Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6575
Peer-review started: April 12, 2021
First decision: April 27, 2021
Revised: May 17, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: August 6, 2021
Processing time: 106 Days and 16.4 Hours
Cutaneous metastasis is a rare event associated with poor prognosis for gastric cancer and has been rarely reported in the literature.
A 69-year-old male patient who had undergone salvage gastrectomy and a few courses of adjuvant chemotherapy 3 mo earlier for recurrent gastric cancer developed widespread cutaneous metastases. Due to the patient’s intolerance to further adjuvant chemotherapy, he was placed in hospice care and expired 1 mo later. In the literature, gastric cancers are rarely reported as the primary malignancies for cutaneous metastasis. We, thus, provide an update on a case review published in 2014 by reviewing 10 more case reports dated from 2014 to 2020. The average age for the new group of patients was 59.4 ± 18.88-years-old. Thirty percent of the patients presented with cutaneous lesions and advanced gastric cancer synchronously while 70% developed cutaneous metastases 1.3 years to 14 years after the initial treatment for primary gastric cancer. Eighty percent of the patients received either local excision or chemo ± radiation therapy to treat their cutaneous metastases.
This report highlights cutaneous metastasis as a late and untreatable metastasis of gastric cancer.
Core Tip: Cutaneous metastasis is a rare and late metastasis associated with poor prognosis for gastric cancer. Here, we report the case of a 69-year-old male patient with recurrent advanced gastric cancer who developed extensive cutaneous metastasis. To enrich the understanding of this clinical entity, we performed an update to a case review published in 2014 by reviewing 10 more newly published case reports dating from 2014 to 2020. We found that it is important to perform thorough work-ups on patients with advanced or recurrent gastric cancer and convince the patient about the benefits of neoadjuvant chemotherapy.
- Citation: Chen JW, Zheng LZ, Xu DH, Lin W. Extensive cutaneous metastasis of recurrent gastric cancer: A case report. World J Clin Cases 2021; 9(22): 6575-6581
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6575.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6575
Cutaneous metastasis from advanced neoplasms is a rare pathological entity with prognostic significance. The condition is reported to have an incidence rate of 0.7%-10.4% and presents metachronously or synchronously in the late course of advanced malignancy[1]. Based on the literature, various types of neoplasms including lung, breast, gastrointestinal, renal, ovarian, brain, and pancreatic cancers have been reported to progress to cutaneous metastases. However, the oncogenesis for the development of cutaneous metastasis remains unclear[2-6]. Theoretically, the cutaneous involvement of malignant diseases can occur through either hematogenous or lymphatic routes following the “soil and seed” hypothesis, but in practice, direct contiguous tissue invasion and iatrogenic implantation are also persuasive mechanisms for the development of cutaneous metastasis.
Historically, the recognition of cutaneous metastasis from gastric cancer can be traced back to the identification of cutaneous metastasis by Mary Joseph. As a surgical assistant to Dr. William J. Mayo, she was the first person to observe a cutaneous nodule of the umbilicus, which was later named the Sister Mary Joseph Nodule, as a result of direct seeding from gastric malignancy[7]. In the most recent literature review on cutaneous metastasis from gastric cancer in 2014, cutaneous metastases in 72 gastric cancer patients were found all over the surface of the body. Even though this kind of metastasis indicates the late stage of cancer, either surgical intervention and adjuvant chemo or radiation therapy were still performed to improve the survival probability of the patients. Here, we present a very unique case with extensive cutaneous metastasis from gastric stump cancer and also perform a literature review to update the clinical experience regarding the management of these pathological entities[8]. We present the following case in accordance with the CARE reporting checklist.
A 69-year-old male patient presented at our clinic complaining of dysphagia from eating and multiple cutaneous nodules.
One year earlier, the patient underwent remnant gastrectomy for gastric stump cancer. Postoperatively, due to the patient’s resistance to chemotherapy, he did not return to the hospital for postoperative adjuvant chemotherapy until 3 mo after the surgery. During the follow-up visit, the patient developed dysphagia from eating and also complained of some painless, red, skin nodules approximately 0.5-1.5 cm in diameter on his forehead, back, neck, and arms. Thus, the patient was readmitted to our hospital for a work up for his dysphagia and multiple cutaneous nodules. We consulted with our dermatologist, who suggested a re-biopsy of the cutaneous nodules, despite the negative pathological results for the subcutaneous nodule, when the patient had a remnant gastrectomy for his recurrent gastric cancer. Based on the medical history as well as pathological findings from the immunostaining study, the cutaneous malignancy was further confirmed to be of gastric origin.
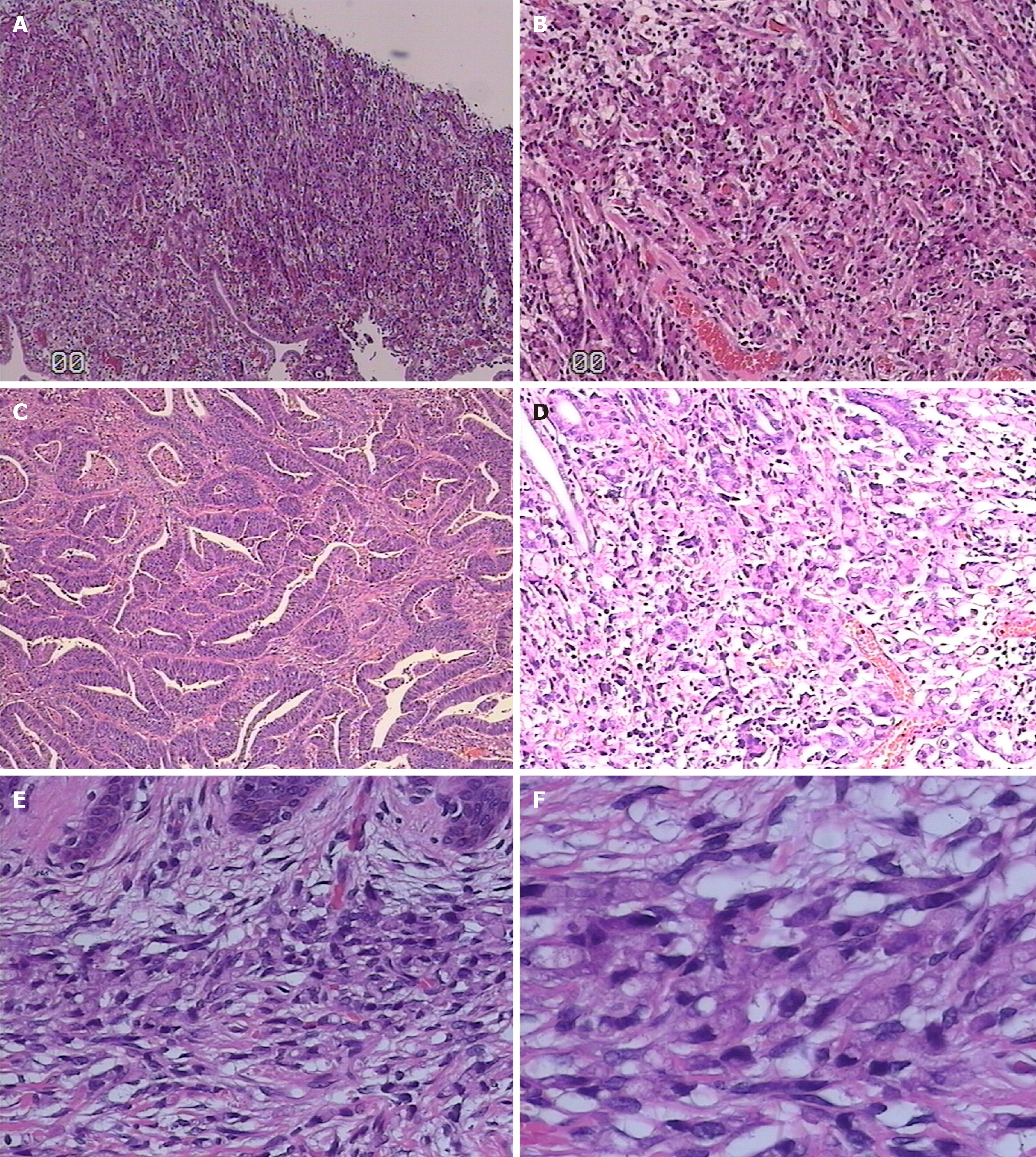
The patient was initially admitted to our department for laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy to surgically manage antral adenocarcinoma 3 years earlier, a pathological stage of pT4aN3bM0 for his antral ulcerated infiltrating gastric cancer with poorly differentiated tubular adenocarcinoma penetrating the serosal layer and invading the nerve (Figure 1), and six cycles of postoperative adjuvant chemotherapy (fluorouracil, folic acid, and oxaliplatin). Additionally, the patient had type 2 diabetes and pneumoconiosis for many years.
He was admitted again 1 year ago for the treatment of gastric stump cancer. Following normal preoperative examinations, the patient underwent remnant gastrectomy, during which a single small subcutaneous nodule in the midline of his abdomen was considered suspicious and removed for a histopathological exam to diagnose the nature of the nodule. Postoperatively, the pathological results revealed ulcerated moderately differentiated adenocarcinoma with signet ring cell carcinoma in the posterior wall of the gastric body, and no cancer tissue was observed in the subcutaneous nodule specimens.
Due to the patient’s resistance to chemotherapy, he did not return to the hospital for postoperative adjuvant chemotherapy until 3 mo after the surgery. During the follow-up visit, the patient complained of some painless, red, skin nodules approximately 0.5-1.5 cm in diameter on his forehead, back, neck, and arms for the past 1 mo. We consulted with our dermatologist, who suggested a re-biopsy of the cutaneous nodules despite the negative pathological results for the subcutaneous nodule in the remnant gastrectomy. Based on the medical history as well as pathological findings from the immunostaining study, the cutaneous malignancy was further confirmed to be of gastric origin. Even though the patient received one cycle of paclitaxel/Tegafur chemo
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
The patient did not have any significant personal history and denied any health issue and genetic problem running in his family.
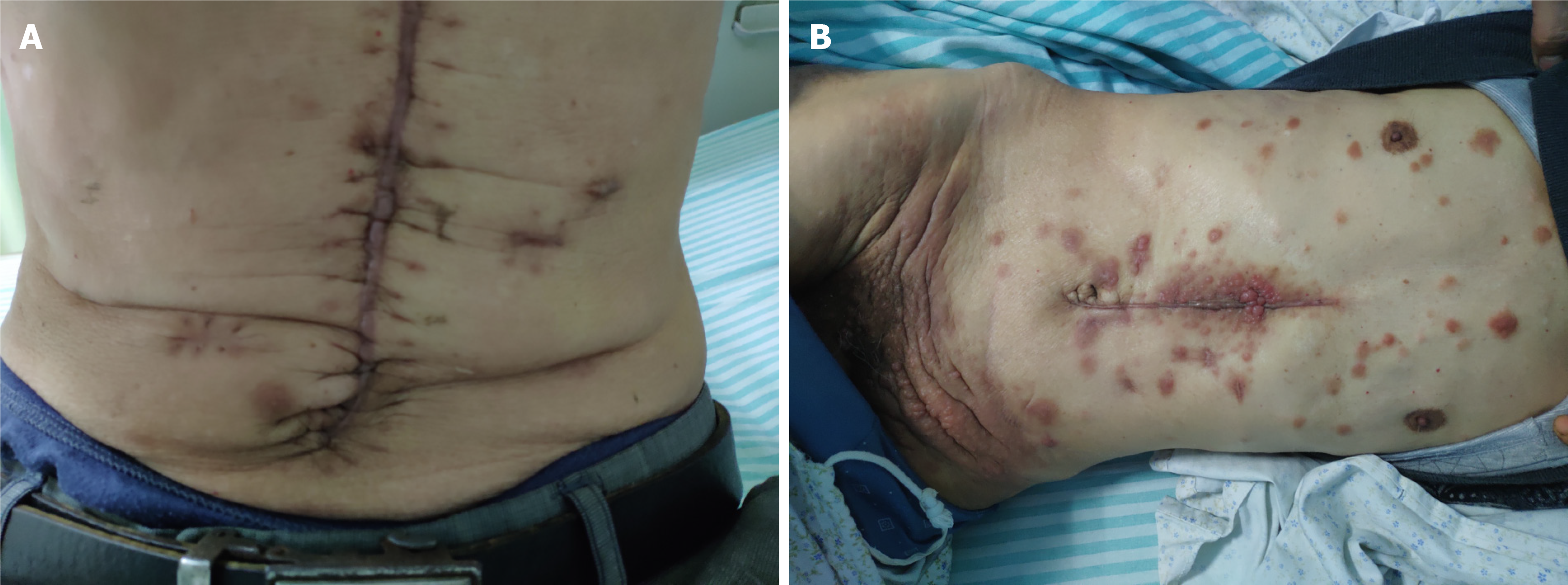
On admission, the patient presented with stable vital signs and a cachexic condition. Focal examinations of the chest, abdomen, and other parts of the body revealed negative findings except for multiple ruby red, tender, cutaneous nodules with diameters of 0.5-1.5 cm along the surgical incisional site and on his forehead, back, neck, arms, and other locations on the abdomen and inguinal/perineal areas, which were significantly enlarged and extensionalized compared to their appearance the previous year. The nodules were hard and fixed without significant tenderness (Figure 2).
On admission, the patient was sent for various laboratory exams including complete blood count, chemistry, liver function, kidney function, and other related tests. However, none of results from the lab test significantly contributed to the diagnosis and management.
Computed tomography also revealed multiple small subcutaneous nodules along the body without metastatic lesions in the important organs.
We performed a biopsy of the cutaneous nodules, which showed signet ring cell carcinoma in the dermis with positive CK7 and Villin, weakly positive CDX2, and negative CK20 negative in the immunostaining study.
Cutaneous metastases from recurrent gastric cancer.
Since the patient was in the terminal stage of advanced cancer, he accepted the recommendation for hospice care and expired a month later.
The patient expired a month later.
In the literature, gastric cancers are rarely reported as the primary malignancies for cutaneous metastasis. The first thorough review on cutaneous metastases from gastric cancer was performed by Cesaretti et al[8] in 2014 and included 72 reported patients with cutaneous lesions at various locations over the body surface. The review demonstrated both the rarity of this kind of metastasis and the location diversity. Based on the case information, even though the presentation of cutaneous metastasis marks the late-stage and poor prognosis for advanced gastric cancer, approximately 61% of patients still undergo aggressive management with surgical, chemo, or radiation therapies[8].
In our review, we aimed to provide an update on the review by Cesaretti’s group after identifying 10 newly published case reports dated from 2014 to 2020 with the keywords “cutaneous metastasis from gastric cancer” (Table 1). For the new set of patients, the general demographic data showed that their average age was 59.4 ± 18.88-years-old with a ratio of six to four male to female patients. Thirty percent of the patients presented with cutaneous lesions and advanced gastric cancer synchronously while 70% developed cutaneous metastases 1.3 years to 14 years after the initial treatments of primary gastric cancer. Interestingly, 80% of the patients received some sort of management approach ranging from local excision to chemo or chemo-radiation therapy to treat their cutaneous metastases[9-15].
| No. | Age | Sex | Site(s) of cutaneous metastasis | MPM classification | Time of occurrence in yr | Management |
| 1 | 89 | M | Axilla | Metachronous | 6 | Palliative surgery |
| 2 | 59 | M | Chest wall | Synchronous | 0 | Palliative surgery |
| 3 | 65 | F | Umbilicus | Metachronous | 5 | Chemotherapy |
| 4 | 55 | F | Breast | Metachronous | 14 | Radiation therapy |
| 5 | 91 | M | Chin | Synchronous | 0 | None |
| 6 | 60 | M | Chest and abdominal wall | Metachronous | 6 | Palliative surgery |
| 7 | 42 | F | Frontal scalp | Metachronous | 1.3 | Palliative surgery with Chemotherapy |
| 8 | 57 | M | Neck and shoulder | Metachronous | 2.5 | Chemotherapy |
| 9 | 33 | M | Neck | Synchronous | 0 | Palliative surgery |
| 10 | 43 | F | Unknown | Metachronous | 2 | None |
In our patient, cutaneous metastasis occurred 3 years after the primary treatment for gastric cancer, with the histopathological results of moderately differential signet ring cell carcinoma. The primary surgery of laparoscopically-assisted subtotal gastrectomy with D2 dissection was satisfactorily performed by our team for curative purposes for this patient. After the rounds of standard postoperative adjuvant therapy with fluorouracil, folic acid, and oxaliplatin, the patient was lost to follow-up until a recurrence at his gastric stump 3 years later. To manage the recurrence, the standard therapeutic process of open remnant gastrectomy followed by postoperative chemotherapy was performed. However, in retrospect, if we had attempted the rounds of neoadjuvant chemotherapy coupled with the surgeries and postoperative adjuvant therapy for our management regimen, the likelihood of recurrence for cancer would have been lower. Before second surgical management for gastric stump recurrence, more careful and detailed work-ups are critical for improving the long-term outcome. Preoperative neoadjuvant therapy should also be considered to downgrade the cancer stage and decrease the cancer cell load, both of which may prevent malignant cells from seeding and further metastasis[16,17].
From the distribution of cutaneous metastasis in our patient, contiguous tissue invasion and iatrogenic implantation cannot be completely ruled out. Oncological principles and techniques for surgery on cancer patients, especially those with advanced malignancies, should be strictly followed to improve the quality of oncological operations. However, if the cancerous cells are overloaded or in a state of metastasis with a breakdown of immunity, iatrogenic seeding may be difficult to avoid even with an incisional protector and appropriate no-touch techniques[18].
Physicians should be aware of the possibility of extensionalized metastasis to the skin from recurrent gastric carcinoma after 3-year remission. Before performing surgery on recurrent gastric cancer at the gastric stump, it is important to perform a work-up for the patient using more diagnostic modalities and convince the patient about the benefits of a round of preoperative chemotherapy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng J S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Alcaraz I, Cerroni L, Rütten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Sittart JA, Senise M. Cutaneous metastasis from internal carcinomas: a review of 45 years. An Bras Dermatol. 2013;88:541-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Wong CY, Helm MA, Kalb RE, Helm TN, Zeitouni NC. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 379] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 7. | Samitz MH. Umbilical metastasis from carcinoma of the stomach. Sister Joseph's nodule. Arch Dermatol. 1975;111:1478-1479. [PubMed] |
| 8. | Cesaretti M, Malerba M, Basso V, Boccardo C, Santoni R, D'Alessandro G, Weiss A, Campisi C, De Cian F. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13. [PubMed] |
| 9. | Kim YS, Lee JH, Park YM, Lee JY. Cutaneous Metastasis of Gastric Cancer Mimicking Primary Inflammatory Breast Cancer. Ann Dermatol. 2015;27:767-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Gallais Sérézal I, Dumitru S, Le Foll C, Hillion B. Cutaneous metastasis of gastric adenocarcinoma at the site of a traumatic ecchymosis. Cutis. 2015;95:E15-E16. [PubMed] |
| 11. | Namikawa T, Munekage E, Munekage M, Maeda H, Yatabe T, Kitagawa H, Kobayashi M, Hanazaki K. Subcutaneous metastasis arising from gastric cancer: A case report. Mol Clin Oncol. 2017;6:515-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kirchberger MC. Unusual presentation of a cutaneous metastasis in the face arising from gastric cancer: a case report. SAGE Open Med Case Rep. 2018;6:2050313X18795080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Koyama R, Maeda Y, Minagawa N, Shinohara T, Hamada T. Late Cutaneous Metastasis Originating from Gastric Cancer with Synchronous Metastasis. Case Rep Gastroenterol. 2019;13:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Yang S, Liu XL, Guo XL, Song B, Li SZ, Sun XF, Feng Y. Solitary metastasis to the skin and colon from gastric cancer after curative gastrectomy and chemotherapy: A case report. Medicine (Baltimore). 2020;99:e21532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Cokgezer S, Samanci NS, Bektas M, Kepil N, Demirelli FH. Cutaneous Metastasis of Signet Cell Gastric Carcinoma. Indian J Dermatol. 2020;65:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Newton AD, Datta J, Loaiza-Bonilla A, Karakousis GC, Roses RE. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol. 2015;6:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 17. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang YJ, Park BJ, Sasako M, Tsujinaka T; REGATTA study investigators. Gastrectomy plus chemotherapy vs chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 508] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 18. | Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-82; quiz 183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 296] [Article Influence: 9.9] [Reference Citation Analysis (0)] |