Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8906
Peer-review started: June 10, 2021
First decision: June 25, 2021
Revised: July 2, 2021
Accepted: July 30, 2021
Article in press: July 30, 2021
Published online: October 16, 2021
Processing time: 127 Days and 1.1 Hours
Although acute pancreatitis associated with hyperparathyroidism has occa
We present a case of a 56-year-old female with upper abdominal discomfort and intermittent nausea and vomiting for 1 wk, without apparent abdominal pain or bloating, no jaundice and decreased blood pressure at the outset. The patient was ultimately diagnosed with moderately severe acute pancreatitis (according to the revised Atlanta classification of acute pancreatitis) combined with metabolic encephalopathy secondary to hypercalcemia caused by primary hyperparathyroidism associated with paraneoplastic syndrome. After active treatment of acute pancreatitis, massive fluid resuscitation, resection of parathyroid and uterine malignant tumors, neoadjuvant chemotherapy and other treatments, her serum calcium eventually returned to the normal level. The patient was successfully discharged from hospital.
This is the first case of acute pancreatitis caused by primary hyperparathyroidism associated with paraneoplastic syndrome.
Core Tip: This is the first case of acute pancreatitis caused by primary hyperparathyroidism associated with paraneoplastic syndrome successfully treated with timely surgery and chemotherapy. This further raises concern for women with refractory hypercalcemia and abnormal vaginal bleeding or abdominal symptoms.
- Citation: Yang L, Lin Y, Zhang XQ, Liu B, Wang JY. Acute pancreatitis with hypercalcemia caused by primary hyperparathyroidism associated with paraneoplastic syndrome: A case report and review of literature. World J Clin Cases 2021; 9(29): 8906-8914
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8906.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8906
Acute pancreatitis is an inflammatory disease of the pancreas[1,2]. Although several causes of acute pancreatitis have been described, including toxins or drugs, neoplastic obstruction of the biliary tract, hyperparathyroidism, hypercalcemia, and trauma[3], primary hyperparathyroidism with paraneoplastic syndrome is rare and infrequently reported in the literature. Here we report a patient with acute pancreatitis associated with refractory hypercalcemia, hyperparathyroidism, and paraneoplastic syndrome. Due to refractory hypercalcemia, the diagnosis is difficult. Only by fully under
A 56-year-old woman visited the emergency department of an affiliated hospital with progressive upper abdominal discomfort, intermittent nausea, and abnormal vaginal bleeding, for 1 wk.
Initially, there was no apparent jaundice or changes in blood pressure. The patient denied any fever, dizziness, headache, or disturbance of consciousness at the beginning of the illness.
The patient had a history of Helicobacter pylori infection for more than 10 years. She had no relevant past interventions.
She denied family or psycho-social history.
The patient had stable vital signs: body temperature was 37.0 °C, blood pressure was 168/87 mmHg, heart rate was 74 bpm, and respiratory rate was 25 breaths/min, with obvious upper abdominal tenderness, slight rebound pain, and muscle tension.
Laboratory examination results were as follows: white blood cell count 26.5 × 109/L, neutrophil percentage 95%, C-reactive protein 109 mg/L, procalcitonin 4.35 ng/mL, serum amylase 1222.5 U/L, creatinine 172.3 µmol/L, urine amylase 1433.0 U/L, platelet 214 × 109/L, hemoglobin 156 g/L, albumin 26.8 g/L, alanine aminotransferase 19.5 U/L, aspartate aminotransferase 26.6 U/L, lactate dehydrogenase 457.3 U/L, ferritin 747 ng/mL, and 25-hydroxyvitamin D < 3 ng/mL. Serum calcium increased significantly to 4.72 mmol/L, with parathyroid hormone (PTH) rising to 366.90 pg/mL and carbohydrate antigen 125 to 142.5 U/mL. An overview of selected initial laboratory values is shown in Table 1.
| WBC (4-10) (× 109/L) | 26.5 |
| Neutrophil percentage (50-70) (%) | 95 |
| PLT (100-300) (× 109/L) | 214 |
| Hb (113-151) (g/L) | 156 |
| Albumin (35-53) (g/L) | 26.8 |
| ALT (4-40) (U/L) | 19.5 |
| AST (4-40) (U/L) | 26.6 |
| CRP (0-10) (mg/L) | 109 |
| PCT (0-0.5) (ng/mL) | 4.35 |
| Serum amylase (0-200) (U/L) | 1222.5 |
| Urine amylase (0-1000) (U/L) | 1433.0 |
| Creatinine (44-97) (μmol/L) | 172.3 |
| Serum calcium (2.13-2.65) (mmol/L) | 4.72 |
| PTH (15-65) (pg/mL) | 366.90 |
| CA-125 (0-35) (U/mL) | 142.5 |
| LDH (80-250) (U/L) | 457.3 |
| Ferritin (11-306) (ng/mL) | 747 |
| 25-OH-VD (20-100) (ng/mL) | < 3 |
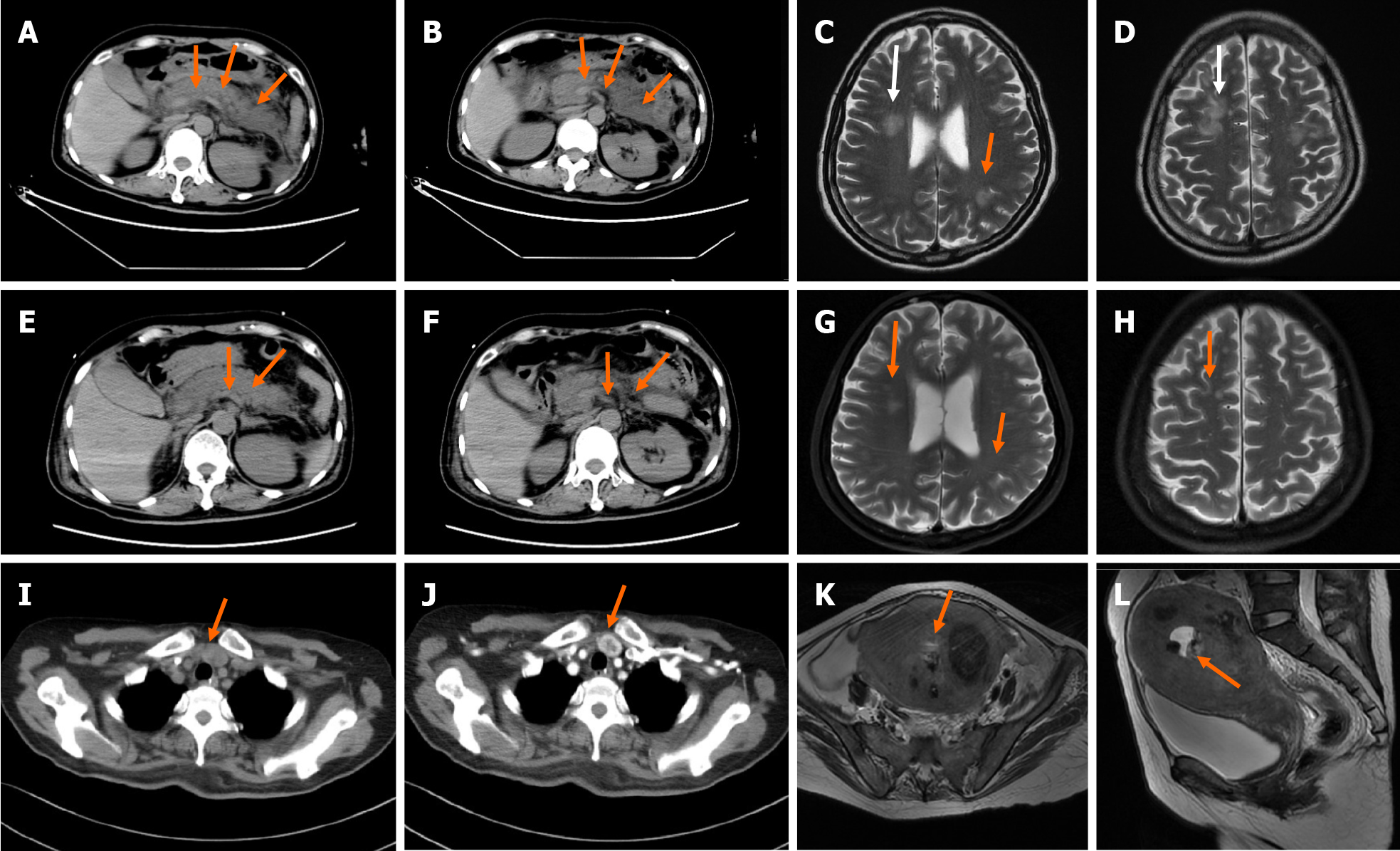
Non-contrast enhanced computed tomography (CT) of the abdomen showed exudation around the pancreas (Figure 1A and B). Ultrasound examination of the neck revealed an inferior thyroid nodule and mild hyperplasia of the parathyroid gland. Head magnetic resonance imaging (MRI) was performed, and abnormal signals were found in the bilateral fronto-parietal-temporal-occipital cortex-medullary junction area and bilateral paraventricular area, which were likely due to metabolic encephalopathy related to pancreatitis (Figure 1C and D). A repeat non-contrast enhanced CT scan of the abdomen (Figure 1E and F) and head MRI (Figure 1G and H) showed a reduction in pancreatic exudation and abnormal head signals after effective treatment. Contrast enhanced CT of the neck (Figure 1I and J) showed nodules located at the junction of the left lobe of the thyroid and parathyroid gland. MRI of the pelvis (Figure 1K and L) suggested malignant lesions of the uterus and multiple uterine fibroids.
Due to the unknown nature of the disease and refractory hypercalcemia, a multidisciplinary expert consultation was conducted, and further workup was proposed. General practitioners believed that refractory hypercalcemia might be caused by parathyroid disease, and exploratory resection of the parathyroid gland was necessary.
Moderately severe acute pancreatitis with refractory humoral hypercalcemia caused by primary hyperparathyroidism associated with paraneoplastic syndrome.
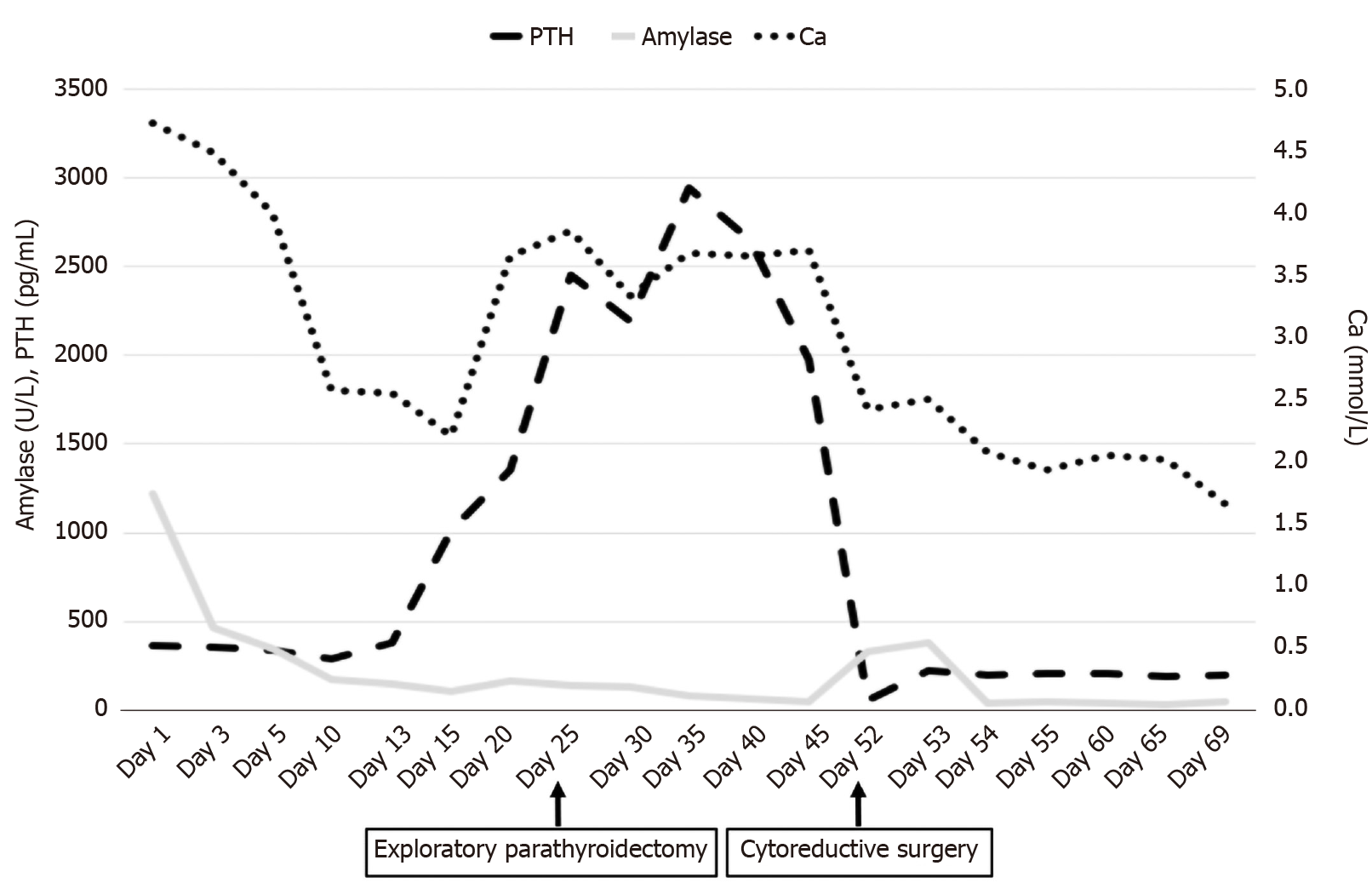
Based on the above laboratory and imaging examinations (initially we did not consider gynecological disease and did not perform pelvic MRI), the preliminary diagnosis was acute pancreatitis secondary to hypercalcemia caused by primary hyperparathyroidism. She was transferred to the emergency intensive care unit and treated with salmon calcitonin, zoledronic sodium, and anti-infection and intravenous fluids. During the hospitalization period, the patient became more lethargic with dry mucous membranes and mild coma. Early use of imipenem and extensive resuscitation effectively controlled her inflammation. As serum calcium treatment was not satisfactory, hemofiltration was then performed twice. Although acute pancreatitis and consciousness gradually improved, serum calcium did not return to normal level (Figure 2).
To determine the cause of the elevated calcium, a multidisciplinary expert was consulted, and further workup was proposed. Finally, although the nature of the parathyroid lesions was not yet clear, to reduce serum calcium, exploratory parathyroidectomy was performed on April 14, 2020 (Figure 2). The pathological result of the removed tissue suggested nodular goiter.
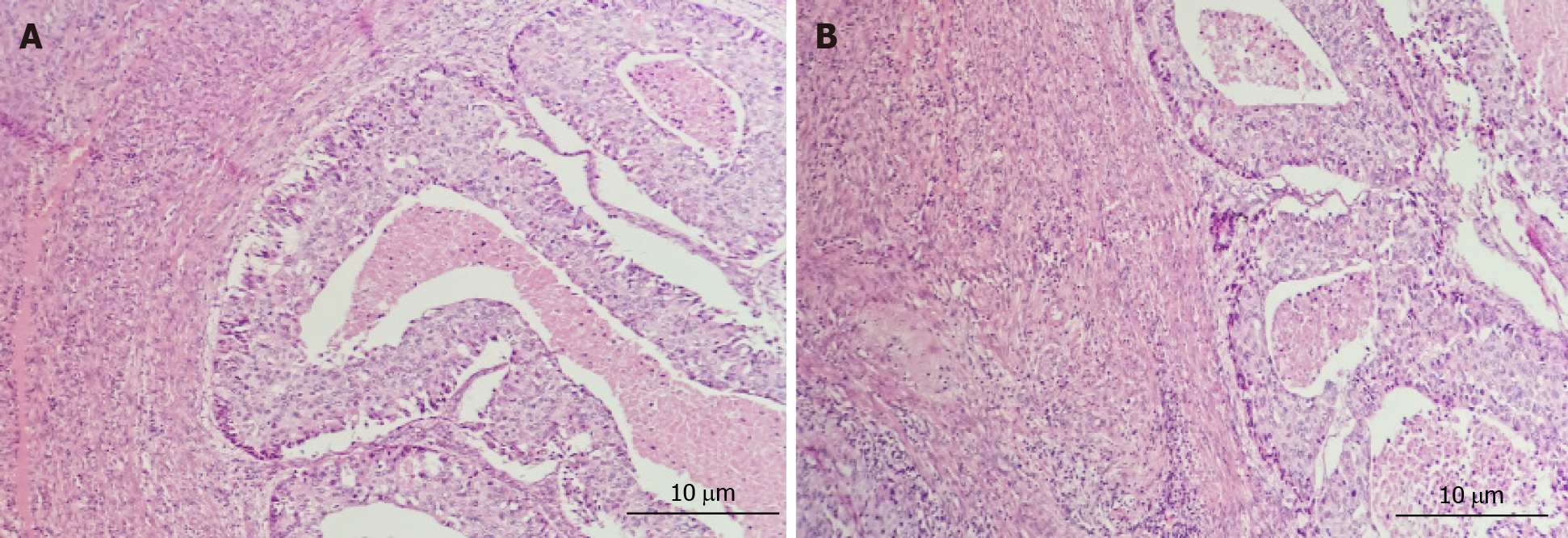
Unexpectedly, removal of the parathyroid gland did not decrease serum calcium; therefore, we speculated that high calcium might not be caused by hyperparathyroidism alone. To verify this hypothesis, an MRI of the pelvis was performed, and the results showed ambiguous, irregular masses and multiple uterine fibroids in the uterine/cervix region. There were multiple peritoneal and omental masses, and the pelvic and para-aortic lymph nodes were enlarged. The pathological results of the vaginal curettage suggested poorly differentiated uterine cancer (Figure 3). She was immediately transferred to the gynecological ward. She was treated with cytoreductive surgery and neoadjuvant chemotherapy (Figure 2). After surgery, supportive care was provided in the intensive care unit (ICU) and she received 3 cycles of carboplatin and paclitaxel.
She did not experience any serious complications or neurologic deficits in the ICU. The patient’s serum calcium eventually decreased to normal levels, and she was successfully discharged from hospital 2 mo later.
Acute pancreatitis is a common disease associated with significant morbidity and mortality. Alcohol and biliary disease are the causes of almost all such cases, with an incidence of approximately 80%-90%[4]. Additionally, uncommon causes include toxic substances, trauma, infection, autoimmune diseases, or metabolic disorders secondary to hypercalcemia, such as primary hyperparathyroidism or malignant tumors[3]. Generally, acute pancreatitis is associated with a decrease in serum calcium, but pancreatitis caused by primary hyperparathyroidism or malignancies is usually associated with hypercalcemia. These two causes are rare in hypercalcemia. Although the relationship and the pathophysiology are unclear, it is possible that the connection between them is not accidental. Inappropriate activation of digestive enzymes in the pancreas, especially trypsinogen in the acetabulum may play an important role in the development of acute pancreatitis[5,6]. An excessive increase in intracellular calcium concentration can lead to over-activation of digestive enzymes and block the pancreatic ducts, causing inflammatory exudation of the pancreas[7].
The above two rare, but well-known causes of hypercalcemia, are discussed in the literature. Many cases of acute pancreatitis caused by hyperparathyroidism with parathyroid adenoma or adenocarcinoma have been described[8-11]. With regard to paraneoplastic syndrome, several cases have shown that pancreatitis was related to Zollinger-Ellison syndrome[12-14]. Four cases diagnosed as pancreatitis were associated with lung cancer[15-18]. One case had pancreatic adenocarcinoma[19]. One case with myelodysplastic syndromes finally led to pancreatitis[20]. One case had breast cancer[21]. One case was associated with an intraductal papillary neoplasm of the bile duct[22]. Another case of Hodgkin lymphoma with paraneoplastic hypercalcemic pancreatitis was reported[23]. Two similar cases of autoimmune-like pancreatitis with a thymoma and myasthenia gravis were described[24,25]. In addition, two rare cases of pancreatitis and ovary carcinoma were reported, where pancreatitis was caused by the gynecological malignancies[26,27]. An overview of previously described cases of pancreatitis associated with malignant paraneoplastic syndromes is shown in Table 2.
| Ref. | Cancer | Along with increased PTH |
| Danne et al[12], 1985 | Gastrinoma | No |
| Yamamoto et al[13], 2003 | Zollinger-Ellison syndrome | No |
| Baffy et al[14], 2000 | Zollinger-Ellison syndrome | No |
| Belhassen-García et al[15], 2009 | Lung cancer | No |
| Saliba et al[16], 2006 | Lung carcinoma | No |
| Akinosoglou et al[17], 2014 | Lung cancer | No |
| Casadei Gardini et al[18], 2016 | Lung cancer | No |
| Leone et al[19], 1998 | Pancreatic adenocarcinoma | No |
| Tanvetyanon et al[20], 2005 | Myelodysplastic syndrome | No |
| Lekakis et al[21], 2012 | Breast cancer | No |
| Miyazaki et al[22], 2019 | Intraductal papillary neoplasm of bile duct | No |
| Mittra and Davidzon[23], 2014 | Hodgkin lymphoma | No |
| Colaut et al[24], 2002 | Thymoma | No |
| Tomiyama et al[25], 2008 | Thymoma | No |
| Wynn et al[26], 2004 | Ovarian carcinoma | No |
| Seifert and Seemann[27], 1967 | Ovarian carcinoma | No |
On the one hand, primary hyperparathyroidism is rare, with a documented incidence of 1.5%-8%[8]. Compared with healthy individuals, patients with hyperparathyroidism have an increased risk of acute pancreatitis. The prevalence of pancreatitis among patients with primary hyperparathyroidism is between 1.5% and 13%[28]. Acute pancreatitis caused by hyperparathyroidism can now be diagnosed by the continuous increase in serum calcium and elevated PTH levels[10]. Primary hyperparathyroidism is mainly associated with a solitary parathyroid adenoma (85%-90%), but it is also associated with parathyroid carcinoma (< 1%)[9]. Malignant tumors of the parathyroid gland are usually rare, with an incidence of less than 0.5%[29]. The clinical manifestations are traditionally secondary to hypercalcemia, including non-specific gastrointestinal symptoms and cardiovascular or neuromuscular dysfunction[30,31]. Imaging studies including ultrasound imaging, radionuclide scanning, CT, and MRI have advantages in detecting ectopic parathyroid lesions and are helpful in diagnosis[32]. Surgery is the most effective and only way to treat parathyroid carcinoma, which provides the best chance for cure and long-term survival[33,34].
On the other hand, paraneoplastic hypercalcemia occurs in patients with malignant tumors, with an incidence of about 0.3%-4.0%, which means that the immune system’s abnormal response to normal tissues is either through the production of auto
In our patient, examinations initially suggested that moderately severe acute pancreatitis was caused by primary hyperparathyroidism. Firstly, the major etiologies of acute pancreatitis include alcohol consumption and biliary stones, which were not present in this patient. Secondly, she did not have a family history of pancreatitis. In addition, PTH and serum calcium were significantly increased, and treatment did not decrease refractory serum calcium. In addition, ultrasound and contrast enhanced CT of the neck revealed nodules located at the junction of the left lobe of the thyroid and parathyroid gland. Therefore, these findings suggested that the pathogenesis of acute pancreatitis was the result of hypercalcemia caused by hyperparathyroidism. Although no adenoma or adenocarcinoma was found, an exploratory resection was performed to decrease refractory serum calcium. Pathological findings of the removed tissue suggested nodular goiter.
Due to this surprising finding, we conducted a further diagnostic workup and finally found gynecological malignancy. A literature review indicated that some malignant tumors might secrete parathyroid hormone-related protein (PTHrP), instead of PTH, causing pancreatitis. The amino terminus of PTHrP has a similar structure to that of PTH. Both activate PTH/PTHrP receptor 1 and decrease the renal clearance of calcium[36].
According to the results of the above analysis, the final diagnosis was moderately severe acute pancreatitis caused by primary hyperparathyroidism associated with paraneoplastic syndrome. Timely surgery and neoadjuvant chemotherapy were performed which extended the survival time of this patient. She was satisfied with the treatment outcome and was successfully discharged from hospital.
To our knowledge, this is the first case of moderately severe acute pancreatitis caused by primary hyperparathyroidism associated with paraneoplastic syndrome. Despite its rare occurrence, hypercalcemia secondary to primary hyperparathyroidism or malignancies usually manifests in the advanced stage of malignancy and has a poor prognosis. Early accurate management can avoid fatal consequences and extend survival time. Therefore, more attention should be paid to the differential diagnosis in women with hypercalcemia and abnormal vaginal bleeding or abdominal symptoms.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab. 2009;94:2115-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ. The association of primary hyperparathyroidism with pancreatitis. J Clin Gastroenterol. 2012;46:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Egea Valenzuela J, Belchí Segura E, Sánchez Torres A, Carballo Alvarez F. Acute pancreatitis associated with hypercalcemia. A report of two cases. Rev Esp Enferm Dig. 2009;101:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Tun-Abraham ME, Obregón-Guerrero G, Romero-Espinoza L, Valencia-Jiménez J. [Acute pancreatitis associated with hypercalcaemia]. Cir Cir. 2015;83:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Gerasimenko JV, Peng S, Tsugorka T, Gerasimenko OV. Ca2+ signalling underlying pancreatitis. Cell Calcium. 2018;70:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10:283-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Otsuka Y, Kamata K, Minaga K, Takenaka M, Watanabe T, Kudo M. Acute Pancreatitis with Disturbed Consciousness Caused by Hyperparathyroidism. Intern Med. 2018;57:3075-3078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Biondi A, Persiani R, Marchese M, Cananzi F, D'Ugo D. Acute pancreatitis associated with primary hyperparathyroidism. Updates Surg. 2011;63:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Yang J, Dong MJ, Chen F. A rare lethal case of severe acute necrotizing pancreatitis due to a parathyroid adenoma in a third-trimester pregnant woman. BMC Endocr Disord. 2019;19:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ma YB, Hu J, Duan YF. Acute pancreatitis connected with hypercalcemia crisis in hyperparathyroidism: A case report. World J Clin Cases. 2019;7:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 11. | Bansal S, Kaushik RM, Kaushik R, Modi S, Raghuvanshi S, Kusum A. Primary hyperparathyroidism presenting as severe hypercalcemia with acute pancreatitis in pregnancy. Gynecol Endocrinol. 2020;36:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Danne PD, Buls JG, Connell J, Bennett RC. A case of pancreatitis associated with gastrinoma. Aust N Z J Surg. 1985;55:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Yamamoto M, Mine H, Maehara Y, Sugimachi K. The Zollinger-Ellison syndrome with acute bleeding pancreatitis. Hepatogastroenterology. 2003;50:430-431. [PubMed] |
| 14. | Baffy G, Boyle JM. Association of Zollinger-Ellison syndrome with pancreatitis: report of five cases. Dig Dis Sci. 2000;45:1531-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Belhassen-García M, Velasco-Tirado V, Carpio-Pérez A, Soler-Fernández MC, López-Bernús A, Pardo-Lledias J, Fuentes-Pardo L, Iglesias-Gómez A. [Acute pancreatitis and obstructive jaundice secondary to metastases from lung cancer]. Gastroenterol Hepatol. 2009;32:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Saliba WR, Dharan M, Bisharat N, Elias M. Eosinophilic pancreatic infiltration as a manifestation of lung carcinoma. Am J Med Sci. 2006;331:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Akinosoglou K, Siagris D, Geropoulou E, Kosmopoulou O, Velissaris D, Kyriazopoulou V, Gogos C. Hyperamylasaemia and dual paraneoplastic syndromes in small cell lung cancer. Ann Clin Biochem. 2014;51:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Casadei Gardini A, Mariotti M, Lucchesi A, Pini S, Valgiusti M, Bravaccini S, Del Monte A, Burgio MA, Marisi G, Amadori D, Frassineti GL. Paraneoplastic lipase and amylase production in a patient with small-cell lung cancer: case report. BMC Cancer. 2016;16:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Leone J, Dehlinger V, Mary V, Malgrange D, Schvartz H, Pennaforte JL, Etienne JC. [Bone localization of Weber-Christian syndrome associated with chronic pancreatitis developing bone metastasis of pancreatic adenocarcinoma]. Ann Med Interne (Paris). 1998;149:305-307. [PubMed] |
| 20. | Tanvetyanon T, Stiff PJ. Recurrent steroid-responsive pancreatitis associated with myelodysplastic syndrome and transformations. Leuk Lymphoma. 2005;46:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Lekakis L, Tryfonopoulos D, Fakinos G, Panopoulos C, Theochari M, Koumakis G, Demiri S, Efremidis A. A case of paraneoplastic autoimmune pancreatitis: mini-review of paraneoplastic syndromes in breast cancer. Anticancer Res. 2012;32:3311-3314. [PubMed] |
| 22. | Miyazaki H, Kuroda K, Fujii M, Shirasaka D, Era Y, Tsuda K, Tanaka S, Nagao K, Kadowaki Y, Okino T. [Intraductal papillary neoplasm of bile duct developed in a patient with IgG4-related sclerosing cholangitis, autoimmune pancreatitis, and myasthenia gravis]. Nihon Shokakibyo Gakkai Zasshi. 2019;116:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Mittra ES, Davidzon G. Case 207: Hodgkin lymphoma with paraneoplastic hypercalcemic pancreatitis. Radiology. 2014;272:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Colaut F, Toniolo L, Sperti C, Pozzobon M, Scapinello A, Sartori CA. Autoimmune-like pancreatitis in thymoma with myasthenia gravis. Chir Ital. 2002;54:91-94. [PubMed] |
| 25. | Tomiyama M, Arai A, Kimura T, Suzuki C, Watanabe M, Kawarabayashi T, Shoji M. Exacerbation of chronic pancreatitis induced by anticholinesterase medications in myasthenia gravis. Eur J Neurol. 2008;15:e40-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Wynn D, Everett GD, Boothby RA. Small cell carcinoma of the ovary with hypercalcemia causes severe pancreatitis and altered mental status. Gynecol Oncol. 2004;95:716-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Seifert G, Seemann N. [Paraneoplastic hypercalcemic syndrome in ovarian carcinoma]. Dtsch Med Wochenschr. 1967;92:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Donovan PJ, Achong N, Griffin K, Galligan J, Pretorius CJ, McLeod DS. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J Clin Endocrinol Metab. 2015;100:2024-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Kearns AE, Thompson GB. Medical and surgical management of hyperparathyroidism. Mayo Clin Proc. 2002;77:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Solimando DA. Overview of hypercalcemia of malignancy. Am J Health Syst Pharm. 2001;58 Suppl 3:S4-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Bilezikian JP, Potts JT Jr, Fuleihan Gel-H, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg SJ, Udelsman R, Wells SA. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353-5361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Phillips CD, Shatzkes DR. Imaging of the parathyroid glands. Semin Ultrasound CT MR. 2012;33:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Fang SH, Lal G. Parathyroid cancer. Endocr Pract. 2011;17 Suppl 1:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Rodrigo JP, Hernandez-Prera JC, Randolph GW, Zafereo ME, Hartl DM, Silver CE, Suárez C, Owen RP, Bradford CR, Mäkitie AA, Shaha AR, Bishop JA, Rinaldo A, Ferlito A. Parathyroid cancer: An update. Cancer Treat Rev. 2020;86:102012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Muggia FM. Overview of cancer-related hypercalcemia: epidemiology and etiology. Semin Oncol. 1990;17:3-9. [PubMed] |
| 36. | Motilal Nehru V, Garcia G, Ding J, Kong F, Dai Q. Humoral Hypercalcemia in Uterine Cancers: A Case Report and Literature Review. Am J Case Rep. 2017;18:22-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Gaur S. Sarcoidosis manifested as hypercalcemic pancreatitis. South Med J. 2001;94:939-940. [PubMed] |