Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9101
Peer-review started: December 11, 2020
First decision: January 17, 2021
Revised: January 31, 2021
Accepted: August 23, 2021
Article in press: August 23,2021
Published online: October 26, 2021
Processing time: 313 Days and 21.6 Hours
Granular cell tumor (GCT) of the pancreas is a rare neurogenic tumor. The first case of pancreatic GCT was described in 1975, and up to now, only 7 cases have been reported.
A 53-year-old male had a pancreatic mass for 1 mo. He was not treated at the local hospital, but referred to Henan Tumor Hospital for surgery. Preoperative imaging revealed a 2.0 cm × 2.5 cm-sized mass located in the body of the pancreas. At the microscopic level, a large number of eosinophilic particles are present in the oval tumor cells. The immunohistochemistry of this tumor cell display CD56 (+), blood vessels CD34 (+), Ki-67 (+) < 10%, and S-100 (+).
GCT of the pancreas should be recognized as a preoperative differential diagnosis of pancreatic tumors. Surgical resection of the tumor should be attempted; however, GCT of the pancreas has a certain rate of tumor metastasis and recurrence. Therefore, GCT of the pancreas requires regular and long-term follow-up.
Core Tip: Granular cell tumor (GCT) of the pancreas is a rare neurogenic tumor. We present a rare case of pancreatic GCT, which recovered successfully after surgical treatment. We reviewed 7 previous cases of the same tumor and conclude that most of the pancreatic GCTs are benign, but there is a potential malignancy. Surgery is an important treatment for this disease, and there is a certain rate of tumor metastasis and recurrence after surgery, so regular reexamination should be insisted on.
- Citation: Zhu MH, Nie CF. Particular tumor of the pancreas: A case report . World J Clin Cases 2021; 9(30): 9101-9107
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9101.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9101
Granular cell tumors (GCTs) arise from Schwann cells[1] and have been located almost everywhere in the body, including visceral or cutaneous locations[2], chest wall regions[3], and in the oral cavity[4], pituitary, central nervous system[5], and respiratory system[6]. Granular cells contain unique acidic protein, S-100 protein, which is present in Schwann cell and satellite cells of ganglia. GCTs of the pancreas are so extraordinarily rare that only 7 cases have been reported in all literatures. Now we report the eighth case of pancreatic GCT, including clinical, imageological, and histological features.
A 53-year-old male patient was referred to our hospital after a physical examination 1 mo earlier revealed a mass in his pancreas.
There's nothing special about his past medical history.
The patient's past medical history is unremarkable.
The patient’s skin and sclera were not yellowish, abdominal muscles were not tense, with/without tenderness and rebound pain. The patient’s liver and spleen were not palpable under the ribs, and the entire abdomen was not palpable. Murphy’s sign was negative. Percussion and auscultation in the abdomen were normal.
Serum tumor marker (carbohydrate antigen 19-9: 15.73 KU/L) was moderately elevated. The liver and kidney function were within the normal range, and the other biochemical tests were within the normal range. Urine analysis results were also normal. An electrocardiogram, chest radiograph and arterial blood gas tests showed no abnormalities.
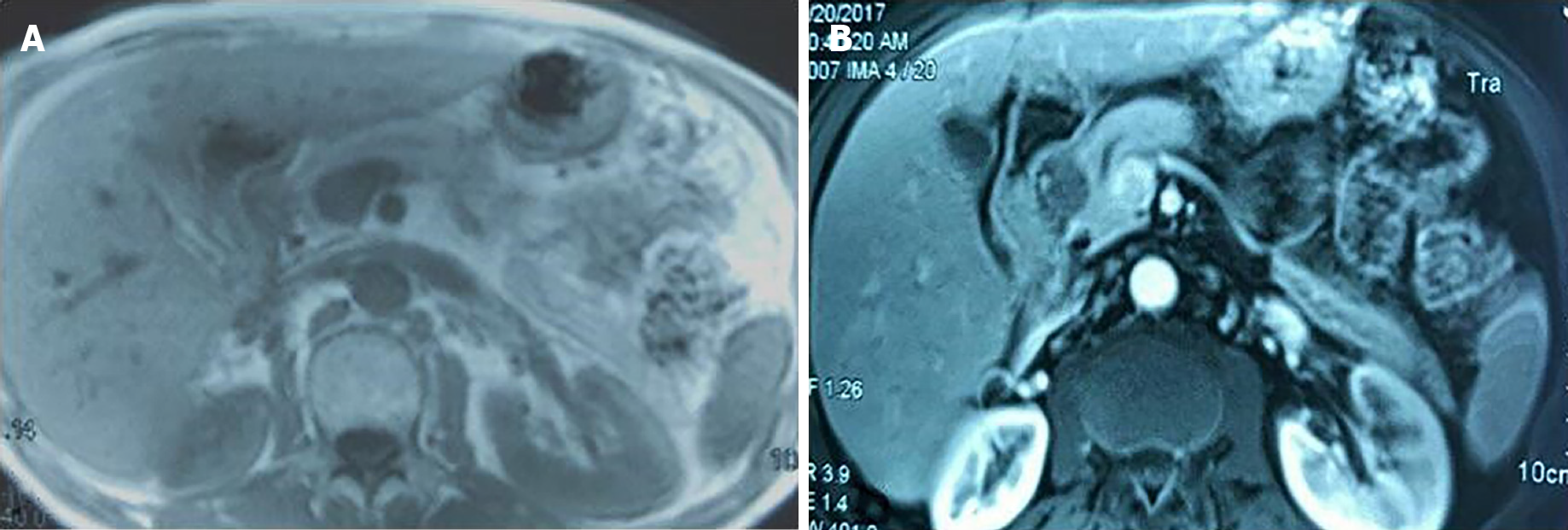
On T1-weighted magnetic resonance imaging (MRI), the tumor in the head of the pancreas showed mild, low signal intensity (Figure 1A). On the contrary, the peripheral tissues around the tumor were equal signal while the central area of tumor was hypointense on the T2-weighted image (Figure 1B). The portal vein was not invaded by the pancreatic head tumor, neither was celiac artery.
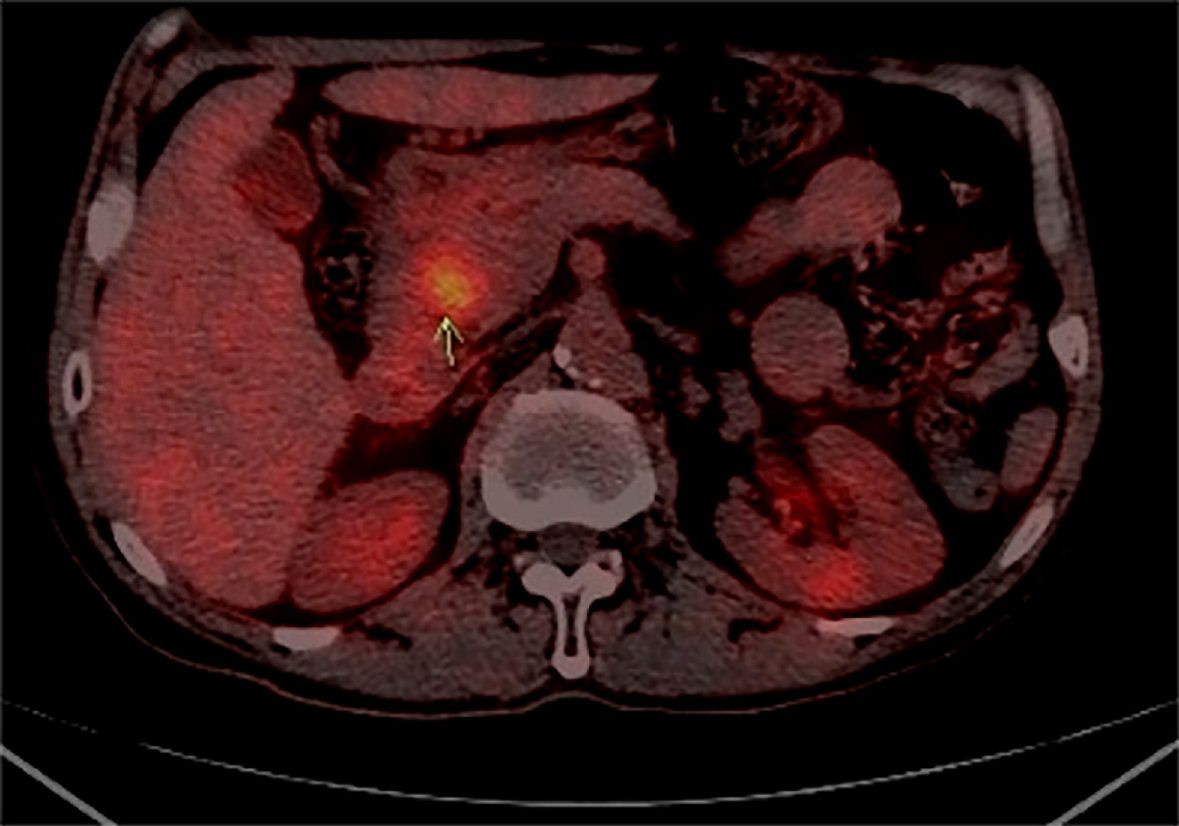
After coming to our hospital, the tumor was assessed by abdominal ultrasound test, and a hypoechoic area whose size was 25 mm × 25 mm was identified. The morphological structure was normal and the boundary was clear in the body of the pancreas. Electronic gastroscope showed a duodenal bulb compressional bulge and surface mucosal hyperemia. Positron emission tomography/computed tomography (CT) examination showed increased metabolism at the L1/2 level of the descending part of the duodenum and head of pancreas and soft tissue nodules indicating the lesion was likely to be a malignant tumor (Figure 2).
The diagnosis prior to operation was pancreatic tumor of unknown properties.
Postoperative pathological results and immunohistochemical results indicated pancreatic GCT.
In view of the above results, the patient was diagnosed with a pancreatic tumor and a pancreatoduodenectomy was performed. Laparotomy showed that the tumor was hard, located in the pancreatic head, with oppression of the duodenum, and was about 3.0 cm × 2.5 cm × 2.0 cm. No capsule was evident around the tumor. Common bile duct was mildly expanded to a diameter of about 1.2 cm and full of green turbidity bile. Gall bladder, intestinal canal, and omentum majus had mutually tight adhesion. There were no metastatic lesions found on the adjacent organs and peritoneum. No swelling lymph nodes were seen around the lesion location. Histological examination confirmed that the tumor was completely removed and the margin of the resection specimen was negative for tumor cells. None of 4 regional lymph nodes examined showed metastasis. After 2 wk of receiving anti-inflammatory, compensation fluid, nutritional therapy, and symptomatic treatment, the patient was discharged from the hospital free of symptoms.
The patient recovered smoothly after surgery, and the postoperative condition was not unstable. The patient was asymptomatic during the 2 years of follow-up after the operation. The patient was advised to continue to follow-up every 3 to 6 mo.
The first report of GCT, localized in the skeletal muscle of the tongue, was in 1926 by Abrikossoff. GCT is a rare tissue tumor derived from Schwann cells. Despite reports that GCTs can be localized anywhere in the body, GCTs of pancreatic origin are rare. The characteristics of the 7 previously reported cases of pancreatic GCT are summarized in Table 1. By reason of the rarity of pancreatic GCT, its epidemiology, clinical symptoms, imaging findings, and serological examination have not been clarified. Therefore, it is difficult to distinguish pancreatic GCT from pancreatic tumor, including pancreatic cancer, pancreatic neuroendocrine tumor, pancreatic cystadenoma, and cystadenocarcinoma. Their clinical manifestations are not typical. On CT and MRI examination, the tumors are round, oval or dumbbell cystic lesion whose cystic wall is thickness. There are some fences that can be obviously enhanced during dynamic CT and MRI inside of cystic lesion. In the past, some experts thought that GCT was benign tumor, as the majority of GCTs are benign However, increasingly the literature has indicated that GCT has some characteristics of malignant tumors, such as metastasis and recurrence, despite having a benign histological appearance. In fact, only about half of reportedly malignant cases, whose incidence is only 1% to 2%, are diagnosed with metastases[7,8]. Previous cases of GCT metastasis to liver, lung, brain, bone, abdominal wall, pancreas, and other sites have been reported[9-15]. Morphological examination cannot predict the biological behavior of GCT. However, when the tumor shows local recurrence, invasive growth, rapid growth over a short period, or a diameter greater than 4 cm, we should be highly alert to the possibility of malignancy.
| Ref. | Age | Sex | Locarization | Size in mm | Treatment |
| Wellman et al[24], 1975 | 29 | M | Head | 6 mm × 4 mm × 3 mm | - |
| Sekas et al[25], 1988 | 31 | F | Head | 5 | Pancreaticojejunostomy |
| Seidler et al[16], 1986 | 62 | F | Tail | 7 mm × 5 mm | Distal pancreatectomy |
| Bin-Sagheer et al[17], 2002 | 50 | F | Body-tail | - | Distal pancreatectomy |
| Méklati et al[22], 2005 | 26 | F | Body-tail | 5 | Distal pancreatectomy |
| Nojiri et al[18], 2001 | 58 | M | Head | 13 | Pancreatoduodenectomy |
| Kanno et al[26], 2010 | 39 | F | Boay | 20 | Distal pancreatectomy |
| Present case | 53 | M | Head | 30 mm × 25 mm × 20 mm | Pancreatoduodenectomy |
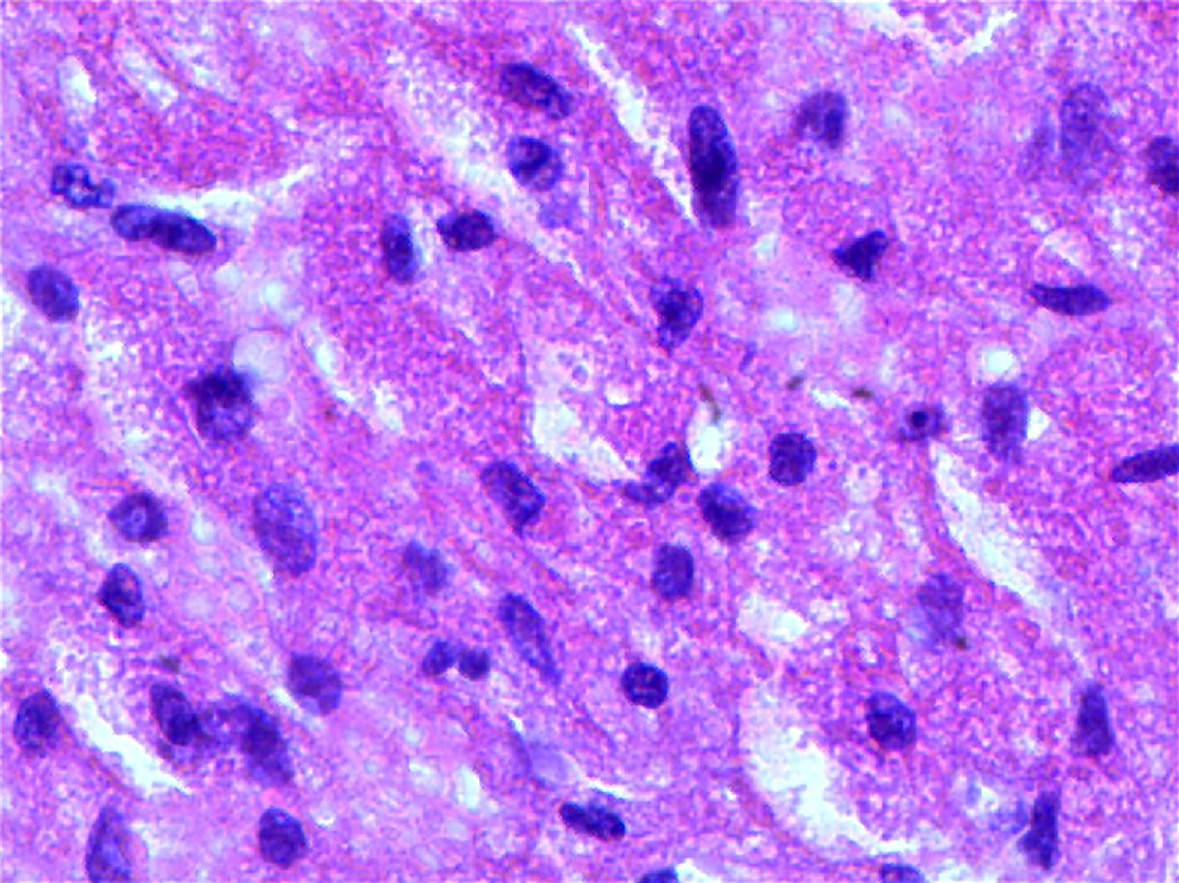
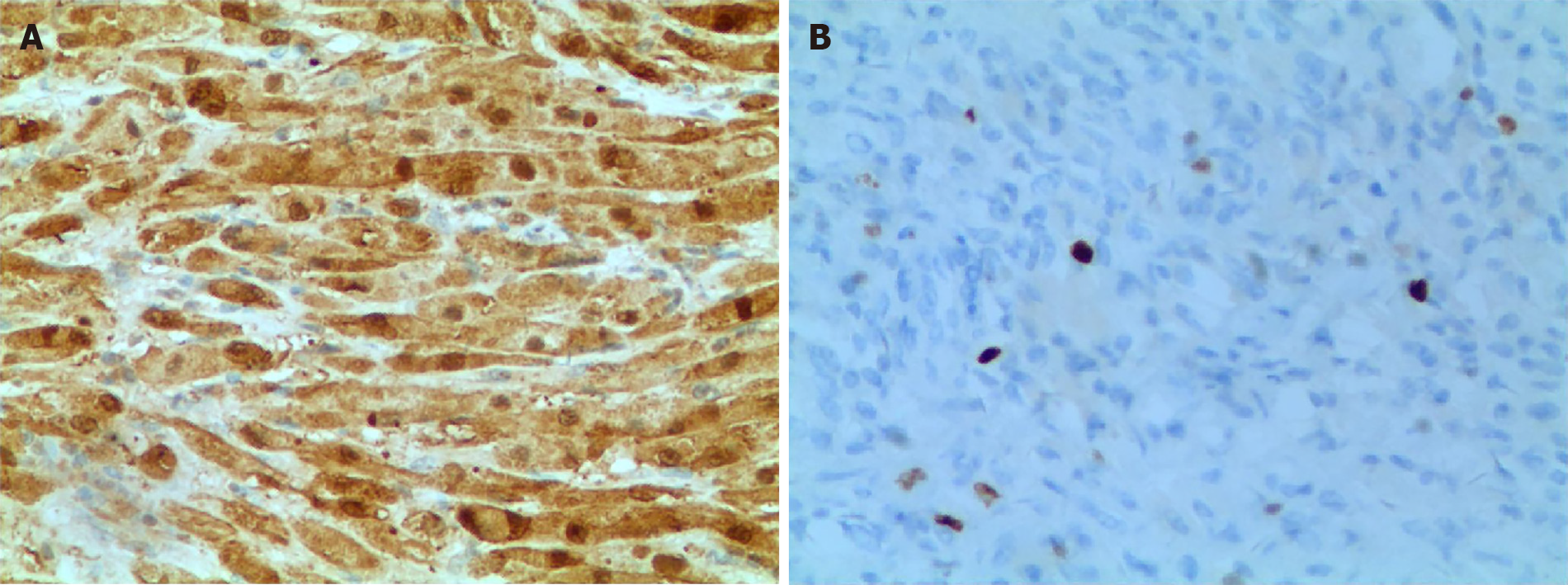
In our patient, diffuse oval tumor cells were seen histopathologically in the presence case of GCT and lots of eosinophilic granules exist in the cytoplasm of tumor cells (Figure 3). Periodic Acid-Schiff staining (Figure 4A), staining for S-100 protein, and neuron specific enolase by immunohistochemistry in GCT cells were positive, which further support for the diagnosis of GCT (Figure 4B). However, it was not easy to make an accurate preoperative diagnosis of pancreatic GCT, because GCT is so rare that its characteristics have been fully elucidated. Not all of the pancreatic GCT can be accurately diagnosed. Among the 7 cases of pancreatic GCT reported in the past, 4 cases of pancreatic GCTs which were misdiagnosed as pancreatic cancer and subsequently surgically removed[16-18]. Given that we did not know enough about imaging characteristics of pancreatic GCT, we also misdiagnosed the case as pancreatic tumor. As far as we know, no literature on the MRI features of pancreatic GCTs has been reported. According to the past literature, the MRI characteristic of GCT are different according to the location, for example, Jagannathan[19] described a GCT of the breast showing as slightly hyperintense lesion in T2 weighted[19], on the contrary, Kudawara et al[20] described a case of GCT that shows hypointense signal on a T2-weighted image[20]. These findings of MRI in other locations were not fully consistent with our report, and therefore we cannot reach an unified conclusion about the MRI characteristics of GCT to suite for all locations. It has been reported that endoscopic ultrasound- or CT-guide fine-needle aspiration, not the most reliable method, may be helpful in diagnosis of pancreatic GCT[21,22], histopathological testing of the tissue specimen is the gold standard for the final and exact diagnosis. Complete resection of the lesion was the main treatment for our patient, and the prognosis is for him is good. However, malignant GCTs has about 32% recurrence rate[23], so postoperative follow-up on a regular basic is also very important.
We diagnosed and treated a case of pancreatic GCT. By imaging examination, we founded a tumor located in patient’s pancreatic head. Subsequently, the patient underwent a surgery and the tumor was completely removed. Finally, the pathology inspection confirmed that the tumor was pancreatic GCT. Because pancreatic GCT is a rare disease, it is quite difficult to make an accurate preoperative diagnosis. In future, we should consider this possibility of the pancreatic GCT in the differential diagnosis in any pancreatic tumor that is less enhanced than the pancreas regardless of pancreatic duct obstruction.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Funel N S-Editor: Fan JR L-Editor: Filipodia P-Editor:Guo X
| 1. | Fisher ER, Wechsler H. Granular cell myoblastoma--a misnomer. Electron microscopic and histochemical evidence concerning its Schwann cell derivation and nature (granular cell schwannoma). Cancer. 1962;15:936-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | De Simone N, Aggon A, Christy C. Granular cell tumor of the breast: clinical and pathologic characteristics of a rare case in a 14-year-old girl. J Clin Oncol. 2011;29:e656-e657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Gavriilidis P, Michalopoulou I, Baliaka A, Nikolaidou A. Granular cell breast tumour mimicking infiltrating carcinoma. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Vered M, Carpenter WM, Buchner A. Granular cell tumor of the oral cavity: updated immunohistochemical profile. J Oral Pathol Med. 2009;38:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Li P, Yang Z, Wang Z, Zhou Q, Li S, Wang X, Wang B, Zhao F, Liu P. Granular cell tumors in the central nervous system: a report on eight cases and a literature review. Br J Neurosurg. 2016;30:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Durán Toconás JC, Obeso Carillo GA, Cañizares Carretero MÁ. Granular cell tumours: an uncommon endobronchial neoplasm. Arch Bronconeumol. 2011;47:214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Aoyama K, Kamio T, Hirano A, Seshimo A, Kameoka S. Granular cell tumors: a report of six cases. World J Surg Oncol. 2012;10:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Donate-Moreno MJ, Pastor-Navarro H, Carrión-López P, Pascual-Martín A, Salinas-Sánchez AS, Lorenzo-Romero JG, Polo-Ruiz L, Virseda-Rodríguez JA. Late recurrence of ovarian granulosa cell tumor at the retroperitoneal and renal hilum level in a single-kidney patient--case report. Eur J Gynaecol Oncol. 2007;28:487-490. [PubMed] |
| 9. | Thirumala SD, Putti TC, Medalie NS, Wasserman PG. Skeletal metastases from a granulosa-cell tumor of the ovary: report of a case diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 1998;19:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Dubuc-Lissoir J, Berthiaume MJ, Boubez G, Van Nguyen T, Allaire G. Bone metastasis from a granulosa cell tumor of the ovary. Gynecol Oncol. 2001;83:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Liu K, Layfield LJ, Coogan AC. Cytologic features of pulmonary metastasis from a granulosa cell tumor diagnosed by fine-needle aspiration: a case report. Diagn Cytopathol. 1997;16:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Williams RJ, Kamel HM, Jilaihawi AN, Prakash D. Metastatic granulosa cell tumour of the diaphragm 15 years after the primary neoplasm. Eur J Cardiothorac Surg. 2001;19:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Abadeer RA, Fleming JB, Deavers MT, Rashid A, Evans DB, Wang H. Metastatic adult granulosa cell tumor mimicking a benign pancreatic cyst. Ann Diagn Pathol. 2010;14:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Ylagan LR, Middleton WD, Dehner LP. Fine-needle aspiration cytology of recurrent granulosa cell tumor: case report with differential diagnosis and immunocytochemistry. Diagn Cytopathol. 2002;27:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Ismi O, Vayisoglu Y, Karabacak T, Unal M. Supraclavicular metastases from a sex cord stromal tumor of the ovary. Tumori. 2009;95:254-257. [PubMed] |
| 16. | Seidler A, Burstein S, Drweiga W, Goldberg M. Granular cell tumor of the pancreas. J Clin Gastroenterol. 1986;8:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Bin-Sagheer ST, Brady PG, Brantley S, Albrink M. Granular cell tumor of the pancreas: presentation with pancreatic duct obstruction. J Clin Gastroenterol. 2002;35:412-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Nojiri T, Unemura Y, Hashimoto K, Yamazaki Y, Ikegami M. Pancreatic granular cell tumor combined with carcinoma in situ. Pathol Int. 2001;51:879-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Jagannathan DM. Benign granular-cell tumor of the breast: Case report and literature review. Radiol Case Rep. 2015;10:1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Kudawara I, Ueda T, Yoshikawa H. Granular cell tumor of the subcutis: CT and MRI findings. A report of three cases. Skeletal Radiol. 1999;28:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 311] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Méklati el-HM, Lévy P, O'Toole D, Hentic O, Sauvanet A, Ruszniewski P, Couvelard A, Vullierme MP, Caujolle B, Palazzo L. Granular cell tumor of the pancreas. Pancreas. 2005;31:296-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 458] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Wellmann KF, Tsai CY, Reyes FB. Granular-cell myoblastoma in pancreas. N Y State J Med. 1975;75:1270. [PubMed] |
| 25. | Sekas G, Talamo TS, Julian TB. Obstruction of the pancreatic duct by a granular cell tumor. Dig Dis Sci. 1988;33:1334-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Kanno A, Satoh K, Hirota M, Hamada S, Umino J, Itoh H, Masamune A, Egawa S, Motoi F, Unno M, Ishida K, Shimosegawa T. Granular cell tumor of the pancreas: A case report and review of literature. World J Gastrointest Oncol. 2010;2:121-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |