Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7682
Peer-review started: February 23, 2021
First decision: March 25, 2021
Revised: April 2, 2021
Accepted: July 27, 2021
Article in press: July 27, 2021
Published online: September 16, 2021
Processing time: 198 Days and 22.3 Hours
There are no studies on the use of roxadustat in patients on regular peritoneal dialysis in China.
To observe the efficacy and safety of roxadustat in treating renal anaemia in peritoneal dialysis patients.
Patients with renal anaemia who were regularly followed at the Peritoneal Dialysis Center of the First Affiliated Hospital of China Medical University from November 1, 2019 to June 30, 2020 were selected. A before-and-after self-control design was performed to retrospectively analyse the treatment effects on anaemia in patients treated with recombinant human erythropoietin (EPO) and roxadustat.
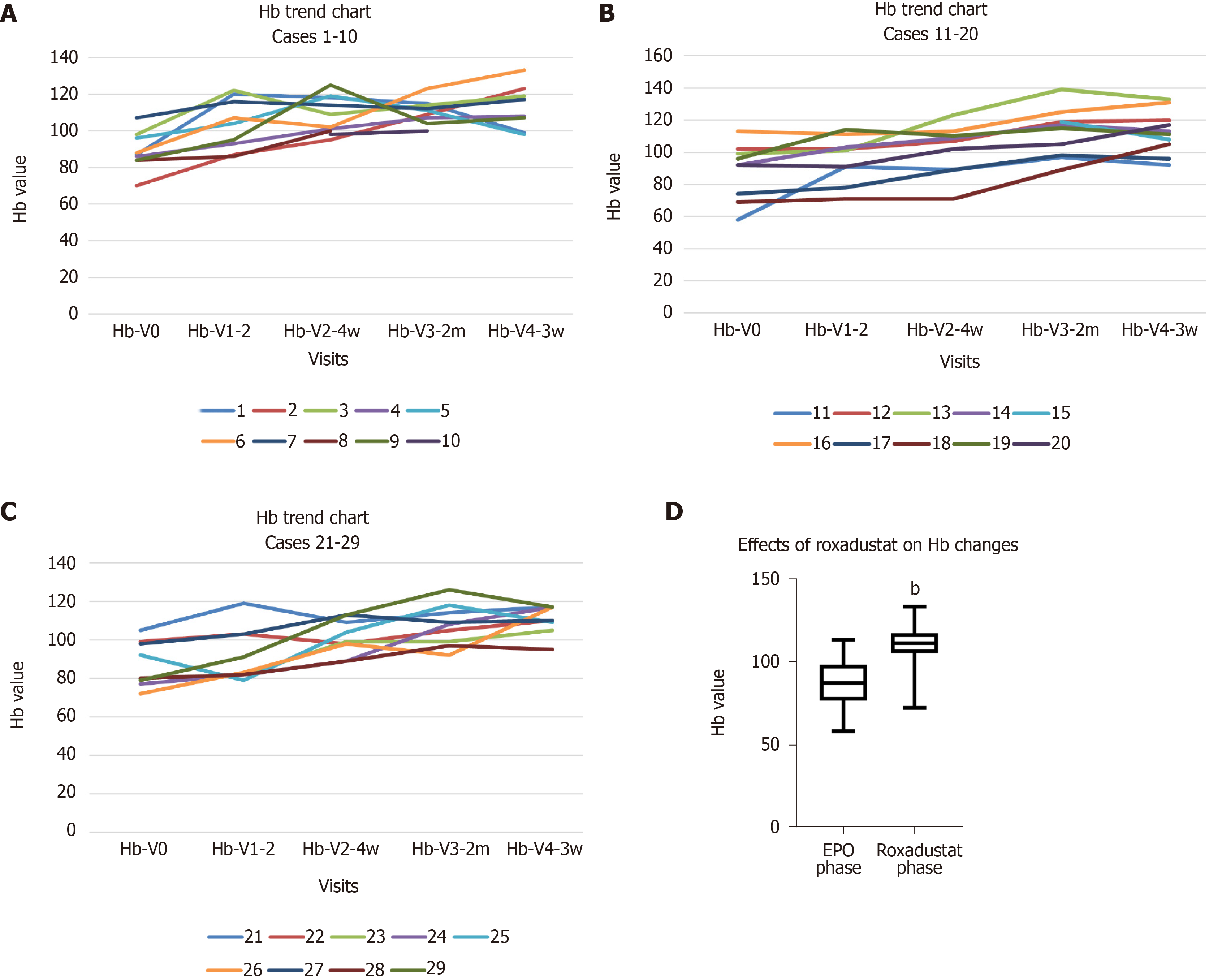
A total of 31 patients with renal anaemia on long-term peritoneal dialysis treated with roxadustat were included. Haemoglobin (Hb) levels were maintained or increased in all patients (100%), and no patients had a decrease in Hb compared with the previous phase. Patients had a mean Hb of 86.2 ± 14.8 g/L with Hb compliance (Hb ≥ 110 g/L) of 16.1% during the EPO phase and a mean Hb of 112.4 ± 18.5 g/L with Hb compliance of 67.7% during the roxadustat phase. No major adverse cardiovascular events occurred in any patient.
The application of roxadustat in peritoneal dialysis patients with renal anaemia can effectively improve the Hb compliance rate.
Core Tip: This was a retrospective study to evaluate the efficacy of roxadustat in the treatment of peritoneal dialysis patients with renal anaemia. A before-and-after self-control design was performed on 31 patients treated with recombinant human erythropoietin (EPO) followed by roxadustat. The haemoglobin compliance rate was 16.1% during the EPO phase and 67.7% during the roxadustat phase. Roxadustat can effectively and safely improve the compliance rate in peritoneal dialysis patients with renal anaemia.
- Citation: Zhu XW, Zhang CX, Xu TH, Jiang GN, Yao L. Efficacy of roxadustat in treatment of peritoneal dialysis patients with renal anaemia. World J Clin Cases 2021; 9(26): 7682-7692
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7682.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7682
The incidence of chronic kidney disease (CKD) in China is 10.8%[1]. Patients with CKD and renal anaemia are highly prevalent, and moderate to severe anaemia is common, which significantly increases the risk of renal deterioration and cardiovascular events in the CKD population[2]. Anaemia not only affects the quality of life of CKD patients but also aggravates other complications. It has been reported as a high-risk factor for cardiovascular and cerebrovascular disease[3,4]. This is more prominent in CKD patients, and correction of anaemia can improve ventricular hypertrophy and reduce cardiovascular and cerebrovascular events in these patients[5].
Currently, the treatment rate of renal anaemia is relatively low, and the compliance rate is only 8.2%[6]. The anaemia non-compliance rate is as high as 60% in China[7], while the non-compliance rate is only 16%-23% in foreign countries[8]. At present, recombinant human erythropoietin (EPO) is mainly used to treat renal anaemia[8]. Unlike haemodialysis patients, who can conveniently use intravenous iron, peritoneal dialysis patients tend to use oral iron agents; therefore, iron deficiency is more prevalent and affects the therapeutic effect in these patients with anaemia[9].
Hypoxia-inducible factor (HIF) is a core transcription factor that regulates hypoxia adaptation, and the prolyl hydroxylase domain (PHD) plays a major role in regulating the HIF oxygen-sensing pathway[10,11]. HIF-1 is composed of two subunits, HIF-1α and HIF-1β, among which HIF-1α is regulated by oxygen[12]. After hypoxia or application of PHD inhibitors or HIF stabilizers, the catalytic function of PHD is inhibited, and HIF-1α is stably expressed, which then translocates to the nucleus, where it binds to the hypoxia response element and exerts its function as a transcription factor. There are many target genes for HIF transcription; therefore, HIF is involved in a wide range of biological functions, including erythrocyte growth, angiogenesis, energy metabolism, iron metabolism, extracellular matrix metabolism, inflammation, and immune regulation[13]. HIF can directly target and regulate the EPO gene and regulate iron metabolism and the status of haematopoietic stem cells by modulating other downstream target genes[14,15].
Roxadustat (HIF prolyl hydroxylase inhibitor, HIF-PHI) is a new generation of oral anaemia treatment drug that can effectively correct renal anaemia in non-dialysis and dialysis patients with CKD[16,17]. However, there are no studies on the use of roxadustat in patients on regular peritoneal dialysis in China. In this study, we retrospectively analysed the therapeutic effect and safety of roxadustat on renal anaemia in peritoneal dialysis patients using a before and after self-control design.
Patients with renal anaemia were regularly followed at the Peritoneal Dialysis Center of the First Affiliated Hospital of China Medical University from January to September 2020. Our study was approved by the Ethics Committee of The First Affiliated Hospital of China Medical University, and informed consent was obtained from all patients.
The inclusion criteria were as follows: (1) Regular peritoneal dialysis treatment for more than 3 mo; (2) Concomitant renal anaemia according to the diagnostic criteria for adults living at sea level [haemoglobin (Hb) < 130 g/L in men, < 120 g/L in non-pregnant women, and < 110 g/L in pregnant women]; and (3) Complete clinical data. The exclusion criteria were as follows: (1) Recent concomitant other active bleeding disorders; (2) Anaemia due to other causes; (3) Concomitant malignant hypertension; and (4) Concomitant neoplasm.
All selected patients who had been treated with recombinant human EPO were administered with roxadustat to correct anaemia 1 wk after discontinuation of EPO 10000 IU per week and 3 d after discontinuation of EPO 3000 IU per week. The initial dose of roxadustat was 100 mg (taken orally 3 times a week) for dialysis patients weighing less than 60 kg and 120 mg (taken orally 3 times a week) for dialysis patients weighing ≥ 60 kg. Dose reductions were reviewed regularly every 2 wk and monthly during the maintenance period. The dose was adjusted according to the degree of Hb change over the past 4 wk, as described in the drug's instructions.
Iron administration was adjusted according to the Chinese Expert Consensus on the Diagnosis and Treatment of Renal Anemia (2018 Revision)[18], i.e., oral iron supplementation was administered for transferrin saturation (TSAT) ≤ 20% or/and ferritin ≤ 100 μg/L. The regimen was 300 mg per day oral administration of polysaccharide-iron complex. Oral administration of the polysaccharide-iron complex was performed as before for patients with TSAT > 20% and ferritin > 100 μg/L but < 500 μg/L. For patients with ferritin ≥ 500 μg/L, no iron supplementation was given[18].
For patients with primary or associated diseases or chronic renal failure complications, such as hypertension, diabetes, disorders of bone mineral metabolism, ionic disorders or acidosis, volume overload, and cardiovascular and cerebrovascular complications, the medications were adjusted according to the appropriate guidelines or consensus diagnoses.
During the initial phase of treatment with roxadustat, blood pressure was measured daily, and the abdominal dose, retention dose and time, automated peritoneal dialysis regimen, and ultrafiltration volume were recorded in an abdominal diary. Routine blood checks were performed every 2 wk, and renal function, potassium, bicarbonate, calcium, phosphorus, lipids [total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)], liver function, and fasting glucose were reviewed monthly. Uric acid, intact parathyroid hormone (PTH), serum ferritin, transferrin, C-reactive protein (CRP), folic acid, and vitamin B12, and electrocardiograms were reviewed every 3 mo. A self-control design was used to compare changes in these parameters from 3 mo before to 3-6 mo after roxadustat administration.
The patient's age, sex, blood pressure, Hb changes, and EPO dosages were collected 3 mo before and 3-6 mo after roxadustat administration.
The laboratory tests included the following: A routine blood check; renal function, potassium, bicarbonate, calcium, phosphorus, and lipids (TC, TG, LDL-C, and HDL-C); liver function; fasting glucose levels; and uric acid levels were reviewed monthly. Intact PTH, serum ferritin, transferrin, CRP, folic acid, vitamin B12, and an electrocardiogram were reviewed every 3 mo. The Hb compliance rate (percentage of patients with Hb > 110 g/L) was calculated.
Adverse drug reactions and complications were recorded. For example, the duration and magnitude of increases in blood pressure compared with those in the premedication period, hyperkalaemia, thromboembolism, headache, nausea and vomiting, abdominal pain, diarrhoea, upper respiratory tract infection, chest discomfort, weakness, hepatic dysfunction, dizziness, and muscle cramps were evaluated[17]. In addition, the time and frequency of occurrence of remedial anaemia treatments were recorded, including blood transfusions and intravenous iron supplementation.
Statistical analysis was performed using IBM SPSS 25 (SPSS Inc., Chicago, IL, United States) and GraphPad Prism 8.3.0 software (GraphPad Software Inc., La Jolla, CA, United States). Normally distributed continuous variables are expressed as the mean ± SD, and categorical variables are presented as the rate (%). The significance level α was set as 0.05.
A total of 31 patients with renal anaemia on long-term peritoneal dialysis and treated with roxadustat were included. Thirteen (31.9%) of the patients were male, and the mean peritoneal dialysis duration was 16.5 ± 7.2 mo. The causes of end-stage renal disease included 8 cases of diabetic nephropathy, 6 cases of hypertensive renal impairment, 5 cases of glomerulonephritis, 1 case of Alport syndrome, and 11 cases of chronic renal failure of unknown aetiology. The clinical indicators evaluated during follow-up are summarized in Table 1.
| Index | EPO phase | Roxadustat phase |
| Haemoglobin (g/L) | 86.2 ± 14.8 | 112.4 ± 18.5b |
| Hb compliance rate (%) | 16.10% | 67.70% |
| Ferritin (μg/L) | 316 ± 236 | 243 ± 260 |
| Iron (μmol/L) | 12.3 ± 4.4 | 15.3 ± 4.5 |
| Transferrin (mg/dL) | 190 ± 39 | 233 ± 42b |
| TSAT (%) | 19.2 ± 5.7% | 26.5 ± 7.6%a |
| Folic acid (nmol/L) | 19.6 ± 13.7 | 24.7 ± 15.3 |
| Vitamin B12 (pmol/L) | 434 ± 147 | 627 ± 314 |
| TC (mmol/L) | 4.72 ± 1.46 | 4.23 ± 0.83 |
| LDL-C (mmol/L) | 2.81 ± 1.05 | 2.45 ± 0.90 |
| HDL-C (mmol/L) | 0.99 ± 0.36 | 0.88 ± 0.26 |
| TG (mmol/L) | 2.67 ± 2.46 | 2.3 ± 1.44 |
| CRP (mg/L) | 16.1 ± 34.3 | 10.3 ± 11.8 |
| Potassium (mmol/L) | 4.26 ± 1.07 | 4.26 ± 1.11 |
| Bicarbonate radical (mmol/L) | 24.4 ± 4.2 | 25.0 ± 2.2 |
| Calcium (mmol/L) | 2.22 ± 0.18 | 2.24 ± 0.20 |
| Phosphorus (mmol/L) | 2.03 ± 0.49 | 1.99 ± 0.66 |
| Parathormone (pmol/L) | 71.9 ± 62.1 | 41.5 ± 26.3 |
| Albumin (g/L) | 37.2 ± 3.5 | 36.8 ± 3.9 |
| PAB (ml/dL) | 38.7 ± 5.5 | 33.1 ± 6.5* |
| Creatinine (μmol/L) | 1058 ± 311 | 1039 ± 217 |
| Urea (mmol/L) | 23.26 ± 9.13 | 20.64 ± 8.18 |
| Cystatin C (mg/L) | 6.75 ± 1.39 | 7.37 ± 1.20 |
| Mean SBP (mmHg) | 136.2 ± 10.5 | 139.2 ± 7.4 |
| Mean DBP (mmHg) | 87.2 ± 7.5 | 88.1 ± 6.9 |
The average Hb was 92.2 ± 16.4 g/L before the use of roxadustat. All patients were treated with recombinant human EPO at an average dosage of 8698 ± 2432 IU/wk, which was subcutaneously injected with the assistance of the patients or their families. Two patients were withdrawn. The remaining 29 cases were followed for ≥ 3 mo with a mean follow-up of 6.2 ± 3.2 mo. Roxadustat was orally administered 3 times a week at a single dose of 20-150 mg (mean of 86.2 ± 21.3 mg).
The Hb levels were maintained or increased in all patients (100%) compared to those in the previous phase, and no patient had a decrease in Hb level. The Hb trend and the effects of roxadustat on Hb changes are shown in Figure 1. The mean Hb in the EPO phase was 86.2 ± 14.8 g/L, with an Hb compliance rate of 16.1% (5/31 cases). The mean Hb in the roxadustat phase was 112.4 ± 18.5 g/L, with an Hb compliance rate of 67.7% (21/29 cases). If Hb ≥ 100 g/L was used as the compliance criterion[8], the Hb compliance rate was 93.1% (27/29 cases). The Hb levels were increased by an average of 25 ± 12.4 g/L in patients.
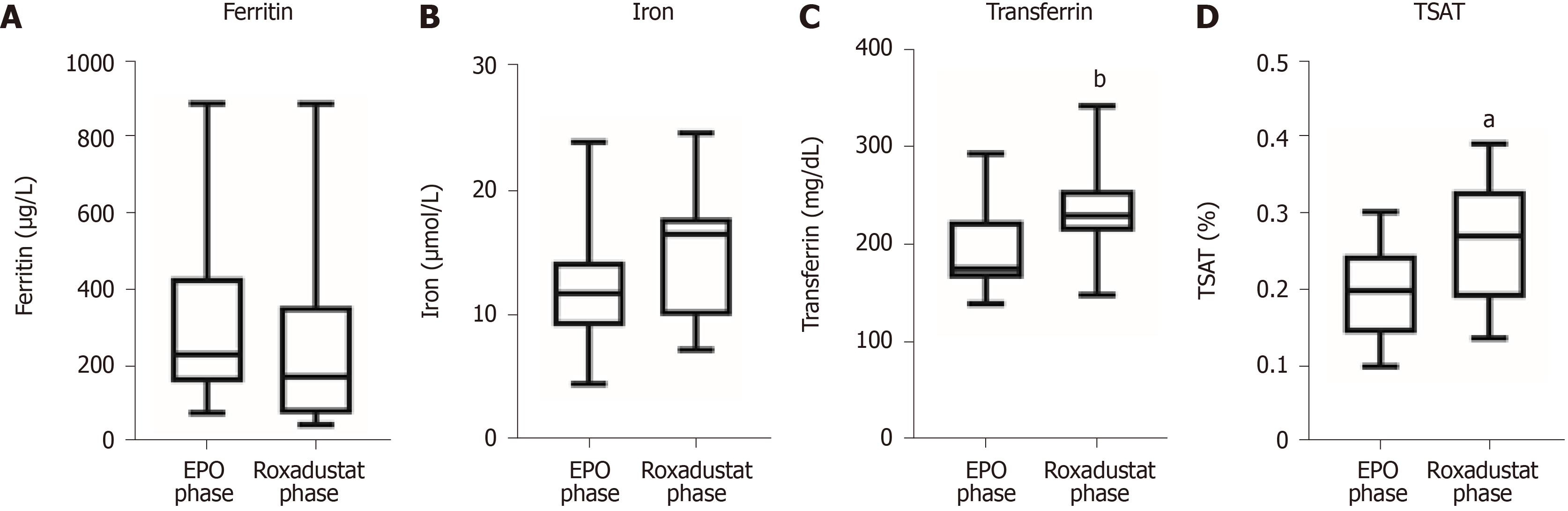
The effects of roxadustat on changes in ferritin, transferrin, and serum iron are demonstrated in Figure 2. Ferritin was 316 ± 236 μg/L in the EPO phase and 243 ± 260 μg/L in the roxadustat phase. A total of 79.3% (23/29) of patients experienced a decrease in ferritin with continuation of previous oral iron supplementation (polysaccharide-iron complex 300 mg once daily); the mean decrease was 57 ± 124 μg/L. Notably, iron deficiency (ferritin < 100 μg/L) was seen in 6 cases before and after administration of roxadustat, and those patients also showed elevated Hb. Serum iron was 12.3 ± 4.4 μmol/L in the EPO phase and 15.3 ± 4.5 μmol/L in the roxadustat phase, and the results indicated that roxadustat promoted iron absorption. In patients with EPO, serum transferrin was 190 ± 39 mg/dL in the EPO phase and 233 ± 42 mg/dL in the roxadustat phase, implying that roxadustat improved the elevation of transferrin. The serum TSAT was 19.2 ± 5.7% in the EPO phase and 26.5 ± 7.6% in the roxadustat phase, which suggested that roxadustat increased TSAT. None of the patients were treated with intravenous iron supplements or blood transfusions. Two patients did not receive iron supplements because their ferritin was above 500 μg/L, and one patient did not receive iron supplements because of iron hypersensitivity. The remaining patients were all treated with polysaccharide-iron complexes, and supplementation was carried out according to the presence of folic acid or vitamin B12 deficiency. Roxadustat was still effective in patients with elevated ferritin, and different decreases in ferritin were observed (601.5 μg/L before and 417 μg/L after using roxadustat in 1 case and 886.8 μg/L before and 818 μg/L after using roxadustat in 1 case).
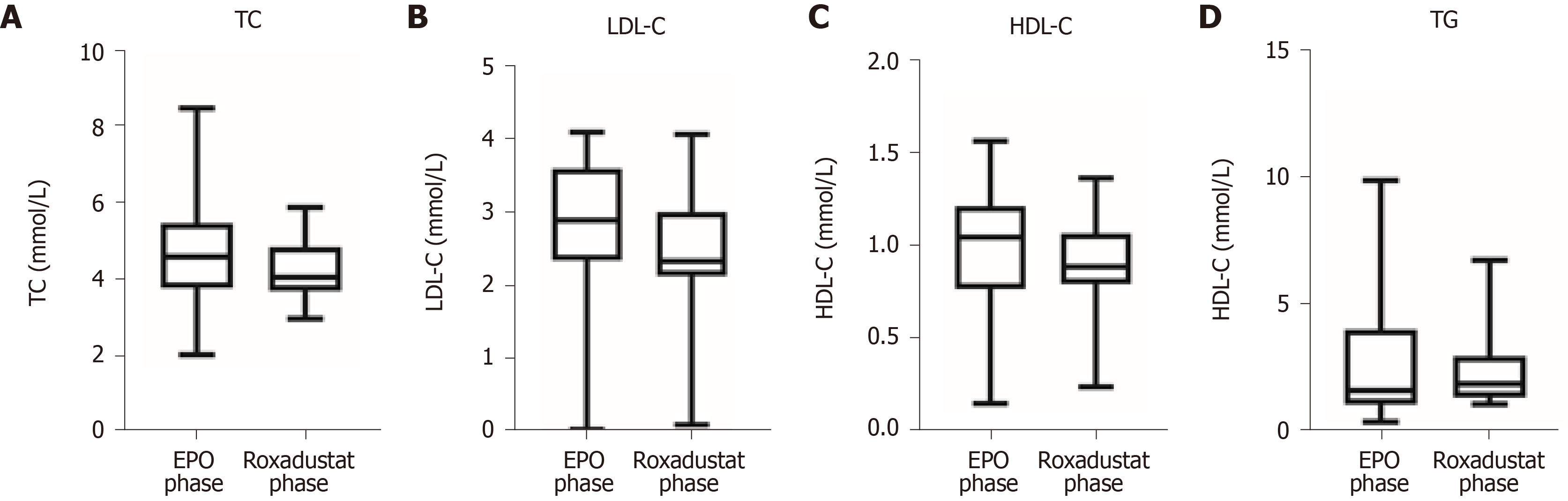
The effects of roxadustat on blood lipids are revealed in Figure 3. TC was 4.72 ± 1.46 mmol/L in the recombinant human EPO phase and 4.23 ± 0.83 mmol/L in the roxadustat phase. LDL-C and HDL-C were 2.81 ± 1.05 mmol/L and 0.99 ± 0.36 mmol/L in the EPO phase and 2.45 ± 0.90 mmol/L and 0.88 ± 0.26 mmol/L in the roxadustat phase, respectively. In addition, TG was 2.67 ± 2.46 mmol/L in the EPO phase and 2.3 ± 1.44 mmol/L in the roxadustat phase. Roxadustat reduced TC, LDL-C, HDL-C, and TG levels on the basis of the original lipid-lowering therapy.
The normal CRP values are 0-6 mg/L. CRP levels were increased in 34.5% (10/29) of the patients during the recombinant human EPO phase. One patient had peritoneal dialysis-associated peritonitis, and an inflammatory state was present. The average CRP level was 30.8 ± 44.0 mg/L, and roxadustat was still effective in increasing Hb levels (from 65 to 119 g/L, with roxadustat maintained at 70 mg). Two patients developed peritoneal peritonitis due to improper operation during medication, including one Neisseria mucosa infection and one Streptococcus dysgalactiae infection, which were cured by vancomycin treatment. Roxadustat continued to increase Hb levels in these two patients. One patient developed external tunnel infection, failed to follow up with a timely exudate culture due to the COVID-19 epidemic, and was cured by oral cefuroxime after local disinfection and a dressing change. Roxadustat continued to increase Hb levels in this patient. The rest of the patients with normal CRP (2.54 ± 2.24 mg/L at the recombinant human EPO phase) did not show an increase after roxadustat administration (2.64 ± 2.55 mg/L).
The mean systolic blood pressure (SBP) was 136.2 ± 10.5 mmHg, and the mean diastolic blood pressure (DBP) was 87.2 ± 7.5 mmHg after application of the recombinant human EPO. The SBP was 139.2 ± 7.4 mmHg, and the DBP was 88.1 ± 6.9 mmHg after the application of roxadustat. There was no significant increase in mean blood pressure. A total of six patients had an increase in blood pressure after application of roxadustat, with a 12.9 ± 7.7 mmHg increase in SBP and 7.2 ± 3.5 mmHg increase in DBP. The blood pressure was mainly increased on the day of application and partially decreased on the next day. The adjusted antihypertensive therapy was effective.
One patient presented with cough, runny nose, and upper respiratory tract infection, which resolved spontaneously. Two patients presented with peritoneal-associated peritonitis, and one presented with external tunnel infection, which was considered to be caused by improper operation and was cured by peritoneal instillation of antibiotics and intensive dressing changes. One diabetic nephropathy patient presented with severely elevated blood glucose and metabolic acidosis with nausea and vomiting, and recovered after hypoglycaemic treatment. One patient presented with facioplegia and recovered after systemic neurological therapy. Patients with changes in condition had clear incentives that were not considered to be drug-related.
The serum potassium level was 4.25 ± 1.07 mmol/L during the recombinant human EPO phase. Two patients had hyperkalaemia (potassium ≥ 5.5 mmol/L). The average potassium was 4.26 ± 1.11 mmol/L during the roxadustat phase, and the mean increase in potassium was 0.006 ± 0.04 mmol/L. No hyperkalaemia occurred.
Patients with CKD have impaired oxygen perception pathways, which can affect EPO and iron metabolism in patients with kidney disease[19,20]. HIF-PHI is a rate-limiting enzyme for the degradation of HIF. Upregulation of HIF can promote EPO production, enhance EPO receptor sensitivity, increase iron absorption, improve iron utilization, and improve renal anaemia through multiple targets[19,21-24].
The compliance rate of Hb was only 16.1% (5/31 cases) during the EPO phase because of a selection bias. The patients who were recommended to use roxadustat in the early stage were mostly patients with severe anaemia because of the poor effect of EPO. Some patients switched to roxadustat at a later stage because it was more convenient to administer the drug orally than to administer EPO subcutaneously. The Hb compliance rate of patients who switched to roxadustat was significantly higher than that of patients using EPO.
Patients on peritoneal dialysis have elevated levels of hepcidin, which affects iron absorption and utilization[25]. In the present study, hepcidin testing was not performed due to the limited laboratory conditions. However, the Hb level was effectively increased in patients with mild iron deficiency prior to administration of roxadustat, even without iron supplementation. Ferritin was decreased in most patients who continued with the original oral iron supplementation regimen, indicating a significant depletion of iron when roxadustat promotes a rapid increase in Hb. Nevertheless, the relationship between the rate of increase in Hb and the rate of decrease in ferritin was inconsistent, which might be due to the different absorption efficiencies of oral iron by patients. In patients who did not receive intravenous iron supplementation and did not increase the oral iron dose, the serum iron, transferrin, and TSAT were all increased, indicating that roxadustat did increase iron absorption and improve iron utilization. This may occur through inhibition of hepcidin and upregulation of intestinal iron transport-related proteins, such as divalent metal transport protein 1 and duodenal cytochrome B[21]. However, iron deficiency can significantly affect the effect of EPO, and severe iron deficiency also affects the rate of Hb elevation after reaching maintenance with roxadustat.
Patients on peritoneal dialysis have varying degrees of inflammation, which can aggravate anaemia by affecting EPO production, downregulating its sensitivity, and inhibiting the absorption and utilization of iron[26,27]. Studies in China on non-dialysis and maintenance dialysis patients have shown that roxadustat can still effectively increase the Hb level of patients in an inflammatory state. In addition, Hb levels were significantly higher in the elevated CRP group than in the normal CRP group, whereas the dose of roxadustat required to maintain Hb within the standard range was lower than that in the normal CRP group[16,17]. Roxadustat was still effective in two cases of peritonitis, one case of extra-tubular infection, and one case of upper respiratory tract infection in our study. These results indicated that although the application of EPO was not suitable for patients with concomitant active inflammation, roxadustat is still effective when used in conjunction with infection control.
Both a large-scale clinical study in China and a previous Japanese clinical study on peritoneal dialysis patients showed that roxadustat reduced the levels of TC, LDL-C, and TG and slightly lowered the levels of HDL-C[17]. This was consistent with our findings. Nevertheless, the exact mechanism is unclear and may be related to the inhibition of PHD to improve insulin resistance and lower serum insulin levels. Insulin increases the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA), and when PHD inhibition decreases serum insulin levels, the activity of HMG-CoA is subsequently decreased, resulting in a decrease in hepatic cholesterol synthesis[28]. Increased hepatic HIF expression can directly inhibit the expression of sterol-regulatory element binding proteins (sREBP) in the endoplasmic reticulum, and increased levels of sREBP can cause an increase in fatty acid synthesis; therefore, HIF-PHI may delay atherosclerosis[29]. The lipid-lowering mechanism of roxadustat needs to be further investigated.
At present, the treatment of anaemia in peritoneal dialysis patients is still dominated by EPO-stimulating agents. Although it can significantly improve the Hb level and quality of life of CKD patients, it will also increase some risks, especially for CKD patients with tumours, diabetes, or cardiovascular diseases[30]. Intravenous iron supplementation is significantly less common in peritoneal dialysis patients than in haemodialysis patients. Oral iron combined with oral roxadustat is more convenient for peritoneal dialysis patients; thus, compliance is higher. No major adverse cardiovascular events occurred in any patient, which was consistent with other studies[17,31].
Our research was a retrospective study, and therefore, EPO concentrations, iron transport-related proteins, and HMG-CoA were not measured. The upregulation of EPO expression and EPO receptor sensitivity, as well as the effects of roxadustat on iron absorption efficiency and lipids, are all indirect evidence. Our team is refining the measurement of the above indicators to support the evidence and to clarify the mechanism of roxadustat, as well as the use of roxadustat in combination with EPO to verify its effect on EPO receptors.
In summary, peritoneal dialysis patients have a low anaemia compliance rate, and roxadustat is more convenient and can effectively improve the anaemia compliance rate of patients without increasing serious cardiovascular and cerebrovascular adverse events.
There are no studies on the use of roxadustat in patients on regular peritoneal dialysis in China.
The recombinant human erythropoietin (EPO) is mainly used to treat renal anemia in peritoneal dialysis patients, but the treatment compliance rate is not satisfactory.
Roxadustat (hypoxia-inducible factor prolyl hydroxylase inhibitor) is a new generation of oral anaemia treatment drug that can effectively correct renal anaemia in non-dialysis and hemodialysis patients. We aimed to research if roxadustat could further improve the compliance of anemia treatment in peritoneal dialysis patients.
This was a retrospective study to evaluate the efficacy of roxadustat in the treatment of peritoneal dialysis patients with renal anaemia. A before-and-after self-control design was performed on 31 patients treated with recombinant human EPO followed by roxadustat.
The haemoglobin (Hb) compliance rate was 16.1% during the EPO phase and 67.7% during the roxadustat phase. Roxadustat also affected the iron reserves and blood lipids. No major adverse cardiovascular events or exacerbation of hypertension occurred in any patient.
The application of roxadustat in peritoneal dialysis patients with renal anaemia can effectively improve the Hb compliance rate.
Roxadustat could safely and effectively improve the anemia in peritoneal dialysis patients, and the mechanisms underlying the effects on or interactions with lipids, iron reserves, and inflammation require further clarification.
We thank Prof. Yao L for the support of this research.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bello BL, Nakada K S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Li X, Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1541] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 2. | Toft G, Heide-Jørgensen U, van Haalen H, James G, Hedman K, Birn H, Christiansen CF, Thomsen RW. Anemia and clinical outcomes in patients with non-dialysis dependent or dialysis dependent severe chronic kidney disease: a Danish population-based study. J Nephrol. 2020;33:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Yogasundaram H, Chappell MC, Braam B, Oudit GY. Cardiorenal Syndrome and Heart Failure-Challenges and Opportunities. Can J Cardiol. 2019;35:1208-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 525] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 5. | Hayashi T, Joki N, Tanaka Y, Hase H. Anaemia and early phase cardiovascular events on haemodialysis. Nephrology (Carlton). 2015;20 Suppl 4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Li Y, Shi H, Wang WM, Peng A, Jiang GR, Zhang JY, Ni ZH, He LQ, Niu JY, Wang NS, Mei CL, Xu XD, Guo ZY, Yuan WJ, Yan HD, Deng YY, Yu C, Cen J, Zhang Y, Chen N. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: First multicenter, cross-sectional study. Medicine (Baltimore). 2016;95:e3872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Zhou QG, Jiang JP, Wu SJ, Tian JW, Chen JH, Yu XQ, Chen PY, Mei CL, Xiong F, Shi W, Zhou W, Liu XS, Sun SR, Xie D, Liu J, Xu X, Hou FF. Current pattern of Chinese dialysis units: a cohort study in a representative sample of units. Chin Med J (Engl). 2012;125:3434-3439. [PubMed] |
| 8. | Perlman RL, Zhao J, Fuller DS, Bieber B, Li Y, Pisoni RL, Robinson BM, Johnson DW, Kawanishi H, Davies SJ, Schreiber MJ, Perl J. International Anemia Prevalence and Management in Peritoneal Dialysis Patients. Perit Dial Int. 2019;39:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Zeidan A, Bhandari S. Anemia in Peritoneal Dialysis Patients; Iron Repletion, Current and Future Therapies. Perit Dial Int. 2017;37:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Liao C, Zhang Q. Understanding the Oxygen-Sensing Pathway and Its Therapeutic Implications in Diseases. Am J Pathol. 2020;190:1584-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Schödel J, Ratcliffe PJ. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. 2019;15:641-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 12. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4719] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 13. | Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 862] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 14. | Tanaka T, Nangaku M. Recent advances and clinical application of erythropoietin and erythropoiesis-stimulating agents. Exp Cell Res. 2012;318:1068-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Liu J, Zhang A, Hayden JC, Bhagavathula AS, Alshehhi F, Rinaldi G, Kontogiannis V, Rahmani J. Roxadustat (FG-4592) treatment for anemia in dialysis-dependent (DD) and not dialysis-dependent (NDD) chronic kidney disease patients: A systematic review and meta-analysis. Pharmacol Res. 2020;155:104747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med. 2019;381:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 17. | Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Cai GY, Hao L, Leong R, Liu C, Neff T, Szczech L, Yu KP. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 18. | Expert Group of Chinese Medical Association Nephrology Branch for diagnosis and treatment of renal anemia. Chinese expert consensus on diagnosis and treatment for renal anemia (2018 revision). Zhonghua Shenzangbing Zazhi 2018; 34: 860-867 . [DOI] [Full Text] |
| 19. | Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21 Suppl 1:S6-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 20. | Jelkmann W. Physiology and pharmacology of erythropoietin. Transfus Med Hemother. 2013;40:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Joharapurkar AA, Pandya VB, Patel VJ, Desai RC, Jain MR. Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases. J Med Chem. 2018;61:6964-6982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Anderson ER, Xue X, Shah YM. Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis. J Biol Chem. 2011;286:19533-19540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055-20062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 310] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu KH, Valone FH. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Niikura T, Maruyama Y, Nakashima S, Matsuo N, Tanno Y, Ohkido I, Yokoyama K, Yamamoto H, Yokoo T. Hepcidin/Ferritin Ratios Differ Among Non-Dialyzed Chronic Kidney Disease Patients, and Patients on Hemodialysis and Peritoneal Dialysis. Ther Apher Dial. 2019;23:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Lankhorst CE, Wish JB. Anemia in renal disease: diagnosis and management. Blood Rev. 2010;24:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Radić J, Bašić-Jukić N, Vujičić B, Klarić D, Radulović G, Jakić M, Jurić K, Altabas K, Grđan Ž, Kovačević-Vojtušek I, Martinović V, Janković N, Gulin M, Ljutić D, Rački S. Anemia Is Correlated with Malnutrition and Inflammation in Croatian Peritoneal Dialysis Patients: A Multicenter Nationwide Study. Perit Dial Int. 2017;37:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, DeBose-Boyd RA. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292:9382-9393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Rahtu-Korpela L, Määttä J, Dimova EY, Hörkkö S, Gylling H, Walkinshaw G, Hakkola J, Kivirikko KI, Myllyharju J, Serpi R, Koivunen P. Hypoxia-Inducible Factor Prolyl 4-Hydroxylase-2 Inhibition Protects Against Development of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Del Vecchio L, Locatelli F. An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2016;15:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther Apher Dial. 2020;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |