Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6435
Peer-review started: April 3, 2021
First decision: April 28, 2021
Revised: May 8, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: August 6, 2021
Processing time: 115 Days and 13.3 Hours
Antisynthetase syndrome (ASS) is characterized by the presence of antisynthetase antibodies coupled with clinical findings such as fever, polymyositis-dermatomyositis and interstitial lung disease. It is, however, rare to observe ASS association with B cell lymphoma presenting severe pneumonia as the first clinical manifestation.
We evaluated a 59-year-old male patient who presented with cough with sputum, shortness of breath and fever for 13 d. A chest computed tomography radiograph revealed bilateral diffuse ground-glass infiltrates in both upper fields, left lingual lobe and right middle lobe. Initially, the patient was diagnosed with severe community-acquired pneumonia and respiratory failure. He was empirically treated with broad-spectrum antibiotics, without improvement. Further analysis showed an ASS panel with anti-PL7 antibodies. Besides, electromyography evaluation demonstrated a manifestation of myogenic damage, while deltoid muscle biopsy showed irregular muscle fiber bundles especially abnormal lymphocyte infiltration. In addition, bone marrow biopsy revealed high invasive B cell lymphoma. Thus, the patient was diagnosed with a relatively rare anti–PL7 antibody positive ASS associated with B cell lymphoma.
This case highlights that rapidly progressive lung lesions and acute hypoxemic respiratory failure associated with heliotrope rash and extremely high lactate dehydrogenase level should be considered as the characteristics of non-infectious diseases, especially ASS and B cell lymphoma.
Core Tip: Antisynthetase syndrome (ASS) is a unique subset of inflammatory myopathy. Patients with inflammatory myopathies carry a higher risk of developing neoplasms, most commonly adenocarcinoma but not lymphoma or other hematologic neoplasms. However, data on the association between ASS and malignancy remain very scant. We present here a rare case of severe pneumonia and acute hypoxemic respiratory failure as the first indicator for anti-PL-7 ASS accompanied by B cell lymphoma.
- Citation: Xu XL, Zhang RH, Wang YH, Zhou JY. Manifestation of severe pneumonia in anti-PL-7 antisynthetase syndrome and B cell lymphoma: A case report. World J Clin Cases 2021; 9(22): 6435-6442
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6435.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6435
Antisynthetase syndrome (ASS) is a systemic autoimmune disorder characterized by the presence of antibodies against aminoacyl-transfer RNA (tRNA) synthetases (antisynthetase antibodies, ASA). It is considered a distinct sub-form of inflammatory myopathy, and Raynaud's phenomenon, arthritis, characteristic cutaneous sign (such as mechanic’s hand) or interstitial lung disease (ILD) is mostly acute and relapsing. Currently, there are eight identified autoantibodies against the amino-acyl tRNA synthetase enzymes: Anti-histidyl tRNA synthetase (anti-Jo-1), anti-alanyl tRNA synthetase (PL-12), anti-threonyl tRNA synthetase (PL-7), anti-glycyl tRNA synthetase (EJ), anti-isoleucyl tRNA synthetase (OJ), asparaginyl-tRNA synthetase (KS), the recently identified tyrosyl-tRNA synthetase (Ha) as well as phenylalanyl-tRNA synthetase (Zo)[1]. The presence of ASA coupled with the typical clinical manifestations signify ASS. About a third of patients with inflammatory myositis are anti-Jo-1 positive, while anti-PL-7 positivity is very rare[2]. Previous studies have shown anti-PL7 ASS frequent involvement of the lungs but not muscular involvement, while the other symptoms associated with ASS such as Raynaud’s phenomenon are rare[3,4]. This phenomenon complicates the diagnosis of the disease.
Patients with inflammatory myopathies (particularly dermatomyositis, DM) carry a higher risk of developing neoplasms, most commonly adenocarcinoma but not lymphoma or other hematologic neoplasms[2]. However, data on the association between ASS and malignancy remain very scant. Here, we present a rare case of severe pneumonia and acute hypoxemic respiratory failure as the first indicator for anti-PL-7 ASS accompanied by B cell lymphoma.
A 59-year-old man was admitted to the Emergency Department of our hospital complaining of cough with sputum, shortness of breath and fever in July 2019.
Patient’s symptoms started 2 wk ago with recurrent cough with sputum, shortness of breath and fever, which had worsened the last 24 h.
He had a history of hypertension and diabetes mellitus.
He smoked one pack of cigarettes daily for almost 30 years and was used to drinking liqueur more than 30 mL daily for 20 years.
Initial blood pressure was 112/76 mmHg, heart rate was 107/min and respiratory rate was 22/min. The patient’s highest body temperature was 39.8 °C, pulse rate was 107 beats/min and PO2 was 8.37 kPa. He breathed (oxygenated) through a nasal catheter at 2 L/min. Crackles were heard in both lungs.
Blood analysis showed a white blood cell count of 18100/μL (neutrophils, 93.8%; lymphocytes, 1.9%; monocytes, 4.1%), platelet count of 278000/ L and a hemoglobin value of 13.9 g/dL. The high C-reactive protein was 324.8 mg/L (normal range: 0-8 mg/L). Further blood tests showed serum alanine aminotransferase 31 U/L, aspartate aminotransferase 81 U/L and normal indexes of kidney function. Whereas albumin was significantly decreased to 23.6 g/L (normal range: 35-55 g/L), the creatine kinase or creatine kinase-MB were within the normal range, while lactate dehydrogenase (LDH) was elevated to 640 U/L (normal range: 109-245 U/L) as well as hydroxybutyrate dehydrogenase, which was at 485 U/L (normal range: 72-182 U/L). He had high blood glucose levels (15-25 mmol/L), with a hemoglobin A1c reading of 7.8%. The procalcitonin concentration was 0.19 ng/mL (normal range: 0-0.5 ng/mL). Seven respiratory viral RNA in sputum (including influenza virus, respiratory syncytial virus, parainfluenza virus) and blood capsular polysaccharide antigen of cryptococcus neoformans were negative. The total immunoglobulin (Ig) E was 371 KU/L (normal range: 0-100 KU/L). The concentration of blood tumor markers was CEA 6.3 ng/mL (normal range: 0-5 ng/mL), cytokeratin 19 fragment 8.5 ng/mL (normal range: 0-3.3 ng/mL), cancer antigen 125 127.4 U/mL (normal range: 0-35 U/mL), and serum ferritin 379.8 ng/mL (normal range: 7-323 ng/mL).
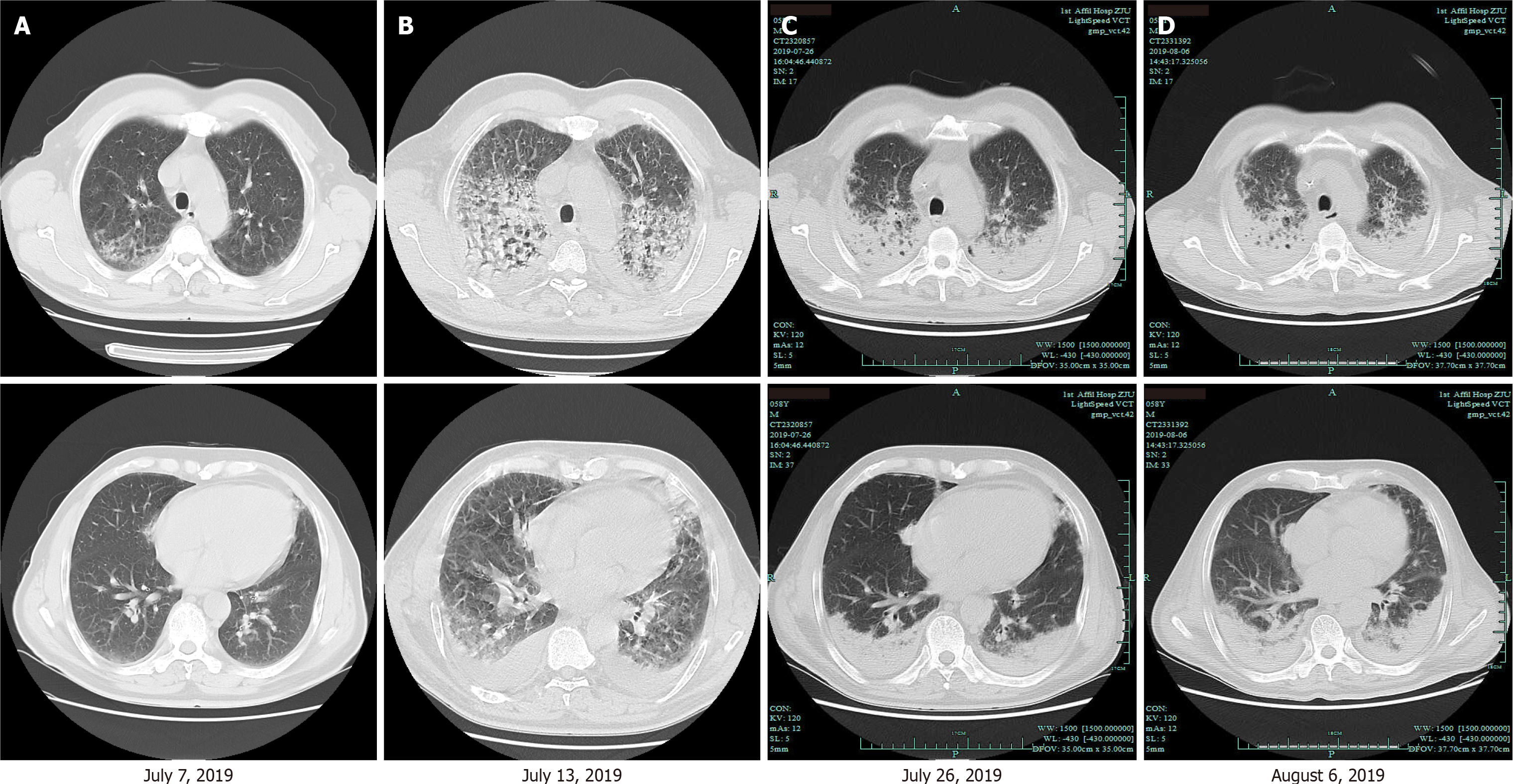
Chest computed tomography (CT) showed bilateral diffuse ground-glass infiltrates in both upper fields, left lingual lobe and right middle lobe (Figure 1A). The result of echocardiography was normal and there was no indication of pulmonary hypertension.
At first the patient was diagnosed with severe community-acquired pneumonia with respiratory failure. He was put under ceftizoxime antibiotics, which was changed to meropenem (1 g every 8 h) plus moxifloxacin (0.4 g daily) and fluconazole injections (200 mg daily). However, neither the symptoms nor the CT images showed any sign of improvement. Besides, there was aggravation of the lung lesions and the bilateral pleural effusion within 6 d (Figure 1B). On July 15, 2019, the patient was on intravenous imipenem-cilastatin (0.5 g three times a day) plus caspofungin (70 mg loading dose, followed by 50 mg daily) and oral oseltamivir (75 mg twice daily) as well as intravenous methylprednisolone (40 mg/12 h, for 3 d, 40 mg/d, for 3 d, 20 mg every day, for 3 d). In addition, he was supported with high-flow oxygen via nasal cannula. The serum IgG4, perinuclear anti-neutrophil cytoplasmic antibodies, cytoplasmic anti-neutrophil cytoplasmic antibodies, myeloperoxidase, proteinase 3 antineutrophil cytoplasmic antibodies or rheumatoid factor was negative, while the 18-item antinuclear antibody (ANA) panel showed low concentrations of ANA (1:20) and a positive soluble nucleoprotein antibody. Next-generation sequencing data from the broncho-alveolar lavage fluid showed the presence of Acinetobacter baumannii (30 series) and Enterobacter cloacae (5 series). We could not exclude these as the pathogen of pneumonia although, its detection series number were relatively low. On the other hand, microbiological assessment provided no reliable evidence for viral, fungal, or tuberculosis infection under sputum and bronchoalveolar lavage fluid cultures. On the 10th day of hospitalization, chest CT revealed bilateral gradual consolidation not only in the upper lobes but also in the inferior lobes (Figure 1C). We then changed the antibiotics to cefoperazone/sulbactam (2 g/8 h) plus tigecycline (100 mg loading dose, followed by 50 mg/12 h) to cover for a resistant gram-negative Bacilli. However, the patient’s shortness of breath persisted and his chest radiography worsened (Figure 1D). With a failed management of pneumonia, blood LDH and hydroxybutyrate dehydrogenase increased to 819 IU/L and 650 IU/L, respectively. Then, the patient underwent positron emission tomography/CT (PET-CT) and bone marrow biopsy. The PET-CT showed multiple alveolar and patchy shadows in the two lungs, bilateral consolidation in the dorsal lung and mild increase in fluorodeoxyglucose metabolism (SUV max 2.3). Besides, there were multiple lymphadenectasis in the clavicular region, mediastinum, hepatic hilum and retroperitoneum, with slight increase in fluorodeoxyglucose metabolism. We then considered inflammation of the lymph nodes. The routine bone marrow examination showed granulocytic hyperplasia and an increase in neutrophil alkaline phosphatase (NAP) score (NAP positive rate 71%, NAP score 171, positive control 68%, score 190). Since both the PET-CT and bone marrow findings were non-specific, we presumed an infectious/inflammatory disease.
Here, we handled a patient whose diagnosis followed a fulminant course. He initially had typical severe pneumonia symptoms such as fever, extremely high white blood cell and C-reactive protein, rapid pulmonary radiographic changes and respiratory failure. The diagnostic criteria for pneumonia is broad and includes non-infectious diseases, such as pulmonary embolism, autoimmune diseases, or malignant tumors such as pulmonary lymphoma.
Professor Zhou found that the patient developed a mild heliotrope rash and proximal myasthenia at the bedside evaluation and physical examination. The medical records showed a mild decrease of muscle strength and walk reluctance. Thus, the patient was recommended to be evaluated for polymyositis (PM)/DM.
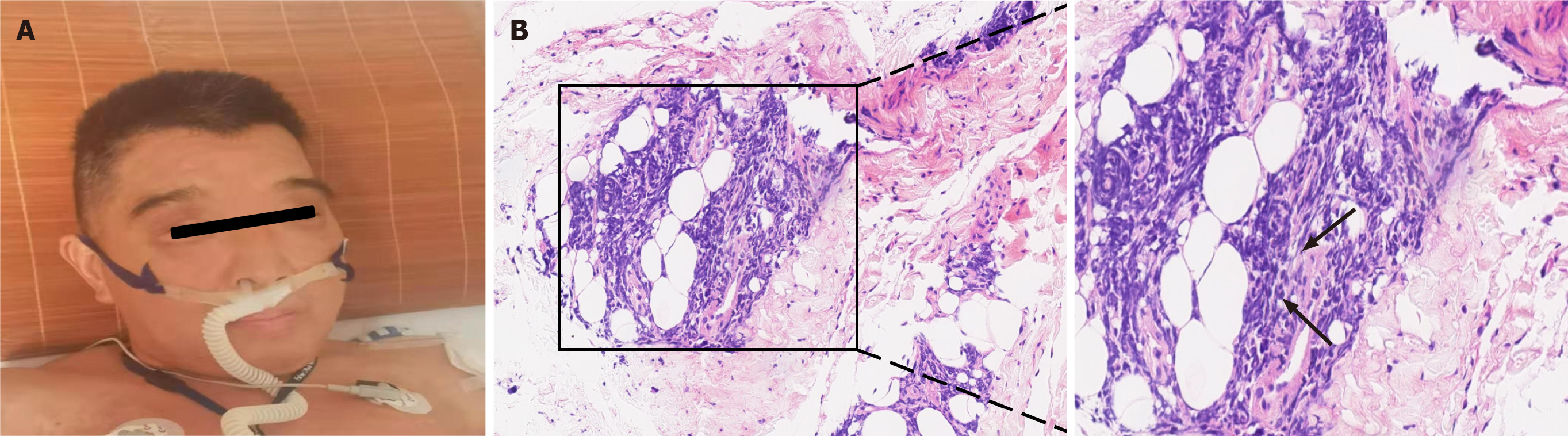
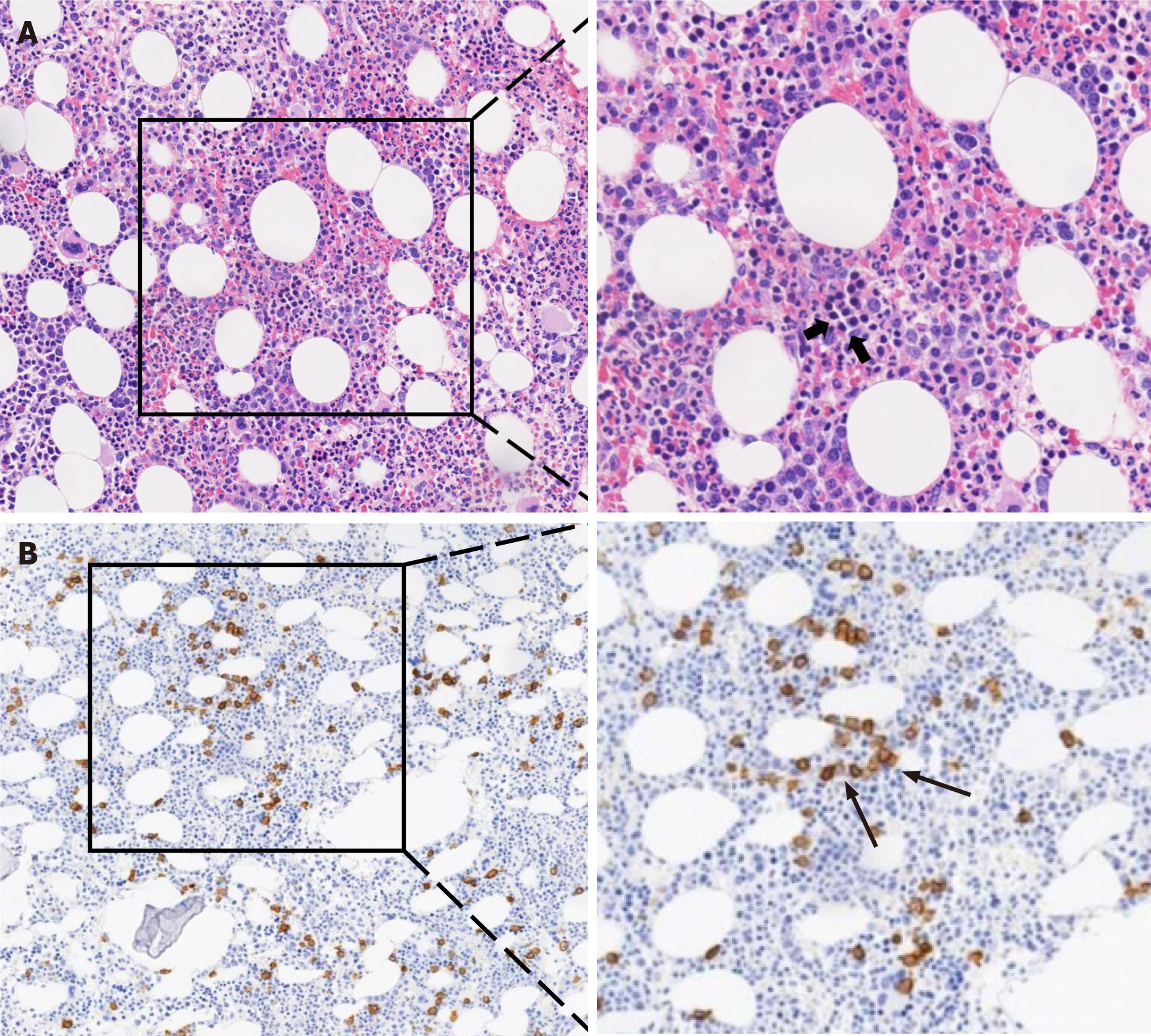
The ASS panel showed positive results for anti-Ro52, anti-Scl75 and anti-PL7 antibodies but not anti-JO1 antibody. This observation triggered our interest to consider ASS diagnosis. The patient underwent a thigh muscle magnetic resonance imaging examination, and the results showed that the soft tissues around the thighs and pelvis were edema. Electromyography data indicated potential myogenic damage. Besides, deltoid muscle biopsy showed irregular muscle fiber bundles, especially abnormal lymphocyte infiltration (Figure 2). On the 24th day of hospitalization, bone marrow biopsy results revealed high invasive B cell lymphoma in the bone marrow, which was confirmed by bright CD138 reactivity on immunohistochemistry assays (Figure 3). The final diagnosis of the presented case is a relatively rare anti-PL7 antibody positive ASS, which was associated with B cell lymphoma.
We added intravenous methylprednisolone treatment (40 mg/8 h) again and discontinued anti-infective treatment.
The patient finally succumbed to respiratory failure, 3 d after the diagnosis.
PM/DM is frequently accompanied with ILD; the classic imaging feature of ILD associated with PM/DM mainly is non-specific interstitial pneumonia (NSIP) or organizing pneumonia (OP), and the lesions are located in bilateral lower lobe[5]. ASS is a unique subset of PM/DM. Patients with ASS-ILD might experience acute and rapidly progressing ILD, which correspond to acute respiratory distress syndrome with the histopathology of diffuse alveolar damage[6]. Different forms of ASS have distinct clinical manifestations. Whereas anti-PL-7 ASS is only detected in 5% of ASS, ILD of anti-PL-7 ASS is much more severe than the anti-Jo-1 ASS and other types of ASS, while its muscle weakness is relatively mild. Respiratory failure accounts for the highest morbidity and mortality cases in ASS. In their study, Marie et al[7] showed that, out of 15 anti-PL-7 patients, none had resolution of ILD, and 35.7% of the patients experienced a deterioration in the ILD, coupled with a worsened functional status. Compared with Jo-1 ASS, the overall survival is significantly poor in non-Jo-1 ASS[8]. Similar to our observation, the presence of the non-Jo-1 antibody, conjugate presence of the Ro52 anti-body, older age, and elevated serum ferritin enhance the development of severe lung disease[9,10].
As described earlier, inflammatory myopathies may carry a risk for malignancy. Independent factors associated with malignancies in PM/DM are male sex, old age, absence of ILD, and a past history of diabetes mellitus[11]. Previous studies have shown that DM has greater association with malignancy than PM[12]. Most neoplasms associated with PM/DM are solid tumors, such as breast, colon, lung, pancreas or ovary cancers. A retrospective review of 32 PM/DM patients with hematological malignancy indicated that the top three malignancies are B-cell lymphoma (62.5%), T-cell lymphoma and Hodgkin's disease. The study also suggested that PM/DM often precedes the onset of hematological malignancy[13]. However, non-Hodgkin’s lymphoma and other hematologic neoplasms associated with ASS are relatively rare[14]. In addition, the anti-Jo-1 positivity may be a protective factor in hematological malignancies in PM/DM patients[13,15]. On the other hand, only a few studies have investigated the risk of cancer onset in ASS, especially in anti-PL7 patients. About 6% of the anti-PL7 patients experienced cancer[7], but literature of anti-PL7 ASS and its association with lymphoma remains very limited.
There are several pathogenic mechanisms that define inflammatory myopathy with lymphoma. It may be linked to immune system changes that lead to muscle destruction and inability to inhibit the proliferation of tumor cells and chronic inflammatory stimulation associated with inflammatory myopathy that activates T- and B- cells to cause malignant transformation of lymphocytes as well as genetic susceptibility and environmental factors. In addition, mutation in the TP53 gene is associated with inflammatory myopathy complicated with lymphoma[16,17].
Corticosteroids have been suggested for the treatment of different types of infection. Glucocorticoids inhibited the action and expression of many relevant cytokines such as interleukin-6 or tumor necrosis factor-α, involved in the inflammatory response associated with pneumonia[18]. According to a study including 17 randomized controlled trials comprising a total of 2264 participants, corticosteroids significantly reduced mortality for patients with severe pneumonia and reduced the time to clinical cure[19]. In this case, the patient had a poor therapeutic response to moderate-dose corticosteroids, and his acute lung injury was considered to be associated with lymphoma and ASS. Previous studies have shown that the therapeutic efficacy of corticosteroids for the ILD in ASS is limited; hence, additional immunosuppressive and immunomodulatory therapies such as cyclosporin A and tacrolimus have been tried with success in recent years[20]. Some evidence supported the use of tacrolimus as salvage therapy for severe respiratory failure due to ILD in ASS[21].
In our study, we highlight an important consideration of the patients with ASS who may be a paraneoplastic manifestation of the underlying malignancy. The mild heliotrope rash and high serum LDH level are the primary indicators of ASS. Through expanding the differential diagnosis of pneumonia, the final diagnosis of anti-PL7 ASS accompanied by B cell lymphoma was confirmed by blood specific myositis antibodies and bone marrow biopsy. Imaging performance of OP pattern presenting with focal consolidation, rather than that of NSIP or usual interstitial pneumonia pattern, increased the difficulty to distinguish the disease from severe community acquired pneumonia. Besides, the acute lung injury was the principal cause of respiratory failure and death of this patient. We inferred that the patient’s lung lesions were mostly caused by ASS, but the involvement of B cell lymphoma was not thoroughly confirmed.
In summary, we reported a case of anti-PL-7 ASS coupled with B cell lymphoma in a patient with the severe pneumonia and acute hypoxemic respiratory failure. Rapidly progressive lung lesions associated with resistance to antibiotics, heliotrope rash and extremely high LDH level should be considered as the characteristics of non-infectious diseases, such as rheumatic diseases and malignancy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roland T S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, Meyer A, Tohmé A, Charuel JL, Musset L, Allenbach Y, Benveniste O. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol. 2018;75:1528-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 2. | Tiniakou E, Mammen AL. Idiopathic Inflammatory Myopathies and Malignancy: a Comprehensive Review. Clin Rev Allergy Immunol. 2017;52:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 3. | Hervier B, Uzunhan Y, Hachulla E, Benveniste O, Nunes H, Delaval P, Musset L, Dubucquoi S, Wallaert B, Hamidou M. Antisynthetase syndrome positive for anti-threonyl-tRNA synthetase (anti-PL7) antibodies. Eur Respir J. 2011;37:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Labirua-Iturburu A, Selva-O'Callaghan A, Vincze M, Dankó K, Vencovsky J, Fisher B, Charles P, Dastmalchi M, Lundberg IE. Anti-PL-7 (anti-threonyl-tRNA synthetase) antisynthetase syndrome: clinical manifestations in a series of patients from a European multicenter study (EUMYONET) and review of the literature. Medicine (Baltimore). 2012;91:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Hozumi H, Fujisawa T, Nakashima R, Johkoh T, Sumikawa H, Murakami A, Enomoto N, Inui N, Nakamura Y, Hosono Y, Imura Y, Mimori T, Suda T. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. 2016;121:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Gasparotto M, Gatto M, Saccon F, Ghirardello A, Iaccarino L, Doria A. Pulmonary involvement in antisynthetase syndrome. Curr Opin Rheumatol. 2019;31:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Marie I, Josse S, Decaux O, Diot E, Landron C, Roblot P, Jouneau S, Hatron PY, Hachulla E, Vittecoq O, Menard JF, Jouen F, Dominique S. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med. 2013;24:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Saketkoo LA, Ascherman DP, Cottin V, Christopher-Stine L, Danoff SK, Oddis CV. Interstitial Lung Disease in Idiopathic Inflammatory Myopathy. Curr Rheumatol Rev. 2010;6:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, Oddis CV. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis. 2014;73:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Chen F, Zuo Y, Li S, Shi J, Wang G, Shu X. Clinical characteristics of dermatomyositis patients with isolated anti-Ro-52 antibody associated rapid progressive interstitial lung disease: Data from the largest single Chinese center. Respir Med. 2019;155:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Motomura K, Yamashita H, Yamada S, Takahashi Y, Kaneko H. Clinical characteristics and prognosis of polymyositis and dermatomyositis associated with malignancy: a 25-year retrospective study. Rheumatol Int. 2019;39:1733-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of Malignancy in Dermatomyositis and Polymyositis. J Cutan Med Surg. 2017;21:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Marie I, Guillevin L, Menard JF, Hatron PY, Cherin P, Amoura Z, Cacoub P, Bachelez H, Buzyn A, Le Roux G, Ziza JM, Brice P, Munck JN, Sarrot-Reynauld F, Piette JC, Larroche C. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun Rev. 2012;11:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 488] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Shi J, Li S, Yang H, Zhang Y, Peng Q, Lu X, Wang G. Clinical Profiles and Prognosis of Patients with Distinct Antisynthetase Autoantibodies. J Rheumatol. 2017;44:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Hoshida Y, Hongyo T, Xu JX, Sasaki T, Tomita Y, Nomura T, Aozasa K. TP53 gene mutation, an unfavorable prognostic factor for malignant lymphomas in autoimmune diseases. Oncology. 2005;69:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Zampieri S, Valente M, Adami N, Biral D, Ghirardello A, Rampudda ME, Vecchiato M, Sarzo G, Corbianco S, Kern H, Carraro U, Bassetto F, Merigliano S, Doria A. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Sibila O, Agustí C, Torres A. Corticosteroids in severe pneumonia. Eur Respir J. 2008;32:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017;12:CD007720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Rigby AL, Plit M, Glanville AR. Tacrolimus rescue therapy for severe respiratory failure in the anti-synthetase syndrome. Respirol Case Rep. 2014;2:70-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Fujisawa T, Hozumi H, Kamiya Y, Kaida Y, Akamatsu T, Kusagaya H, Satake Y, Mori K, Mikamo M, Matsuda H, Yokomura K, Koshimizu N, Toyoshima M, Imokawa S, Yasui H, Suzuki Y, Karayama M, Furuhashi K, Enomoto N, Nakamura Y, Inui N, Suda T. Prednisolone and tacrolimus vs prednisolone and cyclosporin A to treat polymyositis/dermatomyositis-associated ILD: A randomized, open-label trial. Respirology. 2021;26:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |