Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8135
Peer-review started: April 15, 2021
First decision: June 15, 2021
Revised: June 20, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: September 26, 2021
Processing time: 154 Days and 1 Hours
Mucinous gastric carcinoma (MGC) is a rare histological type of gastric car
A 61-year-old man was admitted to our hospital in May 2020 because of a large, tender abdominal mass. Abdominal CT showed diffuse, irregular thickening of the gastric walls, with miliary and punctate calcifications. There were metastases to the perigastric and retroperitoneal lymph nodes and also peritoneal seeding. Histological examination of a specimen obtained by endoscopic biopsy showed poorly differentiated calcified signet-ring cell gastric cancer. The patient was clinically staged with T4N+M1 disease. He was treated with docetaxel, cisplatin, and fluorouracil as first-line therapy, irinotecan combined with S-1 as second-line chemotherapy, and programmed cell death protein 1 as third-line therapy. The patient underwent a total of nine cycles of chemotherapy. Follow-up CT scans every 3 mo showed continually increasing calcifications. As of this writing, the patient has survived almost 1 year.
In this case report, we describe the histopathological and imaging characteristics of a patient with gastric cancer receiving chemotherapy. Multiple punctate calcifications were seen, which gradually increased during chemotherapy. Several possible mechanisms for the calcifications are described, but further research is needed. Future findings may lead to new approaches for the evaluation and treatment of such tumors.
Core Tip: In this report we present a case of gastric mucinous adenocarcinoma with calcification in the gastric wall on computerized tomography evaluation before and after chemotherapy. We explain not only the different theory of the calcification formation and variety, but also the relationship of calcification with prognosis. We hope the results provide new thoughts on tumor evaluation and treatment in the future.
- Citation: Lin YH, Yao W, Fei Q, Wang Y. Gastric cancer with calcifications: A case report. World J Clin Cases 2021; 9(27): 8135-8141
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8135.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8135
Gastric cancer is the fifth most common cancer worldwide and has recently become the third leading worldwide cause of cancer-related deaths[1]. Mucinous gastric carcinoma (MGC) is a rare histological subtype (3%) of gastric cancer. It is often characterized by substantial mucinous lakes within tumors[2]. Calcifications can be seen in tumors of the thyroid, thymus, pancreas, female reproductive system, and others[3-6]. However, calcifications are infrequent in gastric cancer, where they are seen in less than 3% of patients. Several investigators have reported that calcifications are characteristic computerized tomography (CT) features of patients with MGC, showing high specificity (98.7%) for the diagnosis of MGC[7]. In this report, we present serial CT and histopathological features of an advanced calcified signet-ring cell gastric cancer that responded to chemotherapy.
A 61-year-old man was admitted to our hospital in May 2020 because of a tender abdominal mass of increasing size.
The patient had had symptoms of anorexia and stomach pain for almost 1 year. He had lost about 10 kg of weight in the 3 mo before hospitalization.
The patient had suffered from hypertension for years.
No personal or family history was identified.
Physical examination revealed mild tenderness in the upper abdomen.
The carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (Ca199) levels were positive at 12.41 ng/mL, and 196 U/mL, respectively. Serum calcium and phosphorus levels were within the normal ranges.
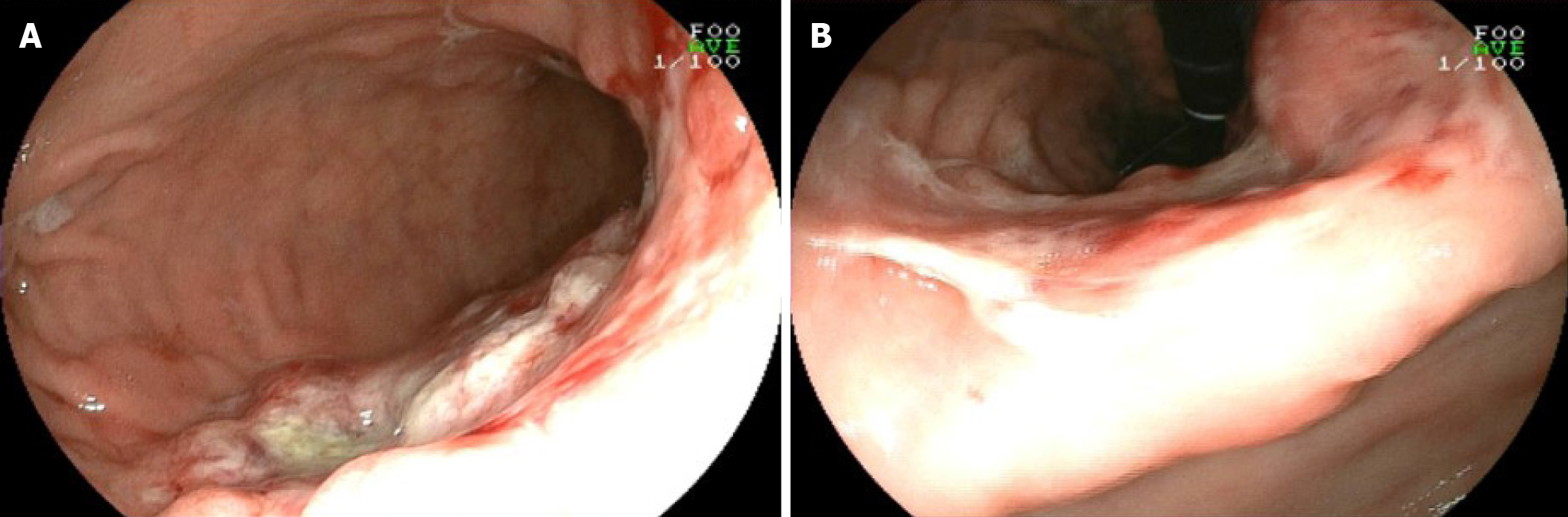
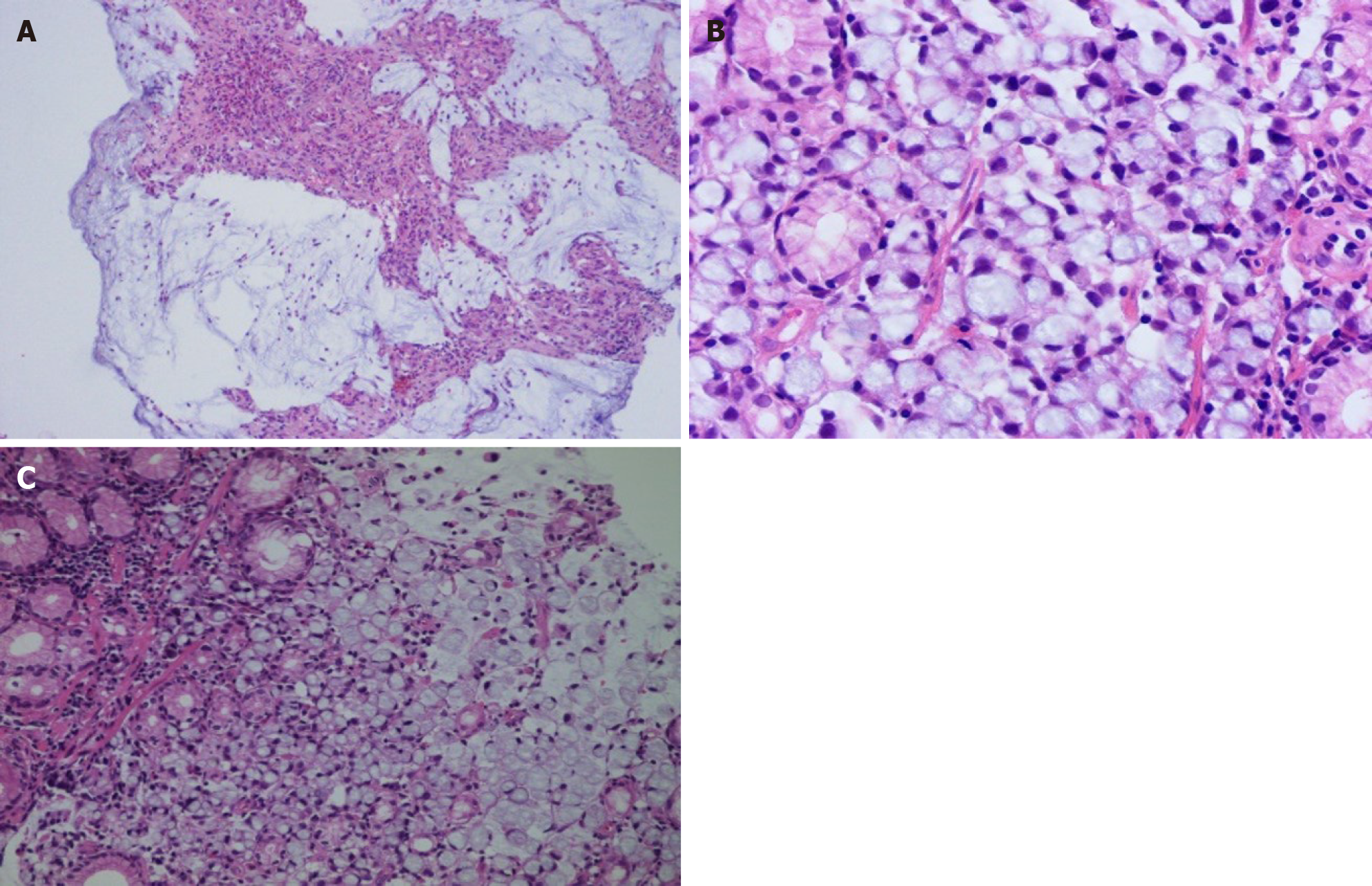
CT of the abdomen showed diffuse and irregular thickening of the gastric wall with miliary and punctate calcifications (Figure 1A). There were metastases to the perigastric and retroperitoneal lymph nodes and peritoneal seeding. Endoscopy and biopsy were performed. Endoscopy showed a protuberant lesion in the posterior wall at the junction of the gastric fundus and body near the cardia. Ulcerative lesions were seen on the side of the protuberant lesion, which extended downward along the anterior wall, lesser curvature, posterior gastric wall to the gastric antrum, and the anterior pylorus (Figure 2). Histologic examination of the specimen revealed a poorly differentiated mucinous adenocarcinoma with scattered signet-ring cells (Figure 3). Immunohistochemistry was positive for mutl homolog 1, postmeiotic segregation increased 2, mutl homolog 2, and mutl homolog 6, and negative for human epidermal growth factor receptor-2, Epstein Barr virus-encoded RNA, and programmed cell death protein ligand 1 expression.
The final diagnosis of the case was poorly differentiated gastric mucinous adenocarcinoma with scattered signet-ring cells (stage IV). The patient was clinically staged to have T4N+M1 disease.
The patient received five cycles of docetaxel, cisplatin, and fluorouracil (DCF). The symptoms associated with the abdominal mass, including anorexia, were relieved. The levels of the CEA and Ca199 tumor markers decreased to 5.95 ng/mL and 87 U/mL, respectively. Abdominal CT showed reduced thickening of the gastric wall. However, in September, after five cycles of DCF chemotherapy, an abdominal CT showed increased thickening of the gastric wall and massive ascites. The patient underwent drainage of the ascites via abdominal puncture and second-line chemotherapy of irinotecan combined with S-1. After four cycles of chemotherapy, the patient's had condition progressed. The patient was started on programmed death-1 (PD1) immunotherapy in December 2020, which is ongoing.
As of this writing, the patient's condition is stable. Over the course of his treatment, the patient's general condition gradually improved, but abdominal CTs revealed progression of the calcifications of the gastric wall after chemotherapy (Figure 1).
Gastric cancer is the second most common cancer and third as the most common cause of cancer-related death in China[8]. Although the morbidity and mortality of gastric cancer has recently decreased, it remains serious public health problem[9]. In China, many patients have an advanced stage of gastric cancer at the initial diagnosis because of poor public health screening programs[10,11].
MGC is a histological type of gastric carcinoma that is classified by the World Health Organization as a poorly differentiated type[12]. It is characterized as a gastric adenocarcinoma with a substantial amount of extracellular mucus (> 50% of tumor volume) within the tumor. Moreover, MGC seems to have a worse prognosis than non-MGC (nMGC), but the mucinous characteristic has not yet been proven to be an independent prognostic marker[13,14].
Calcification in gastric cancer was initially described in 1913 by Gruber et al[15] in Germany. Several other cases were subsequently reported[16-18]. As mentioned previously, calcification can occur in many different tumors, but it is rare in gastric cancer. When present, the tumor is usually proven to be MGC. Our patient was also diagnosed with MGC containing signet-ring cells. It has been reported that the prognosis may be better in patients with MGC and calcifications than in those having gastric cancer without calcification[19].
Based on differences in the pathogenesis, two types of calcification, dystrophic and metabolic, have been described. Dystrophic calcifications are local depositions of calcium salts in necrotic tissue in the presence of a normal serum calcium level. Metabolic calcifications often occur in disorders of mineral metabolism. Dystrophic calcifications are the most frequent in malignant and most benign tumors. Why do the calcifications occur? One theory is that calcification is a result of the degeneration of tumor cells. Factors such as biopsy, chemotherapy, and radiotherapy can lead to ischemia and necrosis. Insufficient blood flow leads to decrease of cellular respiration and carbon dioxide production, resulting in an alkaline environment. Calcium salts precipitate in alkaline solutions, and may be deposited in the necrotic tissue, forming foci of calcification[11]. The other theory is that various factors secreted from the tumor cells or other cells in the tumor microenvironment disturb local calcium metabolism. When calcium metabolism is enhanced, calcium salts gradually accumulate[20].
Calcifications were present in the lesion when the patient was first diagnosed. The calcifications gradually increased during chemotherapy. As mentioned previously, excessive tumor growth at the time of the initial diagnosis may lead to insufficient perfusion of some of the tumor cells, which results in ischemic necrosis. Mucinous adenocarcinomas may also secrete components that interfere with calcium metabolism. With ongoing chemotherapy, more tumor cells undergo ischemic necrosis, leading to increased calcification. The increased calcification may indicate that chemotherapy has been effective. Easson et al[21] reported that the presence of calcifications within a colorectal liver metastasis indicate a markedly improved prognosis. Burkill et al[22] reported that patients with noncalcified ovarian cancers survive longer than the patients with calcified tumors. However, in breast cancer, patients with decreased numbers of calcifications after neoadjuvant treatment had higher rates of complete pathological response compared with patients with no changes in calcification[23,24]. However, whether the degree of calcification is related to the response to chemotherapy and outcome remains unclear. Histological and statistical evidence from a large patient series is needed.
Based on different pathologic characteristics, calcifications can be classified into several types, including mucin pool calcifications, psammomatous calcifications, and heterotopic ossification[25]. Mucin pool calcifications are more frequently seen than the other two types are. As in our patient, they usually appear in advanced diffuse mucinous adenocarcinoma, which usually show miliary and punctate calcifications near the mucin pools. No obvious calcifications were found on hematoxylin-eosin staining of the histopathological preparations. That might partly be accounted for by the fact that the patient did not undergo surgery, and the small specimen that was evaluated was obtained by gastroscopy and was inadequate for a thorough evaluation. Moreover, the initial calcifications were not obvious on the CT scans. Psammomatous calcifications are often seen in nonmucin-producing carcinomas such as renal papillary carcinoma, ovarian serous papillary cystadenoma, and papillary thyroid carcinoma. There have also been a few previous reports of psammomatous bodies observed in ductal adenocarcinomas of the pancreas and gastric carcinomas, in which bone morphogenetic protein (BMP) were found expressed by immunochemistry evaluation[26-29]. The evidence that BMP plays an important role in psammomatous calcifications in other carcinomas is not adequate. Heterotopic ossification refers to calcifications often seen in well-differentiated primary and metastatic adenocarcinomas.
In conclusion, we reported a patient with mucinous gastric adenocarcinoma with signet-ring cell features and calcifications that increased over the course of his illness, both before and after chemotherapy. The increase in calcifications may indicate that the chemotherapy he received was effective and may imply a markedly improved prognosis. We postulated on the mechanisms causing the phenomenon, for which further investigations are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han JH, Toriumi T S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2751] [Article Influence: 550.2] [Reference Citation Analysis (5)] |
| 2. | Choi JS, Kim MA, Lee HE, Lee HS, Kim WH. Mucinous gastric carcinomas: clinicopathologic and molecular analyses. Cancer. 2009;115:3581-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Ferreira LB, Gimba E, Vinagre J, Sobrinho-Simões M, Soares P. Molecular Aspects of Thyroid Calcification. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl). 2013;126:2186-2191. [PubMed] |
| 5. | Tsujimae M, Masuda A, Shiomi H, Toyama H, Sofue K, Ueshima E, Yamakawa K, Ashina S, Yamada Y, Tanaka T, Tanaka S, Nakano R, Sato Y, Ikegawa T, Kurosawa M, Fujigaki S, Kobayashi T, Sakai A, Kutsumi H, Zen Y, Itoh T, Fukumoto T, Kodama Y. Significance of pancreatic calcification on preoperative computed tomography of intraductal papillary mucinous neoplasms. J Gastroenterol Hepatol. 2019;34:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Wen J, Miao Y, Wang S, Tong R, Zhao Z, Wu J. Calcification: A Disregarded or Ignored Issue in the Gynecologic Tumor Microenvironments. Int J Gynecol Cancer. 2018;28:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Zhao J, Ren G, Cai R, Chen J, Li H, Guo C, He W, Wu X, Zhang W. Mucinous adenocarcinoma and non-mucinous adenocarcinoma: differing clinicopathological characteristics and computed tomography features in gastric cancer. Oncotarget. 2017;8:45698-45709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Wang SM, Zheng RS, Zhang SW, Zeng HM, Chen R, Sun KX, Gu XY, Wei WW, He J. [Epidemiological characteristics of gastric cancer in China, 2015]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:1517-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Gao K, Wu J. National trend of gastric cancer mortality in China (2003-2015): a population-based study. Cancer Commun (Lond). 2019;39:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 11. | Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J, Xiang M, Chen MM, Liu BY, Lin YZ. Clinicopathological characteristics and computed tomography features of mucinous gastric carcinoma. J Int Med Res. 2011;39:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2355] [Article Influence: 471.0] [Reference Citation Analysis (3)] |
| 13. | Choi MG, Sung CO, Noh JH, Kim KM, Sohn TS, Kim S, Bae JM. Mucinous gastric cancer presents with more advanced tumor stage and weaker β-catenin expression than nonmucinous cancer. Ann Surg Oncol. 2010;17:3053-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Ryu SY, Kim HG, Lee JH, Kim DY. Prognosis of early mucinous gastric carcinoma. Ann Surg Treat Res. 2014;87:5-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Gruber GB. Knochebildung in einem magen karzinom. Beitrage zur Pathologischen Anatomie und zur Allgemeinen Pathologie. 1913;55:368-370. |
| 16. | Kunieda K, Okuhira M, Nakano T, Nakatani S, Tateiwa J, Hiramatsu A, Mizuno T, Shiozaki Y, Sameshima Y. Diffuse calcification in gastric cancer. J Int Med Res. 1990;18:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Aydemir S, Savranlar A, Engin H, Cihan A, Ustündağ Y, Ozer T, Doğan Gün B. Gastric wall calcification in gastric cancer relapse: case report. Turk J Gastroenterol. 2006;17:50-52. [PubMed] |
| 18. | Kaneko M, Namisaki T, Takaya H, Mori H, Kitade M, Okura Y, Seki K, Sato S, Nakanishi K, Kitagawa K, Ozutsumi T, Shimozato N, Kaji K, Otani T, Nakai T, Obayashi C, Mitoro A, Yamao J, Yoshiji H. Calcified mucinous adenocarcinoma of the stomach metastatic to the iris: a case report. J Med Case Rep. 2019;13:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Balestreri L, Canzonieri V, Morassut S. Calcified gastric cancer--CT findings before and after chemotherapy. Case report and discussion of the pathogenesis of this type of calcification. Clin Imaging. 1997;21:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Wang T, Lu ZY, Tu XF, Zhang SH, Huang F, Huang L. Computerized tomography findings in calcified signet-ring gastric cancer receiving chemotherapy: a case report. BMC Cancer. 2018;18:474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Easson AM, Barron PT, Cripps C, Hill G, Guindi M, Michaud C. Calcification in colorectal hepatic metastases correlates with longer survival. J Surg Oncol. 1996;63:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Burkill GJ, Allen SD, A'hern RP, Gore ME, King DM. Significance of tumour calcification in ovarian carcinoma. Br J Radiol. 2009;82:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Golan O, Amitai Y, Menes T. Does change in microcalcifications with neoadjuvant treatment correlate with pathological tumour response? Clin Radiol. 2016;71:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Yim H, Ha T, Kang DK, Park SY, Jung Y, Kim TH. Change in microcalcifications on mammography after neoadjuvant chemotherapy in breast cancer patients: correlation with tumor response grade and comparison with lesion extent. Acta Radiol. 2019;60:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Imai I, Murayama H, Arima S. Heterotopic ossification and psammomatous calcification in gastric carcinoma: case report and review of literature. Acta Pathologica Japonica. 1979;29:975-984. |
| 26. | Ohike N, Sato M, Kawahara M, Ohyama S, Morohoshi T. Ductal adenocarcinoma of the pancreas with psammomatous calcification. Report of a case. JOP. 2008;9:335-338. [PubMed] |
| 27. | Takahashi T, Hatakeyama S, Machida T. Ductal adenocarcinoma of the pancreas with psammomatous calcification: report of a case with immunohistochemical study for bone morphogenetic protein. Pathol Int. 2011;61:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Kawahara K, Niguma T, Yoshino T, Omonishi K, Hatakeyama S, Nakamura S, Hirota S, Motoi M. Gastric carcinoma with psammomatous calcification after Billroth II reconstruction: case report and literature review. Pathol Int. 2001;51:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Nakasone H, Hokama A, Kochi A, Kinjo F, Saito A, Kinjo T, Muto Y. Gastric cancer with calcification. Gastrointest Endosc. 2000;51:721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |