Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6388
Peer-review started: January 10, 2021
First decision: February 11, 2021
Revised: February 28, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: August 6, 2021
Processing time: 198 Days and 10.4 Hours
Prostatic carcinosarcoma is a very rare and highly aggressive tumor. It may occur after androgen deprivation therapy (ADT) for adenocarcinoma even after a 7-year interval.
A 66-year-old man presented with recurrent symptoms of gross hematuria and urinary retention. The patient had a previous history of combined radical prostatectomy and ADT for prostate cancer 7 years prior. He received total pelvic exenteration for a recurrent pelvic carcinosarcoma. Pathology and immunostaining revealed a carcinosarcoma of prostatic origin with focal spindled cells and bizarre giant cells. The patient subsequently underwent transverse colostomy for carcinosarcoma recurrence and bowel obstruction 3 mo later. Five months after the diagnosis of prostatic carcinosarcoma, the patient died of multiple organ metastases.
Prostatic carcinosarcoma after adenocarcinoma is exceedingly rare. ADT mediated transformation and dedifferentiation of the epithelial components may be the origin of this malignancy.
Core Tip: This is an unusual case of delayed occurrence of prostatic carcinosarcoma 7 years after radical prostatectomy and androgen deprivation therapy (ADT) for prostatic adenocarcinoma. It is very uncommon for a patient to have both neoplasms. ADT mediated transformation and dedifferentiation of the epithelial component may be the origin of this malignancy.
- Citation: Huang X, Cai SL, Xie LP. Prostatic carcinosarcoma seven years after radical prostatectomy and hormonal therapy for prostatic adenocarcinoma: A case report. World J Clin Cases 2021; 9(22): 6388-6392
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6388.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6388
Adenocarcinoma of the prostate is a very common cancer in men worldwide, whereas prostatic sarcoma is rare, with an overall prevalence of 0.1% and greater prevalence at younger ages. It is very rare for an elderly patient to have both neoplasms[1]. When these occur together as a carcinosarcoma, about 50% of the patients have a history of prostatic adenocarcinoma with radiation or androgen deprivation therapy (ADT)[2-4]. We encountered a patient with an unusual delay of prostatic carcinosarcoma occurrence 7 years after radical prostatectomy and ADT for prostatic adenocarcinoma.
A 66-year-old married Asian man, who was retired from the Livestock Bureau, presented with recurrent symptoms of gross hematuria and urinary retention.
The patient’s medical history was significant for prostatic adenocarcinoma [stage T2a, Gleason score 3 + 4; total prostate-specific antigen (PSA): 16 ng/mL] with no familial history of prostate cancer. Bone and chest computed tomography (CT) scans were unremarkable. He had undergone an open radical prostatectomy 7 years ago. Pathological results confirmed an adenocarcinoma (Gleason score 3 + 4) involving 65% of the right side of the prostate volume. The surgical margins were negative for the tumor and there was no extracapsular extension. All lymph nodes from the bilateral obturator area were negative for metastasis. Because the patient’s PSA level was 0.4 ng/mL at 2 wk after the surgery, he underwent one year of continuous ADT with goserelin and flutamide, which reduced the PSA level to 0.002 ng/mL at the end of treatment. Then the PSA level was monitored every 3 mo over 4 years, and each time the results were below 0.2 ng/mL. Unfortunately, the patient stopped returning for follow-up 2 year prior for unknown reasons.
Aside from the prostatic adenocarcinoma, the patient had an illness-free previous medical history.
No familial history of cancer and other illness.
The patient had a large fixed rectal mass by digital rectal examination.
PSA was 1.857 ng/mL.
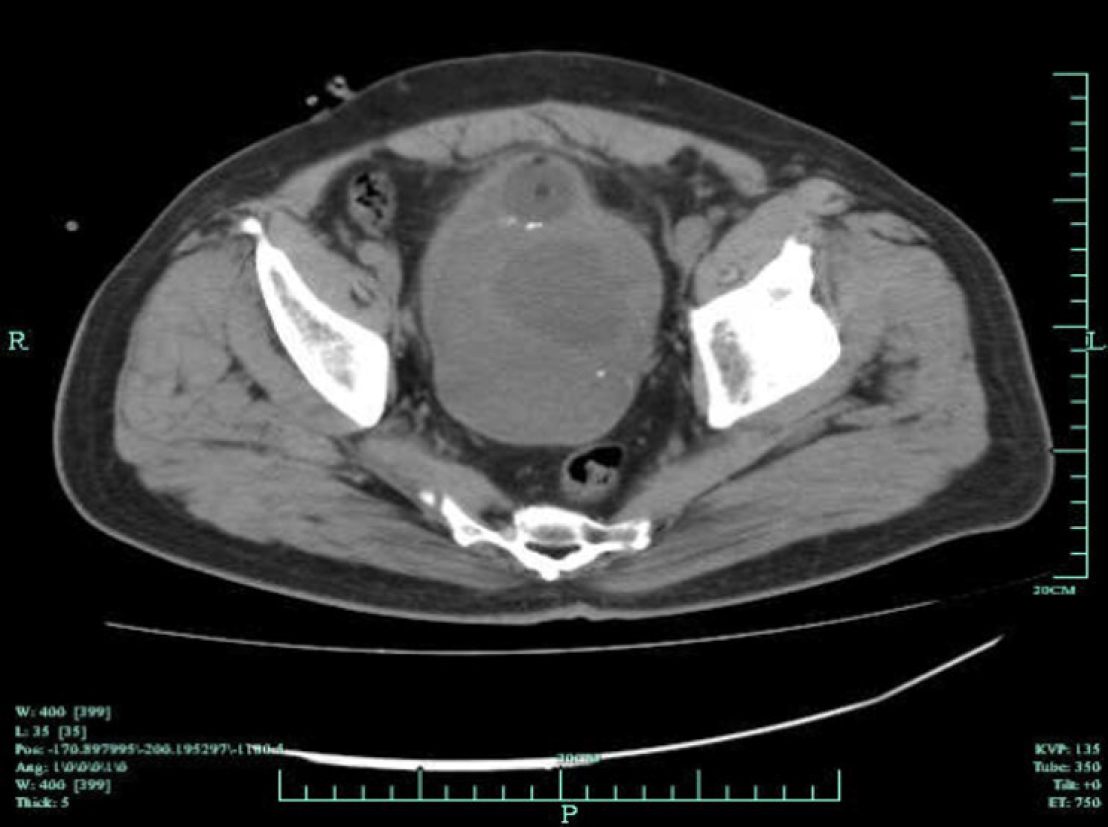
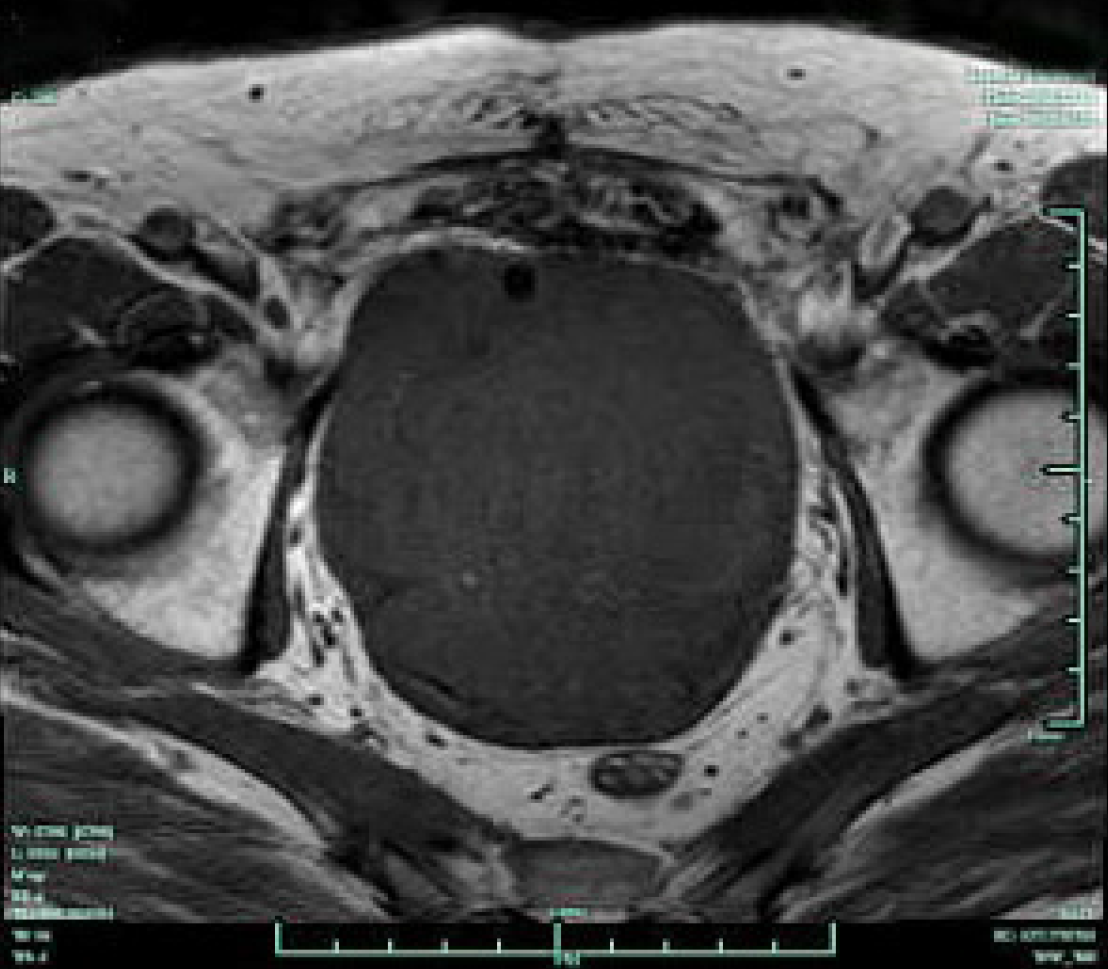
Preoperative pelvic CT imaging demonstrated an approximately 15 cm × 9 cm × 8 cm-large tumor mass with central necrosis distorting the bladder neck, which could only be recognized by a catheter balloon (Figure 1). Magnetic resonance imaging showed a large tumor mass occupying the majority of the pelvic cavity with no evidence of rectal metastasis (Figure 2). Both bone scans and chest X-rays were negative for metastases.
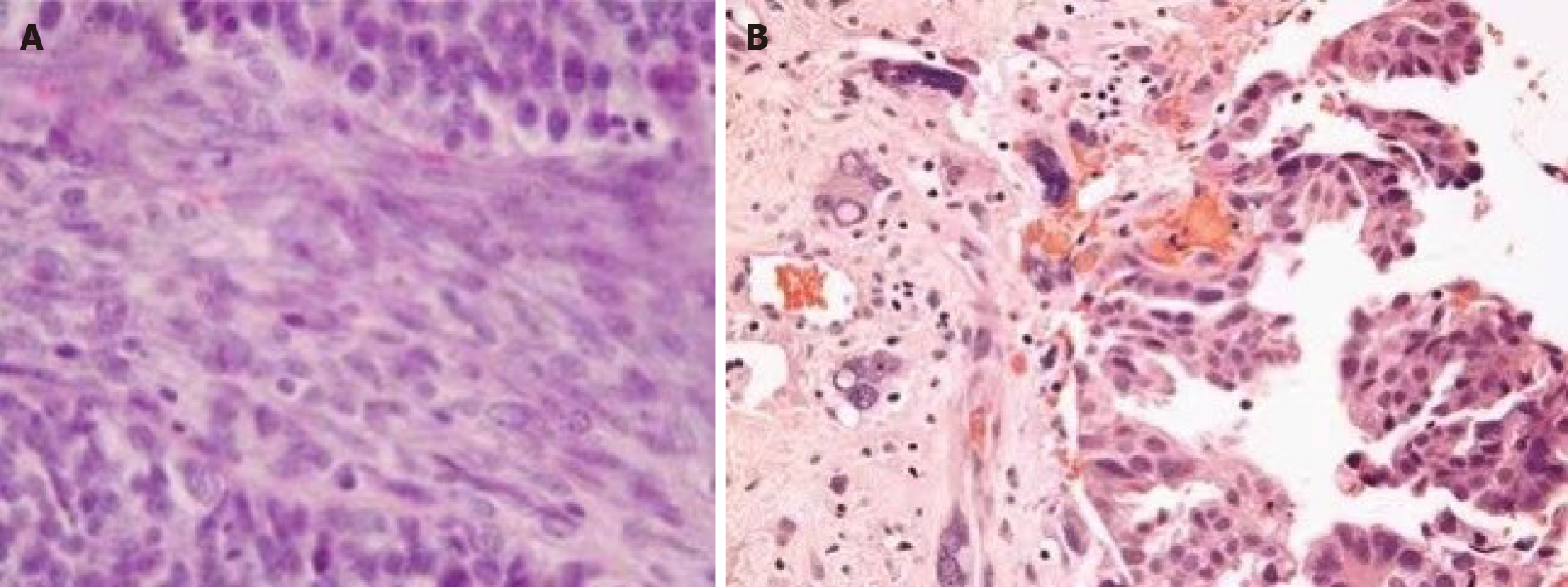
Transrectal biopsy showed a poorly differentiated carcinosarcoma. Surgical pathology demonstrated that the tumor tissue had extensive hemorrhage and central necrosis. The whole bladder wall was invaded at one site. Increased nucleoli and mitotic figures and adenoid structures were seen. Immunostaining confirmed the final pathology as prostatic carcinosarcoma with focal spindled cells and bizarre cells (Figure 3): Cytokeratin (partially positive), epithelial membrane antigen (-), vimentin (+), PSA (less than 30% positive), desmin (-), CD117 (-), S-100 (-), CD34 (-), and BCL-2 (-).
The patient received total pelvic exenteration with ureterostomy for urinary diversion. Nevertheless, the patient subsequently underwent transverse colostomy for carcinosarcoma recurrence and bowel obstruction 3 mo later.
Five months after the diagnosis of prostatic sarcoma, the patient died of multiple organ metastases.
Prostatic carcinosarcoma is an exceedingly rare and aggressive malignant tumor that may develop many years after original presentation with prostatic adenocarcinoma. The average diagnosis age is about 66 years or more in all published cases[3,5,6]. McGee et al[3] found that in fewer than 100 patients, 58% had a previous history of prostatic adenocarcinoma. Most patients (61%) had a previous treatment history of radiation, ADT, or combination thereof. Only 3% of patients had a history of radical prostatectomy. Many theories or hypotheses have been developed regarding the origin of prostate carcinosarcoma, especially transformation of adenocarcinoma into sarcoma[4,7]. Although a variety of hypotheses have been presented regarding the biphasic nature of these tumors, carcinosarcomas seem to represent the best example of the epithelial-mesenchymal transition and dedifferentiation in human cancers, in which the two parts of the tumor are genomically related to one another[8]. As more than half of carcinosarcoma cases have been diagnosed after the diagnosis of adenocarcinoma, it is logical to think that sarcomatous components arise secondarily through metaplastic phenomena or transformation within the adenocarcinoma itself[7]. Prior reports in the literature have raised the possibility that treatments especially radiation or hormonal therapy may influence the subsequent development of sarcomatoid carcinomas[4,6]. Our patient had no history of radiation. However, ADT therapy alone may also lead to the development of a sarcomatous component. However, some investigators discount the role of ADT because of the majority of patients treated with ADT without developing these uncommon carcinosarcomas[2,5]. Thus, the reason for this exceedingly rare ADT mediated adenocarcinoma to carcinosarcoma tran
Patients with carcinosarcoma often have a normal or lower serum PSA level than that of those with adenocarcinoma. This malignant neoplasm may also contain multiple sarcomatoidentities[3,9]. The most commonly found was osteosarcoma (50%), followed by chondrosarcoma (33%) and leiomyosarcoma (17%). Cases of rhabdomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma, spindle cell sarcoma, angiosarcoma, and undifferentiated sarcoma have also been reported[5,6,10]. As previously reported, the prognosis of prostatic carcinosarcoma is extremely poor, with a 20% risk of death 1 year after diagnosis. Radical surgery or pelvic exenteration seems to be the best treatment for patients with prostatic carcinosarcoma. Many patients develop subsequent metastatic disease and a local bulky tumor burden[2,6,10].
Carcinosarcoma of the prostate is a very rare and highly aggressive tumor. ADT mediated transformation and dedifferentiation of the epithelial component may be the origin of this malignancy. Radical surgery is the most viable option for treatment. If the disease has spread beyond the prostatic tissue, the patient typically will not survive for more than 6 mo. The prognosis is dismal regardless of histological or clinical findings.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ichihara K, Scaggiante B, Sha S S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Haddad JR, Reyes EC. Carcinosarcoma of the prostate with metastasis of both elements: case report. J Urol. 1970;103:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Dundore PA, Cheville JC, Nascimento AG, Farrow GM, Bostwick DG. Carcinosarcoma of the prostate. Report of 21 cases. Cancer. 1995;76:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | McGee SM, Boorjian SA, Karnes RJ. Carcinosarcoma of the prostate replacing the entire lower genitourinary tract. Urology. 2009;74:540-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Poblet E, Gomez-Tierno A, Alfaro L. Prostatic carcinosarcoma: a case originating in a previous ductal adenocarcinoma of the prostate. Pathol Res Pract. 2000;196:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Subramanian VS, Coburn M, Miles BJ. Carcinosarcoma of the prostate with multiple metastases: case report and review of the literature. Urol Oncol. 2005;23:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Hansel DE, Epstein JI. Sarcomatoid carcinoma of the prostate: a study of 42 cases. Am J Surg Pathol. 2006;30:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Kubosawa H, Matsuzaki O, Kondo Y, Takao M, Sato N. Carcinosarcoma of the prostate. Acta Pathol Jpn. 1993;43:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Pang A, Carbini M, Moreira AL, Maki RG. Carcinosarcomas and Related Cancers: Tumors Caught in the Act of Epithelial-Mesenchymal Transition. J Clin Oncol. 2018;36:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Rogers CG, Parwani A, Tekes A, Schoenberg MP, Epstein JI. Carcinosarcoma of the prostate with urothelial and squamous components. J Urol. 2005;173:439-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Furlan SR, Kang DJ, Armas A. Prostatic carcinosarcoma with lung metastases. Case Rep Oncol Med. 2013;2013:790790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |