Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6538
Peer-review started: April 15, 2021
First decision: May 10, 2021
Revised: May 15, 2021
Accepted: June 17, 2021
Article in press: June 17, 2021
Published online: August 6, 2021
Processing time: 103 Days and 16.5 Hours
Although the bystander effect and abscopal effect are familiar in medicine, they are relatively rare in clinical practice. Herein, we report the case of a patient who demonstrated an obvious bystander effect and abscopal effect response following carbon-ion irradiation for recurrent thymic carcinoma.
A 44-year-old female presented with shortness of breath. Eleven years prior, she was diagnosed with athymic tumor located in the anterosuperior mediastinum. She underwent extensive tumor resection, and the postoperative pathologic diagnosis was thymic carcinoma. She was administered 50 Gy/25 Fx of postoperative radiation. In 2019, she was diagnosed with a recurrence of thymic carcinoma, with multiple recurrent nodules and masses in the left thoracic chest and peritoneal cavity, the largest of which was in the diaphragm pleura proximal to the pericardium, with a size of 6.7 cm × 5.3 cm × 4.8 cm. She received carbon-ion radiotherapy. After carbon-ion radiotherapy treatment, the treated masses and the untreated masses were observed to have noticeably shrunk on the day of carbon-ion radiotherapy completion and on follow-up imaging. We followed the CARE Guidelines for consensus-based clinical case reporting guideline development and completed the CARE Checklist of information to report this case.
This report is the first of obvious abscopal and bystander effects following carbon-ion irradiation in a human patient, and further research is needed to better elucidate the mechanisms of bystander and abscopal effects.
Core Tip: We presented the case of a patient who demonstrated a bystander effect and an abscopal effect following carbon-ion irradiation for recurrent thymic carcinoma. In this report, obvious abscopal and bystander effects after carbon-ion irradiation in a patient was initially presented, and more research is needed to further elucidate the mechanism of bystander and abscopal effects.
- Citation: Zhang YS, Zhang YH, Li XJ, Hu TC, Chen WZ, Pan X, Chai HY, Ye YC. Bystander effect and abscopal effect in recurrent thymic carcinoma treated with carbon-ion radiation therapy: A case report. World J Clin Cases 2021; 9(22): 6538-6543
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6538.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6538
Radiation-induced bystander effect is an excessive biological phenomenon in unirradiated cells due to the transmission of signals from irradiated cells[1-5]. An abscopal effect is a result of the deterioration of unirradiated metastatic lesions after the irradiation of a distant tumor location[6-10]. The rarity and associated mechanisms of bystander and abscopal effects remain under study. To date, there have been no reports of both effects in one patient, even with photon or proton irradiation. Carbon-ion radiotherapy is a form of heavy-ion radiation modality with stronger effects on tumor cells by physical dose (higher relative biological effect) and better dose distribution compared with photon-based therapies[11,12].
Here, we have followed the CARE Guidelines for consensus-based clinical case reporting guideline development[13]. We present the case of a patient who demon
In December 2019, a 44-year-old female presented shortness of breath and palpitation.
In December 2019, a 44-year-old female presented shortness of breath and palpitation.
Eleven years ago, she transferred to our hospital in February 2009 because of mediastinum tumor. Chest computed tomography (CT) showed a huge mass in the anterosuperior mediastinum, considered a thymic tumor. After detailed workup and multidisciplinary team consultation, she underwent tumor resection and extensive resection of tumor, including part of the left upper lobe of the lung, phrenic nerve and a small part of the pericardium, which were via median sternotomy. Postoperative pathology revealed macroscopic invasion into the pericardium and lung, without great vessel invasion and pathologic diagnosis as Masaoka Staging IIIA, World Health Organization Type C: Thymic carcinoma. On 35 d postoperative, she was administered radiation 50 Gy/25Fx, covering the surgical tumor bed and upper mediastinum. There was no chemotherapy. After that, the patient did not receive any chemotherapy, only regularly thorax CT follow-up.
No similar medical history in the family.
After instructing the patient to inhale deeply, the symptoms worsened.
No abnormalities in routine blood work, biochemistry and electrolytes.
Chest CT revealed multiple nodules and masses in the left thoracic chest and peritoneum cavity, alone with the pleura and peritoneum. The biggest one was at the diaphragm pleura proximity to the pericardium, sized 6.7 cm × 5.3 cm × 4.8 cm, and other multiple masses alone with pleura and peritoneum cavity.
We diagnosed it as recurrence of thymic carcinoma, after fine-needle aspiration of the biggest mass, which was close to the pericardium. The diagnosis still was Masaoka Staging IIIA, World Health Organization Type C: Thymic carcinoma.
Referred to multidisciplinary team consultation, experts considered the patient to have a long disease-free period, and the tumor demonstrated an indolent biological behavior. They decided to irradiate the biggest mass adjacent to the pericardium with carbon ion, which would have probably relieved the patient’s palpitation and shortness of breath, etc. We therefore selected definitive carbon-ion radiotherapy (CIRT) because it could be administered within dose limitations and sparing the lung and heart. Carbon ions can provide a better distribution of physical dose because of lessened lateral scattering, which have higher relative biological effectiveness and a lower oxygen enhancement ratio, with desirable features in eradicating radioresistant, hypoxic tumors[14]. A CIRT plan was developed to deliver 60 Gy [Relative Biological Effectiveness (RBE)] (RBE = 3.0) to the planned target volume in 12 fractions by the broad-beam method. Doses of carbon ions were expressed as photon equivalent doses (GyE), namely, physical doses multiplied by RBE of carbon ions was assumed to be 3.0[15].
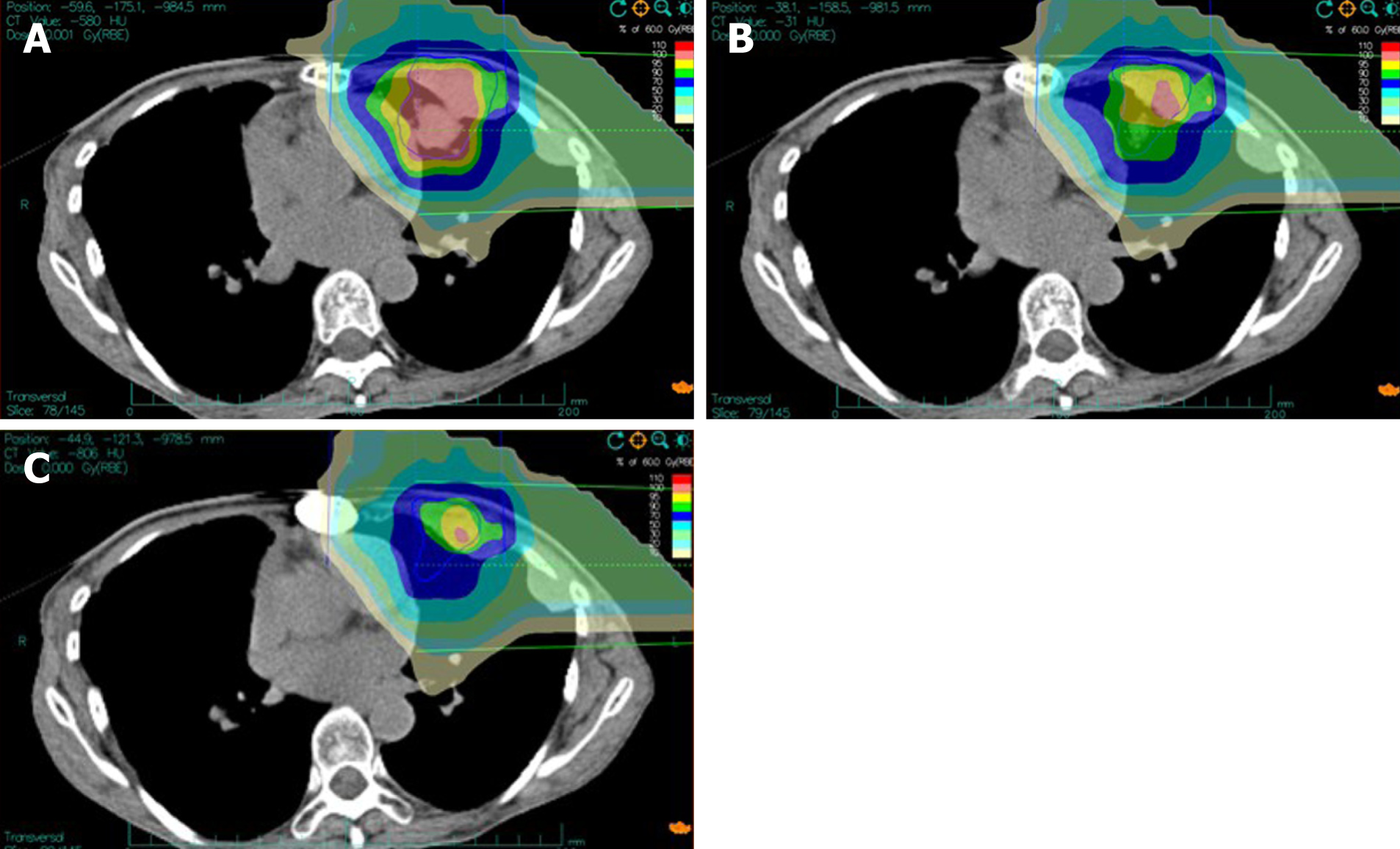
The patient was given CIRT once daily for 5 d within 1 wk (Monday–Friday), 12 fixed fractions (fr.) more than 3 wk in total. Clinical target volume coupling with a safety margin accounting for organ motion (respiratory and heartbeat) and setup inaccuracies were involved in planning target volumes. CIRT planning was conducted by Ci-plan planning software (KJTJ, Lanzhou, China). In order to spare the left ventricle, 1 cm of the tumor was set aside near the left ventricle and was not included in planning target volumes. Treatment planning aims to cover planning target volumes via 90%-isodose lines.
Figure 1 shows the color wash isodose distributions for CIRT. One horizontal and one vertical ports were used for irradiation of the mass with 60 GyE delivered in 12 fractions.
The day after finishing the 60 Gy (RBE) CIRT, we ran a CT review. Amazingly, we found the biggest tumor decreased, including the 1 cm of the tumor that was set aside near the left ventricle, and other masses near or distant of irradiated area were decreased.
Following treatment, the treated masses as well as the untreated masses shrank noticeably the day after finishing CIRT on follow-up imaging. No additional treatment was administered. During and after CIRT, the patient’s shortness of breath and palpitation were relieved. There was only radiation dermatitis grade 1 acute adverse event and mild erythema. There were no ≥ grade 2 Radiation Therapy Oncology Group acute effect. The patient did not develop any later radiation adverse events 10 mo post-treatment, according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criterion.
In the case, the patient exhibited both bystander-like and abscopal-like effects after carbon-ion irradiation, with a disease remission for 3 mo. A reduction in the size of unirradiated tumor was noted when radiation therapy was completed, without any additional treatment, but the reduction in the masses was easily noted on CT. Of note, both high-dose irradiated masses and low-dose irradiated masses as well as masses not irradiated (near or distant masses) obviously shrank. It is still difficult to discern whether this indicates an underlying susceptibility of the patient’s thymic carcinoma or specific characteristics of this patient’s immune system, or whether the bystander and abscopal effects can be taken as advantages of carbon-ion beam system used.
This patient was officially our heavy-ion center’s second patient to receive treatment. The Wuwei Heavy-Ion Center (WWHIC), located in Wuwei City, Gansu Province, is the first Chinese dedicated heavy-ion cancer therapy facility and was designed by the Institute of Modern Physics of the Chinese Academy of Sciences. The device was manufactured by Lanzhou KejinTaiji (KjTj) Corporation Ltd. The WWHIC initiated the clinical application of carbon ions generated by the dedicated heavy-ion medical accelerator in Wuwei on November 2018. On September 29, 2019, the facility’s device was approved by the National Medical Products Administration and registered as a class III medical device. With its high-end medical equipment, the first Carbon-Ion Cancer Therapy Facility in China is a heavy-ion treatment facility designated for the treatment of malignant tumors. The WWHIC is affiliated with Wuwei Cancer Hospital, and the clinical usage of the WWHIC officially started on April 1, 2019. As of January 25, 2020, 9 mo after the operation, the WWHIC has treated 218 patients with CIRT. In the WWHIC, CIRT planning is performed using the carbon-ion Plan (ciPlan, version 1.0, Institute of Modern Physics, Lanzhou, China), including biological plan optimization, taking local values of RBE calculated by ciPlan software based on the local effect model into account. CIRT is delivered using the ciTreat (Institute of Modern Physics, Lanzhou, China). A passive beam and intensity-modulated raster scan system was developed by the WWHIC. For the patient of this case, the passive beam delivery system was involved, together with two different conformal irradiation methods.
Abscopal effects were reported first in 1953[16], and there have been more and more clinical reports for numerous diseases treated with conventional irradiation, such as malignant lymphoma, hepatocellular carcinoma, cervical carcinoma, melanoma and colorectal cancer from then on[17]. There are almost no case reports of the bystander effect, but there are many laboratory studies and literature reviews on the bystander effect[18-20]. Nevertheless, the mechanisms underlying the bystander and abscopal effects remain indeterminate.
The present study shows the development of a post-radiated in situ tumor vaccine, in rare cases, leading to a systemic response to tumor tissues, which involves enhancing the target tumor by irradiation and inducing a strong response of CD8β effector T cells to the target tumor. Radiation can both suppress immunity and stimulate it. After irradiation, tumors can translocate a variety of recognizable antigens, such as calreticulin, to their surface, enhancing recognition and response by the immune system. Durante et al[21] produced evidence recently suggesting that irradiated cells exhibit common T-cell sensitivity, which may boost the enhanced immune system response to primary tumors post irradiation[21]. Nonetheless, the mechanisms by which the bystander and abscopal effects in the tumor are revealed to the immune system remain undetermined.
In this report, obvious abscopal and bystander effects after carbon-ion irradiation in a patient was initially presented, and more work is needed to further elucidate the mechanism by which bystander and abscopal effects occur.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shintani Y S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Heeran AB, Berrigan HP, O'Sullivan J. The Radiation-Induced Bystander Effect (RIBE) and its Connections with the Hallmarks of Cancer. Radiat Res. 2019;192:668-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Hargitai R, Kis D, Persa E, Szatmári T, Sáfrány G, Lumniczky K. Oxidative Stress and Gene Expression Modifications Mediated by Extracellular Vesicles: An In Vivo Study of the Radiation-Induced Bystander Effect. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Du Y, Du S, Liu L, Gan F, Jiang X, Wangrao K, Lyu P, Gong P, Yao Y. Radiation-Induced Bystander Effect can be Transmitted Through Exosomes Using miRNAs as Effector Molecules. Radiat Res. 2020;194:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Tan W, Zhang Y, Li M, Zhu X, Yang X, Wang J, Zhang S, Zhu W, Cao J, Yang H, Zhang L. miR-27a-containing Exosomes Secreted by Irradiated Skin Keratinocytes Delayed the Migration of Unirradiated Skin Fibroblasts. Int J Biol Sci. 2019;15:2240-2255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Deng C, Wu J, Wang T, Wang G, Wu L, Wu Y, Bian P. Negative Modulation of Bystander DNA Repair Potential by X-Ray Targeted Tissue Volume in Arabidopsis thaliana. Radiat Res. 2019;191:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Espenel S, Vallard A, Rancoule C, Garcia MA, Guy JB, Chargari C, Deutsch E, Magné N. Melanoma: Last call for radiotherapy. Crit Rev Oncol Hematol. 2017;110:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Ishiyama Y, Takagi T, Yoshida K, Iizuka J, Kakuta Y, Okumi M, Ishida H, Tanabe K. Possible abscopal effect in urothelial carcinoma of the upper urinary tract after treatment with immune checkpoint inhibitors. IJU Case Rep. 2020;3:25-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | D'Andrea MA, Reddy GK. Extracranial systemic antitumor response through the abscopal effect induced by brain radiation in a patient with metastatic melanoma. Radiat Oncol J. 2019;37:302-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Trommer M, Yeo SY, Persigehl T, Bunck A, Grüll H, Schlaak M, Theurich S, von Bergwelt-Baildon M, Morgenthaler J, Herter JM, Celik E, Marnitz S, Baues C. Abscopal Effects in Radio-Immunotherapy-Response Analysis of Metastatic Cancer Patients With Progressive Disease Under Anti-PD-1 Immune Checkpoint Inhibition. Front Pharmacol. 2019;10:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Abbas W, Goel V, Verma A, Gupta VG, Rao RR. Harnessing the Immunomodulatory Effects of Radiation in Urinary Bladder Cancer. Cureus. 2019;11:e4108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 312] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Haefner MF, Verma V, Bougatf N, Mielke T, Tonndorf-Martini E, König L, Rwigema JM, Simone CB 2nd, Uhlmann L, Eichhorn F, Winter H, Grosch H, Haberer T, Herfarth K, Debus J, Rieken S. Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol. 2018;57:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, Makishima H, Choy H, Yamada S, Morishima T, Tsuji H, Miyashiro I, Kamada T. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol. 2019;20:674-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, Jäkel O, Mayer R, Orecchia R, Pötter R, Vatnitsky S, Chu WT. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93-e100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 15. | Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka T, Furusawa Y, Ando K, Suzuki M, Soga F, Kawachi K. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 599] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | MOLE RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 810] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Ebner DK, Kamada T, Yamada S. Abscopal effect in recurrent colorectal cancer treated with carbon-ion radiation therapy: 2 case reports. Adv Radiat Oncol. 2017;2:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Ariyoshi K, Miura T, Kasai K, Akifumi N, Fujishima Y, Yoshida MA. Radiation-induced bystander effect in large Japanese field mouse (Apodemus speciosus) embryonic cells. Radiat Environ Biophys. 2018;57:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Jia R, Chen Y, Jia C, Hu B, Du Y. Suppression of innate immune signaling molecule, MAVS, reduces radiation-induced bystander effect. Int J Radiat Biol. 2021;97:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Singh AP, Seigel GM, Guo L, Verma A, Wong GG, Cheng HP, Shah DK. Evolution of the Systems Pharmacokinetics-Pharmacodynamics Model for Antibody-Drug Conjugates to Characterize Tumor Heterogeneity and In Vivo Bystander Effect. J Pharmacol Exp Ther. 2020;374:184-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Durante M, Brenner DJ, Formenti SC. Does Heavy Ion Therapy Work Through the Immune System? Int J Radiat Oncol Biol Phys. 2016;96:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |