Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6798
Peer-review started: January 13, 2021
First decision: May 11, 2021
Revised: May 24, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 16, 2021
Processing time: 204 Days and 4.3 Hours
Psoriasis is a chronic autoimmune disease that usually manifests as a red scaly epidermis, induration, and hyperproliferation of basal keratinocytes. About 2% of the world’s population suffers from psoriasis but there are no clear therapeutics yet. Recently, mesenchymal stem cells (MSCs) have been regarded as a thera
The patient was a 47-year-old male, diagnosed with psoriasis in 1995. He had received various treatments for 25 years, but the psoriatic condition was not significantly improved. He was given three rounds of minimally manipulated umbilical cord-derived MSCs over 2 wk. The erythema gradually disappeared. Three months after the 1st round, all erythema completely disappeared, and the psoriasis did not recur.
Minimally manipulated umbilical cord-derived MSC transplantation can potentially treat patients who suffer from psoriasis.
Core Tip: In recent decades, interest in biomedical applications of mesenchymal stem cells (MSCs) has increased due to their anti-inflammatory, immunosuppressive, and immunomodulatory abilities. MSCs have been used to study a variety of human diseases without side effects. The human umbilical cord is an excellent source of MSCs. We sought to improve a patient’s psoriatic condition by using minimally manipulated umbilical cord-derived MSCs. In this case study, we report the efficacy of the minimally manipulated umbilical cord-derived MSC therapy to treat a patient with psoriasis.
- Citation: Ahn H, Lee SY, Jung WJ, Pi J, Lee KH. Psoriasis treatment using minimally manipulated umbilical cord-derived mesenchymal stem cells: A case report. World J Clin Cases 2021; 9(23): 6798-6803
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6798.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6798
Psoriasis is an incurable autoimmune disease exhibiting a wide spectrum of clinical symptoms. Psoriasis typically presents with a red scaly epidermis, induration, and hyperproliferation of basal keratinocytes[1]. This serious skin condition most commonly occurs on the head, upper limbs, hands, trunk, and lower limbs, though it can appear on any location. About 2% of the world’s population suffers from psoriasis[1]. Despite the wide variety of treatment options available to treat psoriasis, there are currently no definitive treatment methods[2-4]. Therefore, there is a need for a treatment method that can replace the existing treatment methods.
Previous studies about the effectiveness of mesenchymal stem cells (MSCs) for patients with autoimmune diseases showed positive results because they have anti-inflammation and immunomodulation[5-8]. For this reason, studies using MSCs to treat psoriasis have been conducted[9-12]. As a result, the possibility of treating psoriasis using MSCs has been demonstrated. Previous case reports have shown that psoriasis did not recur after MSC treatment[11,12].
In this study, we present a safer and more effective treatment method than previous studies. It is well known that genetic mutations and aging-related modifications increase as the MSCs are cultured[13-15]. These results show the increasing occurrence of various risks, including tumor formation, and decreasing treatment effect. Therefore, we treated psoriasis using uncultured, minimally manipulated umbilical cord-derived (MM-UC)-MSCs. We evaluated the severity of psoriasis in the patient using the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI)[16]. As a result of three rounds of MM-UC-MSCs transplantation, the PASI and DLQI score of the patient decreased from 9.9 to 1.7 and from 27 to 3, respectively. We confirmed that there were no side effects and no relapse during the 5-mo follow-up period. Therefore, in this case study, we report the efficacy of MM-UC-MSC therapy for a psoriasis patient.
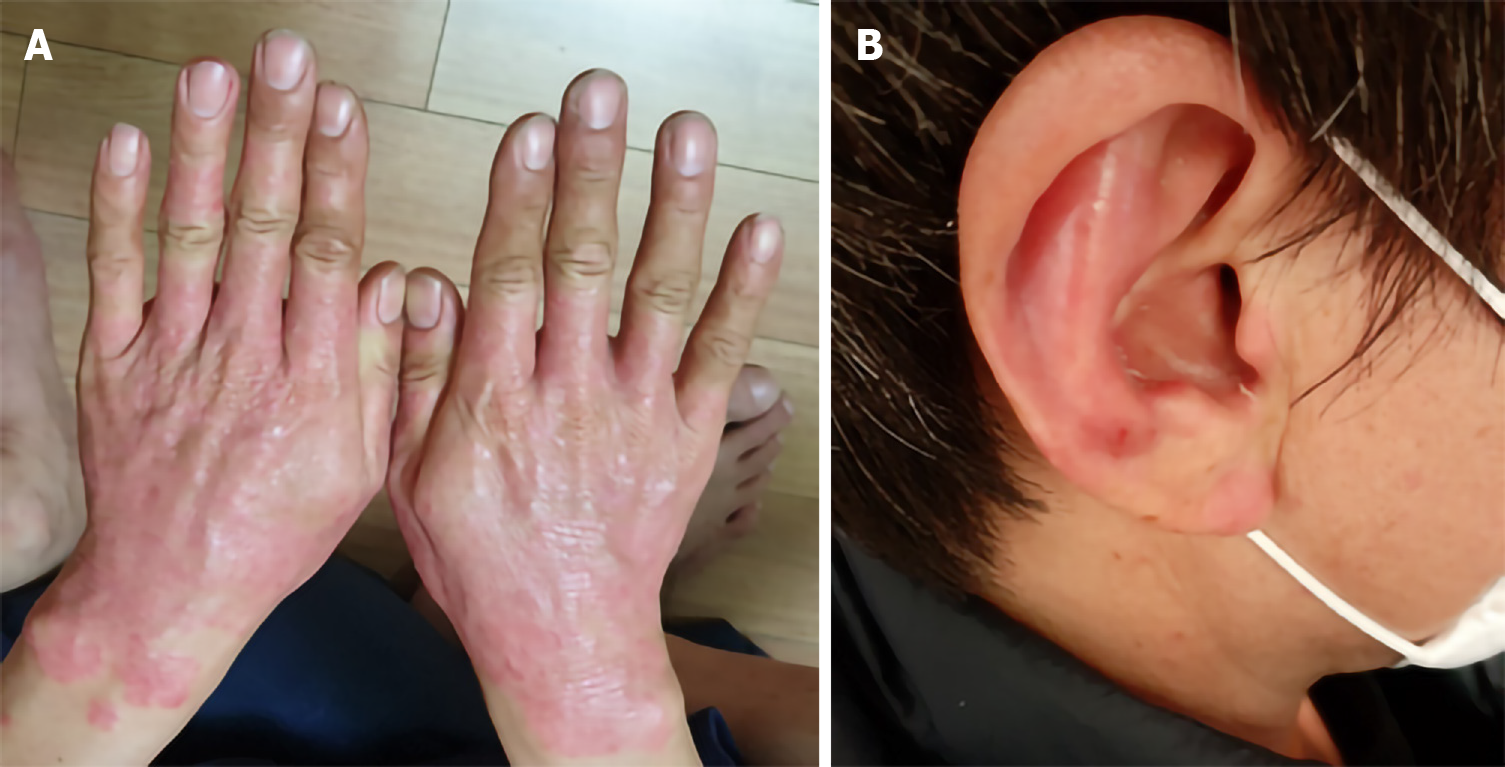
On April 1, 2020, a 47-year-old man, suffering from psoriasis, visited our clinic. The patient had inflammation on the fingers, the backs of the hands, both wrists, and both ears. Erythema was widely spread on both hands, all fingers, and both wrists. Erythema was also found on the inside of the auricle and on the lower part of the earlobe (Figure 1A and B). Also, he complained of itchiness.
He had been diagnosed with psoriasis in 1995. Subsequently, over the next 25 years, he had received herbal treatment, dermatological laser treatment, and drug treatment.
The patient has been no specific disease excepted psoriasis.
The patient had no diseases personal and family histories.
When the patient visited the hospital, the lesion area was spread over several parts of the body.
The severity of psoriasis was evaluated using the PASI and DLQI at the time of the patient’s visit. The PASI and DLQI scores were 9.9 and 27, respectively.
The patient was diagnosed with psoriasis based on the PASI score and previous diagnosis results.
Disinfected UCs were donated by the Obstetrics and Gynecology Department at Lynn Woman’s Hospital. Informed consent was obtained from the mothers donating the umbilical cord. Seven blood and urine tests were performed on the mothers to confirm the safety of the UCs. These tests included assessment for hepatitis B surface antigen, hepatitis B surface antibody, hepatitis C antigen, hepatitis C antibody, human immunodeficiency virus, syphilis rapid plasma reagin, and human T-cell lymphotropic virus type I and II antibody.
The donated UCs were isolated, evaluated, and prepared as described in our former work[8,17]. Briefly, the UC tissues were ground and treated with a mixture of collagenase and hyaluronidase. After filtration, the cells were immediately frozen at
For local transplantation, the isolated cells were used at a concentration of 1 × 106 cells/mL in 0.9% physiological saline. For intravenous transplantation, cells were used at a concentration of 3 × 106 cells/mL in 0.9% physiological saline.
In the 1st round, the injection solution containing MM-UC-MSCs was transplanted intravenously and by local transplantation on April 1, 2020. For intravenous transplantation, a 10-mL injection solution containing MM-UC-MSCs was mixed with 100 mL of 0.9% sodium chloride injection, USP. Then, the 110 mL mixture was intravenously transplanted for 1-1.5 h. Immediately after the transplant was over, the same process was repeated once more. In the case of local transplantation, 12 Ultra-FineTM II Insulin Syringes containing the injection solution were prepared. Each syringe containing the injection solution was injected in and around the lesion, at 2 cm intervals with 0.25 mL. The injected volume of injection solution for each hand and wrist was 4 mL, for each ear was 1 mL, and for the face was 2 mL. The 2nd and 3rd rounds were local transplantation only and were performed each at 1 wk intervals.
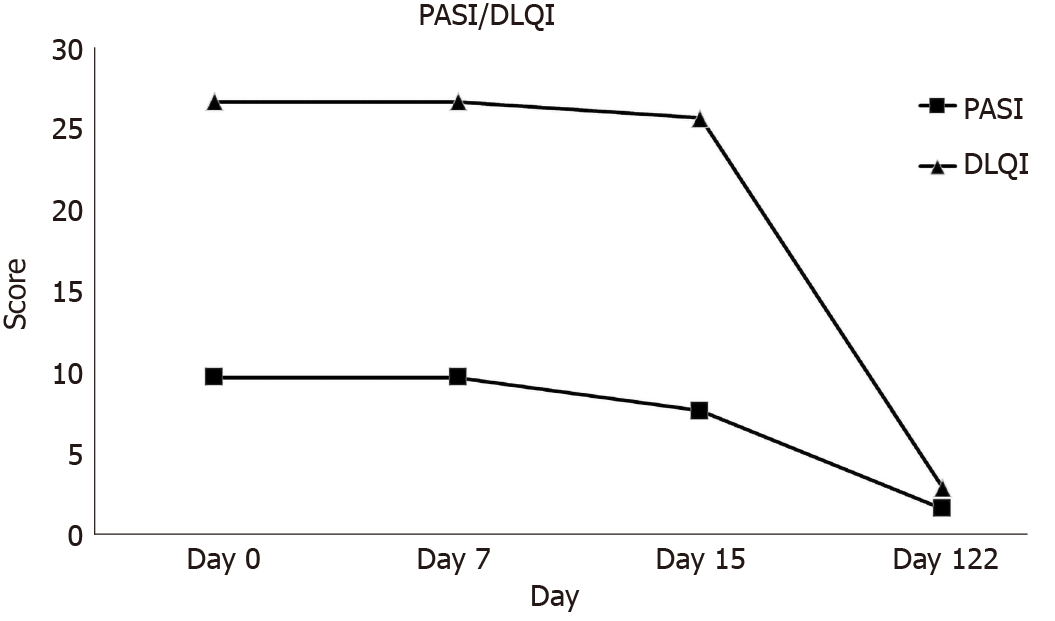
At the 1st hospital visit on April 1, 2020, the severity of psoriasis was diagnosed using PASI and DLQI. The PASI score was 9.9. The extent of psoriasis was determined to be not severe, but the DLQI score was 27, indicating that the skin disease had an enormous influence on daily life. The PASI and DLQI score of the patient decreased from 9.9 to 1.7 and from 27 to 3, respectively, after 122 d from the 1st transplantation (Figure 2).
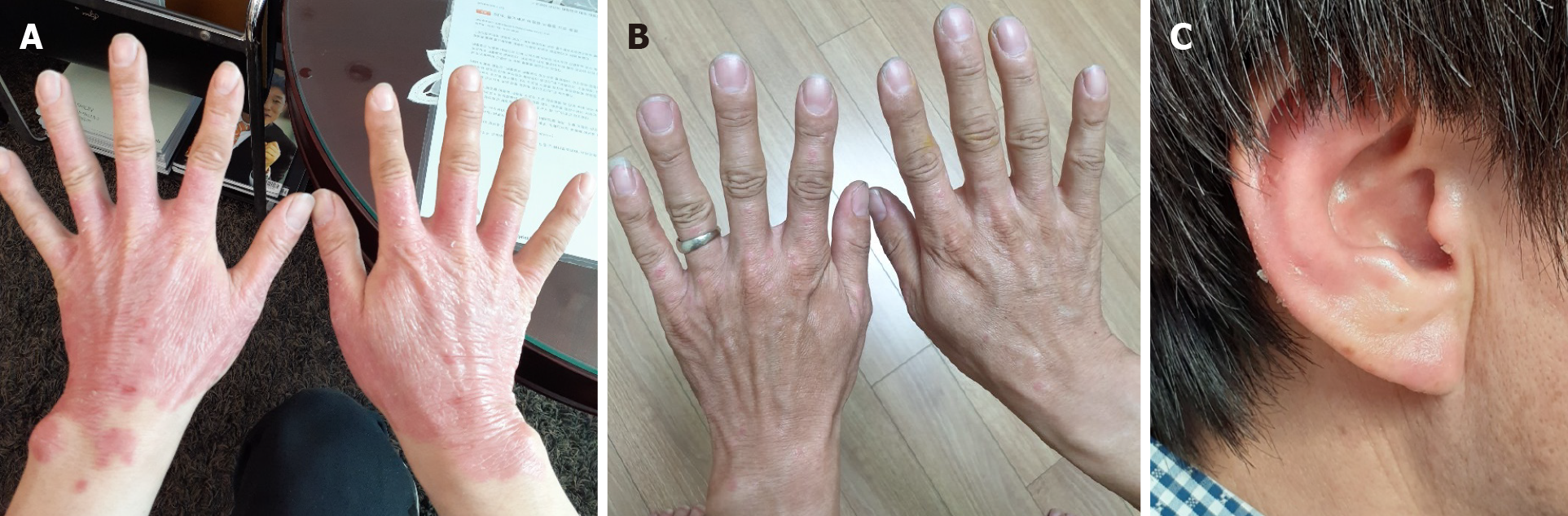
The erythema on both hands was slightly reduced, especially around the wrist area at day 7 after the 1st transplantation (Figure 3A). Itching also decreased, and exfoliation increased. After the 1st transplantation, the erythema gradually disappeared, and all erythema was almost resolved at day 122 after the 1st transplantation. Ultimately, the erythema was not observed on either hand, any fingers, or either wrist (Figure 3B). It was also confirmed that erythema of the ears was significantly reduced (Figure 3C).
The patient reported having experienced no adverse reactions or side effects, including dermatitis, fever, chills, or nausea.
Psoriasis is one of the autoimmune diseases that can reduced a patient’s quality of life[2]. Currently, there is no adequate treatment for psoriasis[2-4]. Therefore, there is a need for a new treatment method for psoriasis.
Previous studies have shown that MSCs may be a new alternative to psoriasis treatment[9-12]. However, previous studies have used cultured MSCs. It is well known that genetic mutations and aging-related modifications increase as the MSCs are cultured[13-15]. This results in an increase in the occurrence of various risks, including tumor formation and decreasing the treatment effect.
Here, we describe a case of psoriasis treated using MM-UC-MSCs. The MM-UC-MSCs safer materials than cultured MSCs because MM-UC-MSCs have few genetic mutations by expansion in vitro. Also, the MM-UC-MSCs are expected to be more effective materials than cultured MSCs because of no aging-related modifications[13-15]. The patient was transplanted with the MSCs three times at 1-wk intervals. The patient had not received any other therapies during or after the MM-UC-MSCs treatment. Gradual clearing of the erythema was observed 122 d from the 1st treatment. Follow-up for 5 mo showed the patient’s psoriasis lesions to be mostly cleared. Interestingly, there was no significant change after the 1st and 2nd treatments. This result is hypothesized to be due to the time required for transplanted MM-UC-MSCs to engraft and function.
In general, treatment for psoriasis is aimed at a 75% improvement based on the PASI score[16]. In addition, there is a strong correlation between the PASI score, which represents severity of psoriasis, and the DLQI score, which represents quality of life. Therefore, a reduction in the PASI score of 75% or more can significantly improve the quality of life of the treated patient, which means that the treatment method is highly effective. In this study, the PASI score of the psoriasis patient treated by MM-UC-MSC transplantation decreased from 9.9 to 1.7 at day 122 after the 1st transplantation. During this period, the patient’s DLQI score dropped from 27 to 3, confirming a significant improvement in quality of life as well.
In this patient, treatment of psoriasis by MM-UC-MSC transplantation was safe and effective. However, further studies should be performed in a larger number of patients with psoriasis and for a longer follow-up time to confirm our observations.
Based on the PASI and DLQI scores, MM-UC-MSC transplantation had a great effect on treating psoriasis. After 5 mo, psoriasis did not recur and the patient did not report any side effects. Clinical trials with more patients and a longer follow-up time are needed before introducing this method as a safe and efficient psoriasis treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leung PC, Onsun N S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2361] [Cited by in RCA: 2129] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 2. | Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Winterfield LS, Menter A, Gordon K, Gottlieb A. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis. 2005;64 Suppl 2:ii87-90; discussion ii91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 4. | Radi G, Campanati A, Diotallevi F, Bianchelli T, Offidani A. Novel Therapeutic Approaches and Targets for Treatment of Psoriasis. Curr Pharm Biotechnol. 2021;22:7-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 7. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 564] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 8. | Ahn H, Lee SY, Jung WJ, Lee KH. Alopecia treatment using minimally manipulated human umbilical cord-derived mesenchymal stem cells: Three case reports and review of literature. World J Clin Cases. 2021;9:3741-3751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 9. | Owczarczyk-Saczonek A, Krajewska-Włodarczyk M, Kruszewska A, Placek W, Maksymowicz W, Wojtkiewicz J. Stem Cells as Potential Candidates for Psoriasis Cell-Replacement Therapy. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Paganelli A, Tarentini E, Benassi L, Kaleci S, Magnoni C. Mesenchymal stem cells for the treatment of psoriasis: a comprehensive review. Clin Exp Dermatol. 2020;45:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Chen H, Niu JW, Ning HM, Pan X, Li XB, Li Y, Wang DH, Hu LD, Sheng HX, Xu M, Zhang L, Zhang B. Treatment of Psoriasis with Mesenchymal Stem Cells. Am J Med. 2016;129:e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Wang SG, Hsu NC, Wang SM, Wang FN. Successful Treatment of Plaque Psoriasis with Allogeneic Gingival Mesenchymal Stem Cells: A Case Study. Case Rep Dermatol Med. 2020;2020:4617520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Wang J, Dang J, Zhu W, Chen Y, Zhang X, Xie J, Hu B, Huang F, Sun B, Bellanti JA, Zheng SG. A preclinical study-systemic evaluation of safety on mesenchymal stem cells derived from human gingiva tissue. Stem Cell Res Ther. 2019;10:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bao X, Wang J, Zhou G, Aszodi A, Schönitzer V, Scherthan H, Atkinson MJ, Rosemann M. Extended in vitro culture of primary human mesenchymal stem cells downregulates Brca1-related genes and impairs DNA double-strand break recognition. FEBS Open Bio. 2020;10:1238-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Liu J, Ding Y, Liu Z, Liang X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front Cell Dev Biol. 2020;8:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 16. | Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |