Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6943
Peer-review started: May 7, 2021
First decision: June 6, 2021
Revised: June 6, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: August 16, 2021
Processing time: 89 Days and 22.3 Hours
Gastric mucosal hypertrophy, also known as Menetrier's disease (MD), is more common in men over 50 years of age, and the cause is unknown. The symptoms of the disease are atypical, mostly accompanied by hypoproteinemia and edema, and sometimes accompanied by symptoms such as epigastric pain, weight loss, and diarrhea. Most experts believe that the site of the disease is mainly located in the fundus of the stomach and the body of the stomach. We found that the site of the disease in this patient involved the antrum of the stomach.
We introduced the case of a 24-year-old woman who had repeated vomiting for 5 d and was admitted to our hospital. After various examinations such as computed tomography and pathology in our hospital, the final diagnosis of the presented case is MD. The salient feature is that the mucosal folds in the fundus and body of the stomach are huge and present in the shape of gyrus. The greater curvature is more prominent, and there are multiple erosions or ulcers on the folds. The patient did not undergo gastric surgery and did not undergo re-examination. She is drinking Chinese medicine for treatment, and her vomiting and abdominal pain symptoms have improved. This disease is relatively rare in clinical practice, and it is easy to be misdiagnosed as gastric cancer, chronic gastritis and gastric lymphoma, etc.
MD can occur in the antrum, it is necessary to raise awareness of the disease and reduce misdiagnosis.
Core Tip: Giant hypertrophy of the gastric mucosa is a proliferative gastric disease that was first discovered by French pathologist Pierre Ménétrier in an autopsy and reported in 1888, and was named Ménétrier disease.
- Citation: Wang HH, Zhao CC, Wang XL, Cheng ZN, Xie ZY. Menetrier’s disease and differential diagnosis: A case report. World J Clin Cases 2021; 9(23): 6943-6949
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6943.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6943
Giant hypertrophy of the gastric mucosa is a proliferative gastric disease that was first discovered by French pathologist Pierre Ménétrier in an autopsy and reported in 1888, and was named Ménétrier disease (MD). Dr. Ménétrier reported four cases of MD, which were mainly located in the fundus and body of the stomach, two of which were combined with gastric cancer[1-3]. Therefore, he believed that the disease involved the stomach and the fundus of the stomach, and the antrum was preserved. The disease has a certain connection with gastric cancer[4,5]. With the continuous reports of cases, we have found that a few cases can involve the gastric antrum. For example, the lesions in this patient are mainly located in the fundus and body of the stomach, and the antrum is also involved. Pathologically, MD mainly manifests as the proliferation of epithelial cells in the gastric mucosa, the deepening of the gastric pit, the proliferation of mucous cells in the pit, the atrophy of the glands, and the increase in the ratio of gastric pit to glands[6,7].
The patient, female, 24 years old, self-reported that she had repeated vomiting without obvious inducement 5 d ago.
The vomit was food, occasionally bloodshot, accompanied by abdominal pain, and alleviated after eating, no acid reflux, no belching. Gastroscopy in the outside hospital indicated that the Gastric occupying lesions. Laboratory examination: Blood and biochemical test index are close to normal, Tumor indicators and coagulation function are negative; Helicobacter pylori staining was positive.
During the course of the disease, there was no fever, no chills, no diarrhea, no hematemesis, no cough or sputum and good spirits, good diet and sleep, normal stools, and no significant weight loss.
No history of cancer in individuals and families.
The skin and sclera were not yellowish, the liver and spleen were not touched under the ribs, the abdomen was soft, no tenderness, no rebound pain, and mobile dullness was negative.
The blood analysis revealed that the average hemoglobin was a mild decreased, the test result was 26.7 pg (normal range 27-34 pg), and the reticulocytes were slightly decreased, the test result was 0.023 × 1012/L (normal range 0.024 × 1012/L-0.084 × 1012/L) Prothrombin and partial thromboplastin times were normal. The blood biochemistries, as well as urine analysis were normal. Electrocardiogram, chest X-ray and arterial blood gas were also normal.
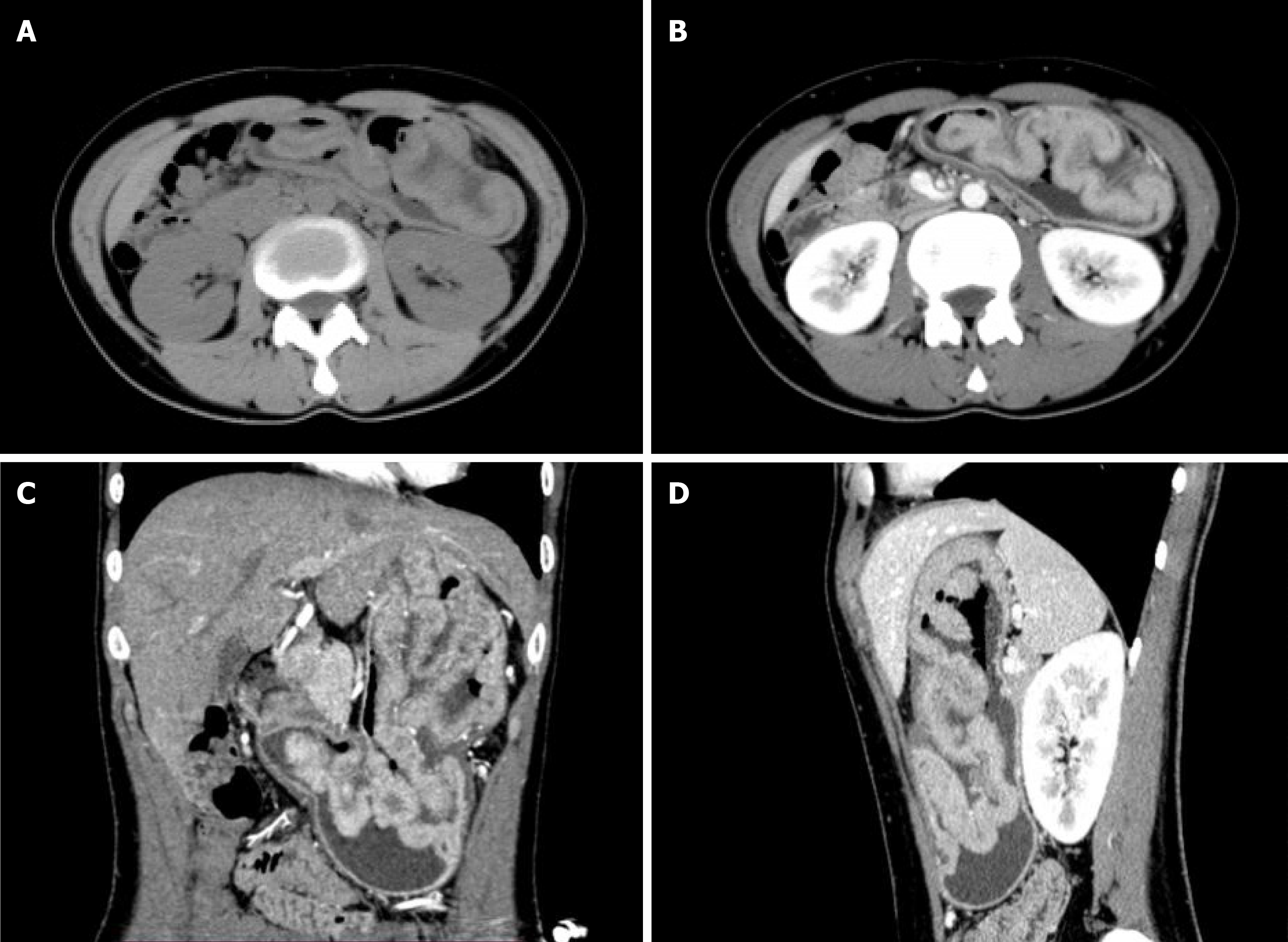
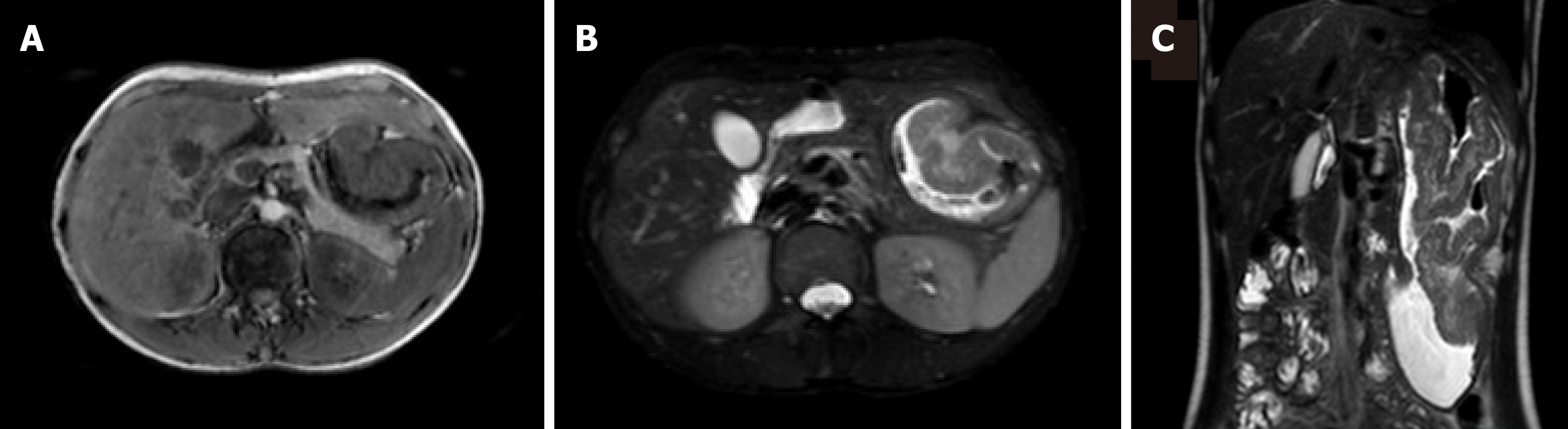
Plain computed tomography(CT) scan and contrast enhancement CT scan examination showed that the gastric body, stomach fundus, and gastric antrum were diffusely and uniformly thickened, showing gyrus-like changes, focusing on the greater curvature side. After enhancement, it was significantly enhanced. The plain scan, arterial phase and venous phase CT values were about 50 HU, 129 HU, and 113 HU, respectively. The serosal surface of the gastric wall showed smoothness and the density of perigastric fat was clear (Figure 1). Magnetic resonance imaging (MRI) showed that the gastric body, fundus and gastric antrum were diffusely and uniformly thickened, showing slightly longer T1 and T2 signal shadows. DWI showed high signal. The measured ADC value ranged from 1.387 × 10-3 mm2/s-1.467 × 10-3 mm2/s. The mucosa showed smoothness (Figure 2).
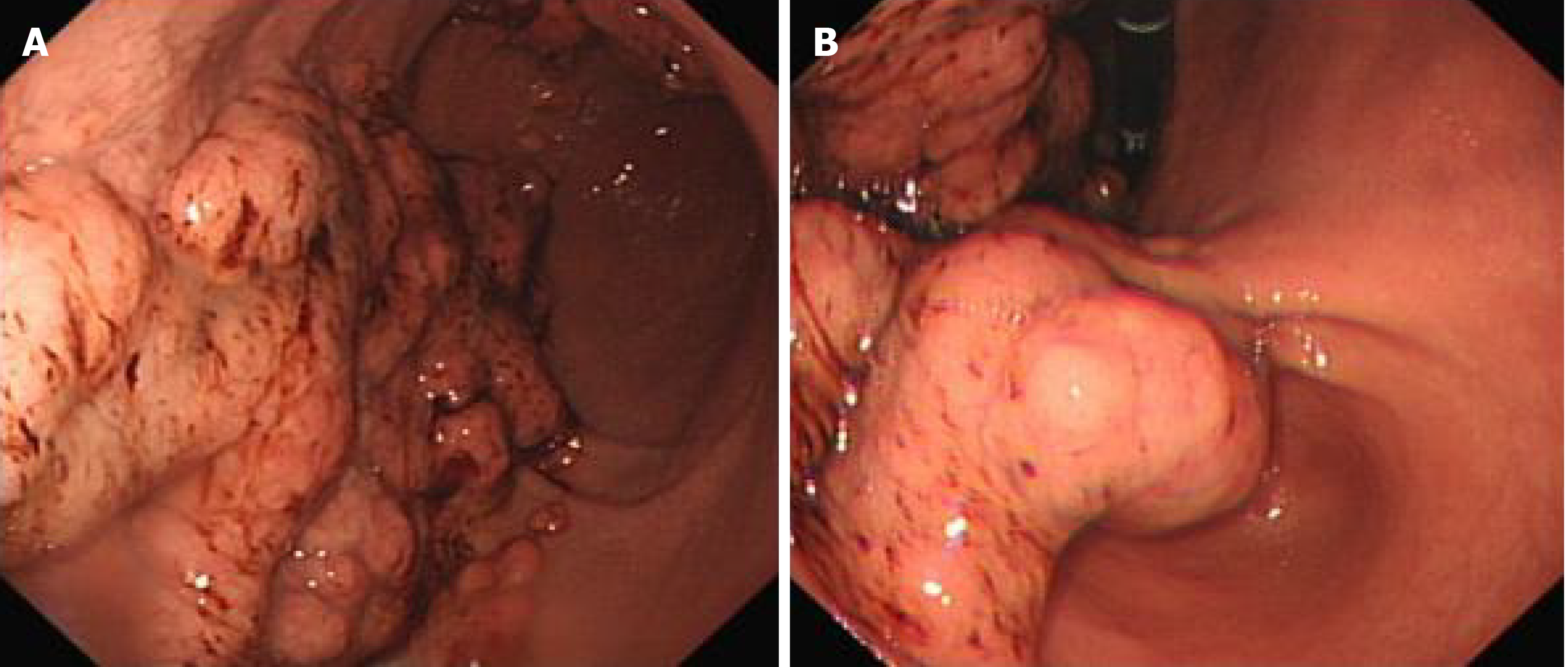
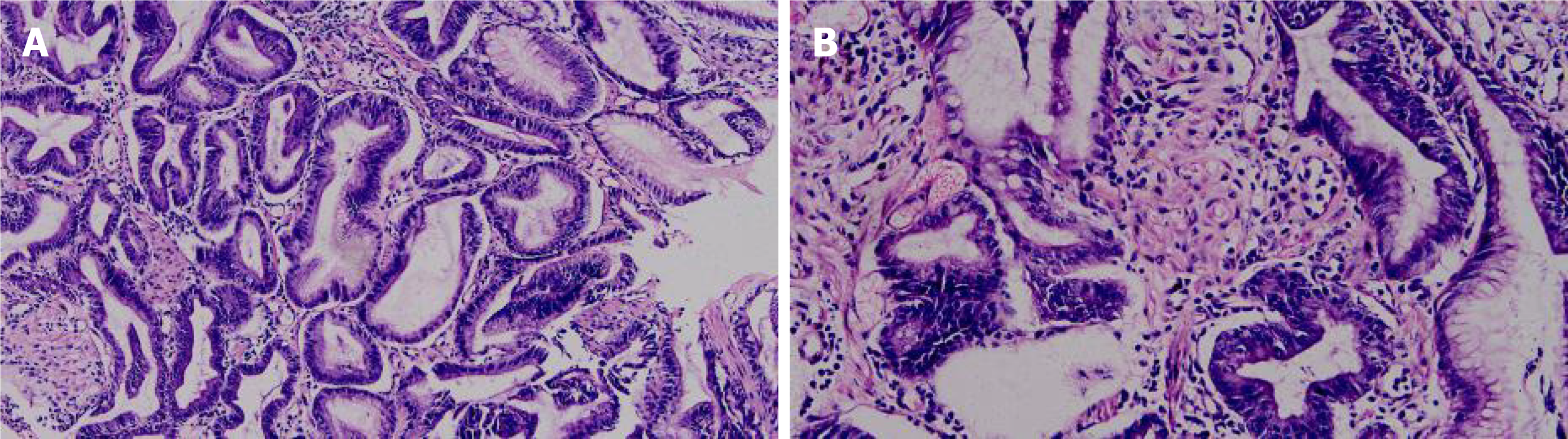
Gastroscopy and pathological: The cardia extends to the stomach, fundus and antrum, with irregular mucosal bulges, medium texture, poor peristalsis, and narrow gastric cavity. The pylorus is round and can be opened and closed (Figure 3). The diagnosis suggests that the Gastric occupying lesions, possibly gastric cancer. The pathological results showed: Chronic inflammation of the gastric mucosa, hyperplasia and lengthening of the gastric pits, and a small number of glands with mild atypical hyperplasia (Figure 4).
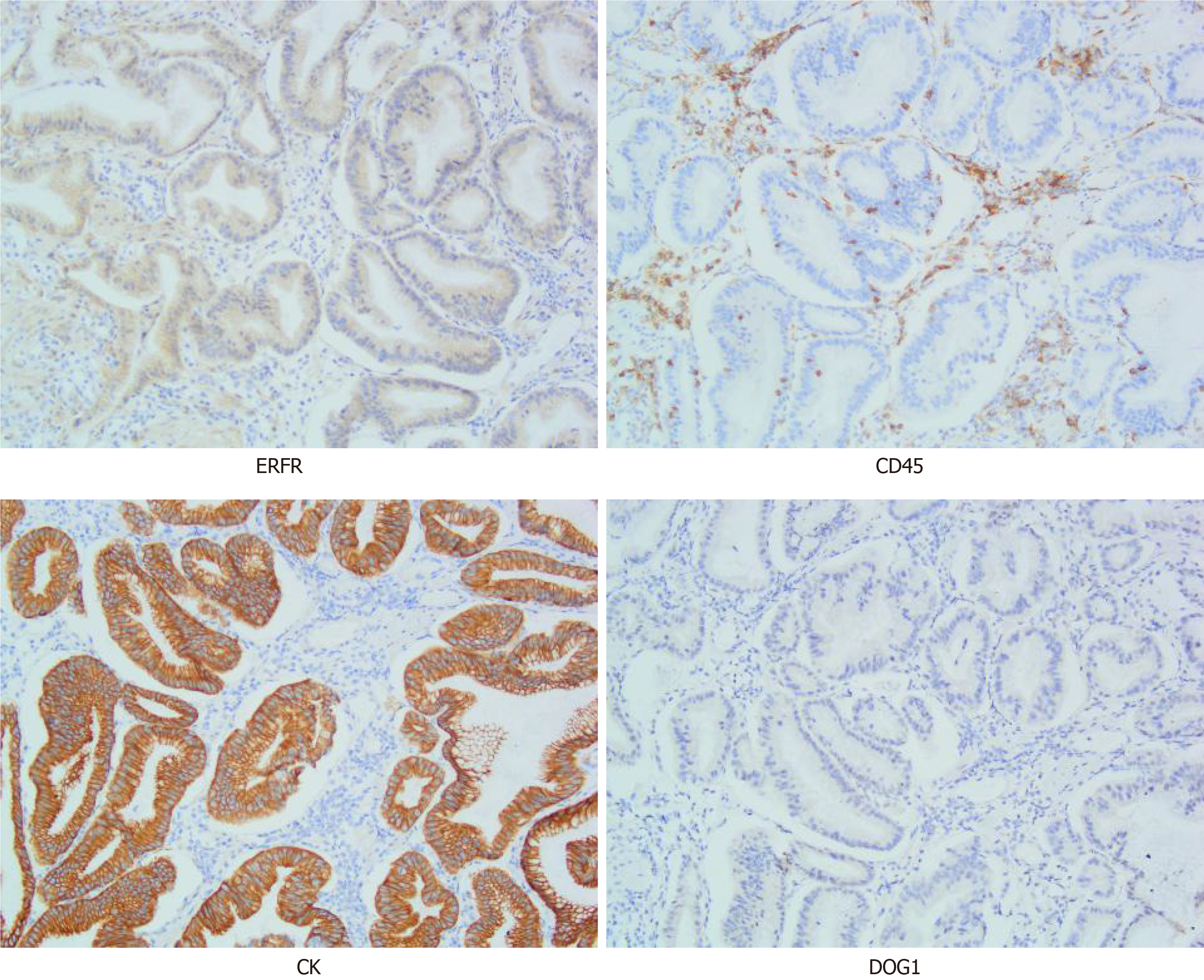
Gastric lymphoma: It is a tumor originating from the lamina propria and submucosal lymphoid tissues of the gastric mucosa. This case is characterized by a small number of CD45-positive lymphocytes scattered among the hyperplastic glands (Figure 5), while the lymphoma is characterized by uniform round tumor cells diffusely distributed under the pathological microscope, so this case does not match.
Gastric cancer: It is a malignant tumor that originates from the gastric mucosa, the structural and cellular atypia of the glands are very obvious, accompanied by obvious invasive growth, so this case does not meet. Figure 5 is an immunohistochemical picture of CK, showing that the hyperplastic glands are arranged regularly.
Gastric polyp: It is a benign tumor derived from gastric epithelium or gastric interstitium. The characteristics of lesion distribution and enhancement characteristics are not consistent with this disease.
Stomach stromal tumor: In gastric stromal tumors, proliferating spindle cells can be seen arranged in bundles and weaves. In this case, only a few proliferating spindle cells are seen between the hyperplastic glands, which is not consistent with this case. The immunohistochemical marker DOG1 was negative (Figure 5), which ruled out the possibility of gastric stromal tumor.
Gastric mucosal prolapse: It refers to the abnormally loose gastric mucosa passing through the pylorus or cardia, protruding into the duodenal ampulla or protruding into the esophagus, which can be distinguished by observation of different positions of upper gastrointestinal angiography.
The final diagnosis of the presented case is MD.
The patient did not undergo gastric surgery and did not undergo re-examination. She is still drinking Chinese medicine for treatment, and her vomiting and abdominal pain symptoms have improved.
The weight of the patient increased by 3-4 kg compared to the previous time when he came to the hospital for treatment. Her vomiting symptoms improved significantly, and she would eat less and more meals in her daily life. The patient will have symptoms of vomiting and abdominal pain in a hungry state. However, the patient herself will pay special attention and try not to let herself be hungry. The patient had undergone an operation due to a hernia deformity under the cerebellar tonsil recently. This is also the reason why she did not go for a review recently. I wonder if these two diseases are related?
According to the age of onset, the disease is divided into child type and adult type. The age of onset of child type is mostly under 10 years old, and about 70% is related to cytomegalovirus infection[8-10]. It is acute and self-limited, usually can heal itself. The clinical manifestations of MD in children are usually characterized by acute systemic edema caused by hypoproteinemia, accompanied by gastrointestinal symptoms such as abdominal pain and vomiting. The adult type is mostly 40-60 years old, and Helicobacter pylori infection may be related to adult type MD. Adult-type lesions are characterized by gradual progress and have the potential to become cancerous[11,12]. The main symptoms are abdominal pain, nausea, vomiting, anemia, hypochlorhydria, and edema of surrounding tissues[13,14]. These clinical symptoms are mainly determined by the pathological basis of the disease. A large number of mucous cells proliferate in the gastric pit, leading to increased mucus protein secretion, resulting in hypoalbuminemia and edema in surrounding tissues[15,16]. The glands shrink, and the secretion of principal cells and parietal cells decreases, resulting in a decrease in gastric acid secretion.
Some scholars found that the expression of transforming growth factor (TGFα) and epidermal growth factor receptor (EGFR) in gastric mucosa of MD patients was higher than that of normal controls, and it was overexpressed in gastric mucosa. TGFα transgenic mice showed some characteristics of MD, so they speculated that the pathogenesis of this disease is caused by the overexpression of TGFα and EGFR. In addition, infection, allergies, poisons and other factors may be related to this disease, and cytomegalovirus and Helicobacter pylori may be the triggering factors of this disease. There are also few reports of familial clusters in the literature. The immunohistochemical picture of this case shows the positive expression of ERFR (Figure 5).
The lesions are mainly seen in the body of the stomach and the bottom of the stomach, with the greater curvature as the side, which can be localized or diffuse, and less involve the gastric antrum. An upper gastrointestinal angiography showed thickening of the gastric mucosa, good or poor gastric motility, soft stomach wall, and delayed emptying. CT or MRI showed that the gastric mucosal folds were thickened and twisted, showing gyrus-like changes. After enhancement, the gastric wall was normal and the serosal surface was smooth. Positron emission tomography-CT showed increased nuclide uptake in the lesion area, which may be caused by chronic inflammation of the mucosa. This disease is relatively rare in clinical practice, and it is easy to be misdiagnosed as gastric cancer, gastric lymphoma, etc. Therefore, it is necessary to raise awareness of the disease and reduce misdiagnosis.
The disease is relatively rare and the cause is unknown. The symptoms and signs are atypical. It should be differentiated from chronic hypertrophic gastritis, gastric polyps, gastric cancer, gastric stromal tumor, gastroptosis, especially gastric lymphoma. Although the mucosa is hugely thickened, the lesions are only limited to the mucosal layer, so the morphology changes after compression, the stomach wall is soft, and emptying is delayed. Further gastroscopy can be performed, when the identification is difficult, and confirming the diagnosis depends on pathological examination. This disease is relatively rare in clinical practice. It is necessary to improve the under
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arshad N S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Li JH
| 1. | Waisberg DR, de Mello ES, Tustumi F, Szor DJ, Charruf AZ, Fuhro FE, Waisberg J, Dias AR. A case report of diffuse hyperplastic gastropathy with multiple polypoid formations in a patient with pernicious anemia, Helicobacter pylori infection, hypergastrinemia and hypoalbuminaemia: Do not forget of Ménétrier's disease. Int J Surg Case Rep. 2020;77:498-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Rallis TS, Patsikas MN, Mylonakis ME, Day MJ, Petanides TA, Papazoglou LG, Koutinas AF. Giant hypertrophic gastritis (Menetrier's-like disease) in an Old English sheepdog. J Am Anim Hosp Assoc. 2007;43:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Juglard R, Rimbot A, Stéphant E, Paoletti H, Talarmin B, Arteaga C. [Gastroduodenal intussusception complicating Menetrier's disease]. J Radiol. 2006;87:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kamal MU, Tariq H, Mehak V, Azam S, Kumar K, Niazi M, Dev A. A Rare Etiology of Abnormally Large Gastric Folds: Menetrier's Disease. Case Rep Gastrointest Med. 2019;2019:7927083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Brautbar A, Paz J, Hadas-Halpern I, Reinus C, Rosenmann E, Zimran A, Elstein D. Menetrier's disease presenting as an acute protein-losing gastroenteropathy in a 27-year-old man with Gaucher disease. Eur J Gastroenterol Hepatol. 2005;17:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kim J, Cheong JH, Chen J, Hyung WJ, Choi SH, Noh SH. Menetrier's disease in korea: report of two cases and review of cases in a gastric cancer prevalent region. Yonsei Med J. 2004;45:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 7. | Hong J, Lee S, Shon Y. Ménétrier's Disease as a Gastrointestinal Manifestation of Active Cytomegalovirus Infection in a 22-Month-Old Boy: A Case Report with a Review of the Literature of Korean Pediatric Cases. Clin Endosc. 2018;51:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Son KH, Kwak JJ, Park JO. A case of cytomegalovirus-negative Ménétrier's disease with eosinophilia in a child. Korean J Pediatr. 2012;55:293-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Akita C, Saikawa Y. Gastric Gyri - Pediatric Ménétrier's Disease. N Engl J Med. 2017;376:774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 10. | Fiori R, Velari L, Di Vito L, Della Gatta F, Bianchi M, Capurso L, Simonetti G. Ménétrier's disease diagnosed by enteroclysis CT: a case report and review of the literature. Abdom Imaging. 2011;36:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Silva PH, Rigo P, Batista RP, Toma RK, Oliveira LA, Suzuki L. Ménétrier's disease associated with gastric adenocarcinoma in a child - imaging aspect. Rev Assoc Med Bras (1992). 2016;62:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Rodríguez Gonzalez O, José R, Génesis J, Luis M, Liumariel V, Raquel F, Alexis S. Robot-assisted laparoscopic gastrectomy for Menetrier's disease. J Robot Surg. 2015;9:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Pryczynicz A, Bandurski R, Guzińska-Ustymowicz K, Niewiarowska K, Kemona A, Kędra B. Ménétrier's disease, a premalignant condition, with coexisting advanced gastric cancer: A case report and review of the literature. Oncol Lett. 2014;8:441-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Di Nardo G, Oliva S, Aloi M, Ferrari F, Frediani S, Marcheggiano A, Cucchiara S. A pediatric non-protein losing Menetrier's disease successfully treated with octreotide long acting release. World J Gastroenterol. 2012;18:2727-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, Piessevaux H, Washington MK, Coffey RJ. Distinguishing Ménétrier's disease from its mimics. Gut. 2010;59:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Parianos C, Aggeli C, Sourla A, Zografos GN. Total gastrectomy for the treatment of Menetrier's disease persistent to medical therapy: A case report. Int J Surg Case Rep. 2020;73:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |