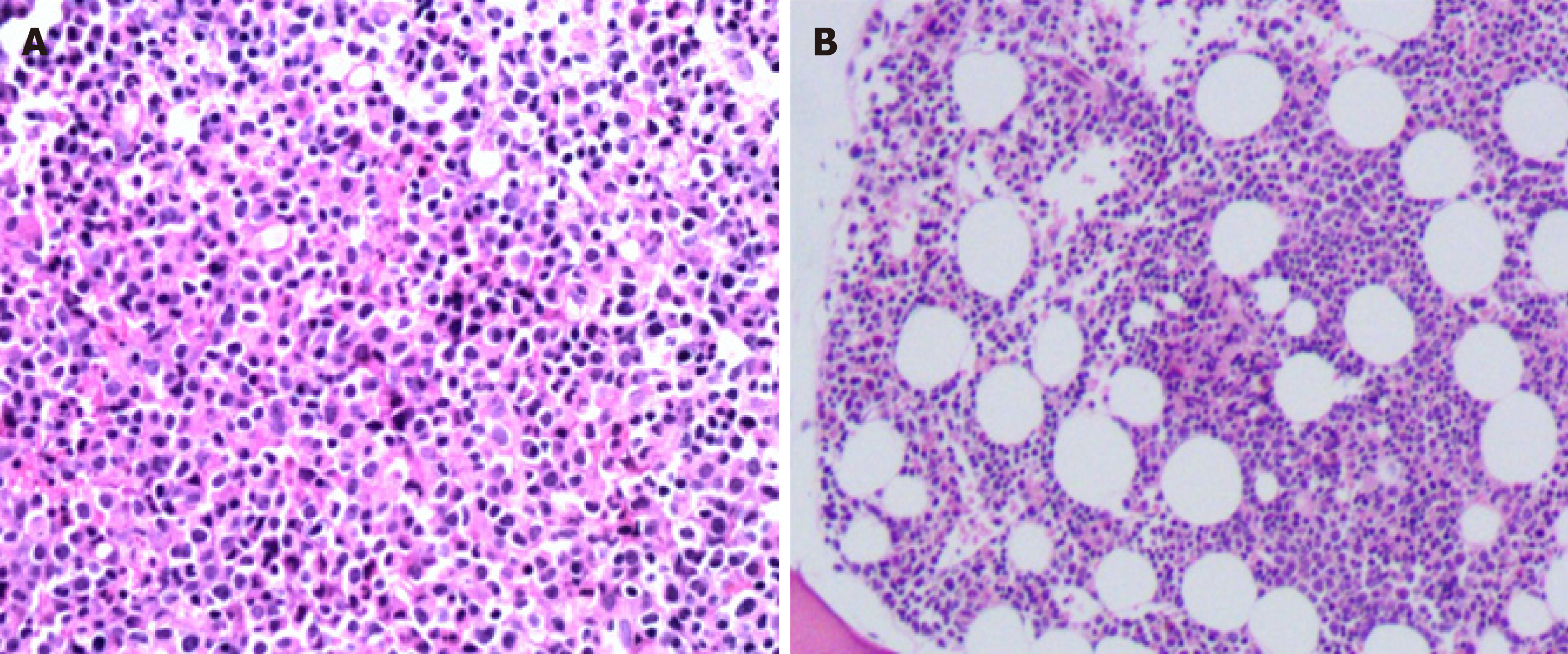
Case 1: The patient developed a mass on his left neck accompanied by fever five months ago. Then progressive enlargement of the right parotid gland followed. The patient went to a local hospital, and ultrasound indicated substantial mass in the right parotid gland and bilateral cervical lymph node enlargement. The mass shrank slightly after hormone and anti-infective therapy. The patient was later transferred to our hospital for further treatment. Right neck lymph node biopsy was performed, and the lymph node biopsy results returned a pathologic diagnosis of CD5-positive B cell lymphoma (small lymphocytic lymphoma (SLL)/CLL was considered first) with myeloid leukemia invasion (Figure 1A). Bone marrow examination indicated that the number of nucleated cells was increased, and myeloblasts accounted for 52% of cells. The FAB classification was M2a.
Case 2: The patient felt fatigue one week ago, and then went to the hospital for blood routine examination which indicated that the three blood lines were reduced. Bone marrow and immunophenotyping were performed. Bone marrow assessment showed that the number of nucleated cells was increased, and type I+II immature granulocytes accounted for 38% of these cells. The pathology results of the bone marrow biopsy showed diffuse distribution of immature cells and lamellar proliferation of lymphoid cells (Figure 1B).
Case 1: The patient had a history of hypertension for 20 years.
Case 2: The patient had no prior history of a hematolymphoid neoplasm or exposure to toxins or radiation.
Case 1: There is nothing special about personal history. His parents died of "cancer". His sister suffered from bowel cancer.
Case 2: The patient had undergone cholecystectomy for gallbladder polyps and hysterectomy for hysteromyoma. There is nothing special about family history.
Case 1: Progressive enlargement of the right parotid gland.
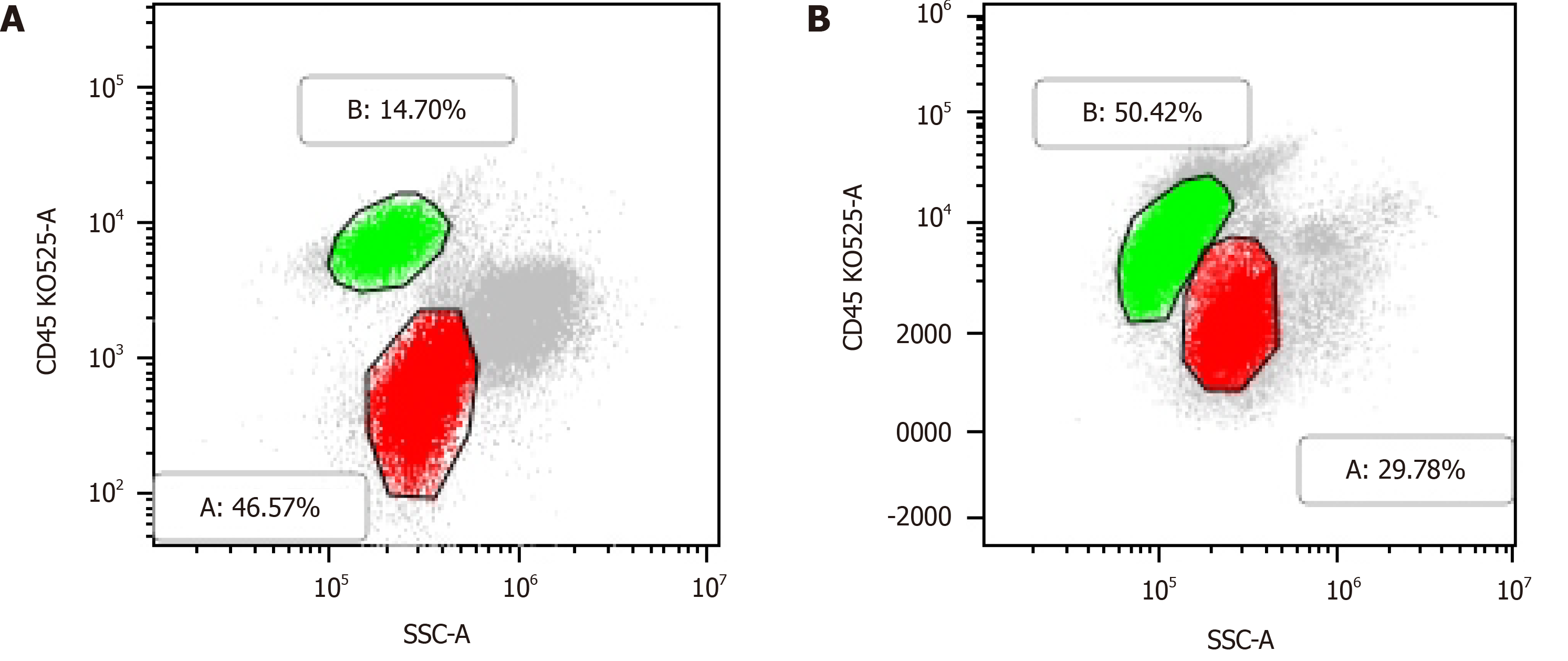
Case 1: Routine blood examination showed the following: white blood cells (WBCs) 3.04 × 109/L, lymphocyte ratio 58.9%, proportion of monocytes 10.9%, hemoglobin (HB) 93 g/L and platelets (PLTs) 68 × 109/L. His peripheral blood smear showed a few primitive naive cells (6%). Flow cytometric analysis showed a population of cells with moderately low side scatter (red, Figure 2A) and a separate population with high side scatter (green, Figure 2A): one population was positive for myeloblast markers (CD117, CD34, CD33, CD13, HLADR, CD38, and MPO), and the other population was positive for B lymphoid markers (CD19, CD5, CD22, CD20, CD23 (dim), CD200, and kappa light chain-restricted expression) and did not express CD103, FMC7, CD10, CD25, or sIgM. Thus, the patient’s flow cytometry results clearly showed the concurrence of CLL and AML.
Immunohistochemical analysis of lymph node tissue showed the following: lymphocyte CD3 (-), CD20 (+), Ki-67 (+20%), Bcl-2 (+), CD5 (+), CD10 (-), CD23 (dim), cyclin D1 (-), CD21 (-), kappa (κ) (dim), lambda (λ) (dim), SOX11 (-), and TDT (-). The analysis of myeloid cells yielded the following results: MPO (+), KI67 (+70%), CD34 (-), and CD117 (dim). Chromosome analysis revealed an abnormal karyotype: 45, XY, -7[10]/46, XY[5]. NGS showed the following mutation frequencies in cells: ASXL1 34.3%, TET2 40.5%, STAG2 81.8%, CEBPA 38.9%, and ETV6 1.7%. The WHO classification was AML with CEBPA mutation. Bone marrow cells showed IgHV gene mutation, and the IgH rearrangement-positive mutation rate was 9.8%, suggesting a good prognosis.
Case 2: Peripheral blood analysis showed WBCs 24.4 × 109/L, HB 113 g/L, PLTs 17 × 109/L and abnormal cells 5%. Immunotyping of the leukemia cells showed the following: A primitive myeloid cell population (red, Figure 2B) (expressing CD117, CD34, CD 33, CD13, HLA-DR, and CD38) accounted for 29.78% of cells, and an abnormal B lymphocyte population (green, Figure 2B) (expressing CD19, CD5, CD22 (dim), CD20 (dim), CD23, CD200, and kappa light chain) accounted for approximately 50.42% of cells. NGS showed the following mutation frequencies in cells: CEBPA mutation 7.23%, GATA2 6.5%, NOTCH1 28.47%, and SF3B1 1.14%.
(1) Synchronous CLL and AML; and (2) Pulmonary infection.
The results of the bone marrow morphology and flow cytometric analyses indicated a diagnosis of AML-M1 and CLL.
The pulmonary infection, which was diagnosed at admission, partially responded to therapy with dexamethasone 10 mg/d, which was administered before leukemia treatment. Considering the poor physical condition of the patient and the desire for combination therapy, a low dose of combination cytarabine and azacytidine (azacytidine 130 mg d1–7 + cytarabine 20 mg Q12H d1–10) was used initially. Partial hematological remission after the first induction therapy was achieved by administration of the same chemotherapy regimen a second time. However, routine bone marrow monitoring after two cycles of this regimen indicated that immature granulocytes accounted for 38% of bone marrow cells. Minimum residual disease (MRD) monitoring showed a residual cancer cell proportion of 36.149%, suggesting that the patient was not in remission. Therefore, the treatment regimen was changed. Two courses of the Bcl-2 inhibitor Venclexta (100 mg d1–28) combined with azacytidine (130 mg d1–7) were administered; surprisingly, both bone marrow and molecular remission were obtained. Pulmonary fungal infection occurred during the patient’s hospital stay, and the dose of Bcl-2 inhibitor was adjusted for concomitant use with the antifungal drug posaconazole.
The HA regimen (homoharringtonine (HHT) 4 mg d1–5 and cytarabine 150 mg d1–5) combined with a low dose of hormonal therapy (30 mg/d) was used as induction chemotherapy. Complete remission of the AML component was obtained after the first course of chemotherapy (with bone marrow immature granulocytes accounting for 2% of cells). Then, cytarabine and VP-16 (cytarabine 4 g d1/3/5 and VP-16 100 mg d1–5) treatment was administered once as consolidation chemotherapy. Next, HA+VP (HHT 4 mg d1–5, cytarabine 150 mg d1–7, vincristine 4 mg d1/8 and dexamethasone 10 mg d1–8) was administered as maintenance therapy, and MRD negativity (abnormal B lymphocytes < 0.01%) occurred after three cycles of HA+VP.
The patient had low PLTs although bone marrow remission was achieved, and he is currently waiting for a bone marrow transplant.
The patient achieved remission but refused a bone marrow transplant.
With flow cytometry becoming a reliable detection method to diagnose the coexistence of CLL and AML, understanding the mechanisms underlying such cases and exploring treatments have become urgent. Patients with CLL and AML always have poor prognoses. The clinical features, as Rui Zhang et al summarized, include a male predominance and predilection for older patients, and AML-M2 is the most frequent subtype[7]. The unique aspects of our cases include the following: (1) The NGS results of our cases provide insight into the underlying mechanisms; and (2) The complete chemotherapy regimens were administered in the process of treatment.
The majority of recent studies support the idea that CLL and AML originate from two separate cell types[6,8,9]. However, some researchers maintain that AML and CLL cells share the same origin[7]. The myeloid and lymphoid lineages evolve from common progenitor cells called primitive HSCs. The occurrence of simultaneous hematologic tumors may be triggered by an early defect at the level of pluripotent stem cells, resulting in the simultaneous development of two tumorigenic processes[10-12]. To date, no specific genetic or chromosomal mutations that are clearly associated with the synchronous development of CLL and AML have been identified. However, complex chromosomal rearrangements occur in most cases, as do cytogenetic abnormalities[4]. Mutations affecting epigenetic modulators (TET2 40.5% and ASXL1 34.3%), cohesion proteins (STAG2 81.8%), and the cell cycle and differentiation (CEBPA 38.9%) have been discovered by previous NGS studies. TET2, a member of the ten-eleven translocation (TET) family, largely participates in regulating lymphoid and myeloid differentiation and function and may contribute to the development of multiple hematological malignancies[13,14]. Viny et al[15] proposed that STAG2 mutation may represent a transformative event in a primed premalignant clone. The other genetic mutations observed in the NGS results of the second case (CEBPA 7.23%, GATA2 6.5%, and NOTCH1 28.47%) occur primarily in AML. Our study supports the view that early mutation and dysregulation at the HSC stage and the accumulation of multiple rearrangements cause the concurrence of CLL and AML.
Timely treatment of AML is necessary due to the nature of the disease. However, CLL is associated with profound immunosuppression, which results in both impaired antitumor responses and increased susceptibility to infection[16]. To date, few successful chemotherapy attempts have been made. The difficulty in finding effective therapy may be related to the fact that patients with both AML and CML often have concurrent opportunistic infections and older age. Therefore, anti-infection and combination treatment for the CLL component are equally important in the management of these patients.
Two effective therapeutic options were used in our cases (Venclexta combined with azacytidine and HA+VP). DiNardo et al[17] concluded that azacitidine-venetoclax significantly improved the response and prolonged overall survival (OS) compared with azacitidine alone in previously untreated AML patients. BCL-2 inhibitors have been proven to provide good therapeutic responses in CLL patients[18,19]. TET-2 mutation was detected by NGS in the first case, and epigenetic silencing is known to play an important role in the pathogenesis of CLL[20]. Therefore, BCL-2 inhibitors plus azacytidine might be a good treatment option for CLL patients. Because patients similar to the one in case 2 generally have advanced age and susceptibility to infection, a simple, modified combination regimen for first-line treatment of AML and CLL (HA+VP) was chosen as intensive chemotherapy in the second case. However, for this rare high-risk subtype of hematologic tumor, allo-HSCT is still the ultimate goal of therapy.
Our data support the view that early mutation and dysregulation at the HSC stage and the accumulation of multiple rearrangements may cause the concurrence of CLL and AML. The treatment of infection and combination therapy aimed at CLL are significant in the management of patients with concurrent CLL and AML.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giordano A S-Editor: Ma YJ L-Editor: A P-Editor: Li JH
| 1. | Schöllkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Robertson LE, Estey E, Kantarjian H, Koller C, O'Brien S, Brown B, Keating MJ. Therapy-related leukemia and myelodysplastic syndrome in chronic lymphocytic leukemia. Leukemia. 1994;8:2047-2051. [PubMed] |
| 3. | Lai R, Arber DA, Brynes RK, Chan O, Chang KL. Untreated chronic lymphocytic leukemia concurrent with or followed by acute myelogenous leukemia or myelodysplastic syndrome. A report of five cases and review of the literature. Am J Clin Pathol. 1999;111:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Shoyele O, Gupta G. Synchronous Diagnosis of De Novo Acute Myeloid Leukemia with inv(16)(p13q22) and Chronic Lymphocytic Leukemia: A Case Report and Review of the Literature. Ann Clin Lab Sci. 2018;48:790-796. [PubMed] |
| 5. | DeFilipp Z, Huynh DV, Fazal S, Sahovic E. Allogeneic stem cell transplantation for acute myeloid leukemia with del(7q) following untreated chronic lymphocytic leukemia. Hematol Oncol Stem Cell Ther. 2012;5:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lu CM, Murata-Collins JL, Wang E, Siddiqi I, Lawrence H. Concurrent acute myeloid leukemia with inv(16)(p13.1q22) and chronic lymphocytic leukemia: molecular evidence of two separate diseases. Am J Hematol. 2006;81:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Zhang R, Kim YM, Lu X, Wang X, Pang H, Li Y, Li S, Lee JY. Characterization of a novel t(2;5;11) in a patient with concurrent AML and CLL: a case report and literature review. Cancer Genet. 2011;204:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ornellas De Souza MH, de Souza Fernandez T, Diamond HR, Maioli MC, Pitanga Bacha PC, De Lucena SB. Cytogenetic and immunophenotypic evidence of independent clonal origins of concomitant chronic lymphocytic leukaemia and acute myeloid leukaemia. Eur J Haematol. 2001;66:281-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Gottardi M, Gattei V, Degan M, Bomben R, Zucchetto A, Tecchio C, Laurino L, Zanatta L, Dei Tos AP, Mordacchini M, Canal F, Gherlinzoni F. Concomitant chronic lymphocytic leukemia and acute myeloid leukemia: evidence of simultaneous expansion of two independent clones. Leuk Lymphoma. 2006;47:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Lima M, Porto B, Rodrigues M, Teixeira MA, Coutinho J, Ribeiro AC, Malheiro MI, Justiça B. Cytogenetic findings in a patient presenting simultaneously with chronic lymphocytic leukemia and acute myeloid leukemia. Cancer Genet Cytogenet. 1996;87:38-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Kikushige Y, Miyamoto T. Hematopoietic stem cell aging and chronic lymphocytic leukemia pathogenesis. Int J Hematol. 2014;100:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kikushige Y, Miyamoto T. Pre-malignant lymphoid cells arise from hematopoietic stem/progenitor cells in chronic lymphocytic leukemia. Int J Hematol. 2015;102:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cong B, Zhang Q, Cao X. The function and regulation of TET2 in innate immunity and inflammation. Protein Cell. 2021;12:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Lio CJ, Rao A. TET Enzymes and 5hmC in Adaptive and Innate Immune Systems. Front Immunol. 2019;10:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Viny AD, Levine RL. Cohesin mutations in myeloid malignancies made simple. Curr Opin Hematol. 2018;25:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 17. | DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, Koller E, Havelange V, Leber B, Esteve J, Wang J, Pejsa V, Hájek R, Porkka K, Illés Á, Lavie D, Lemoli RM, Yamamoto K, Yoon SS, Jang JH, Yeh SP, Turgut M, Hong WJ, Zhou Y, Potluri J, Pratz KW. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 1753] [Article Influence: 350.6] [Reference Citation Analysis (0)] |
| 18. | Cang S, Iragavarapu C, Savooji J, Song Y, Liu D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J Hematol Oncol. 2015;8:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1482] [Article Influence: 164.7] [Reference Citation Analysis (0)] |
| 20. | Tong WG, Wierda WG, Lin E, Kuang SQ, Bekele BN, Estrov Z, Wei Y, Yang H, Keating MJ, Garcia-Manero G. Genome-wide DNA methylation profiling of chronic lymphocytic leukemia allows identification of epigenetically repressed molecular pathways with clinical impact. Epigenetics. 2010;5:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |