Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6308
Peer-review started: April 8, 2021
First decision: April 28, 2021
Revised: May 12, 2021
Accepted: June 7, 2021
Article in press: June 7, 2021
Published online: August 6, 2021
Processing time: 110 Days and 17.3 Hours
A growing amount of evidence provides support for the hypothesis that acute myocardial infarction (AMI) patients should go through cardiopulmonary exercise testing (CPET) about 3-5 d after AMI is diagnosed, make reasonable exercising prescription, and conduct exercise training under guidance.
To investigate the effect of exercise training (ET) on left ventricular systolic function and left ventricular remodeling (LVRM) and to study the possible mechanisms of LVRM by the changes of matrix metallopeptidase 9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) in patients with acute ST-segment elevation myocardial infarction (STEMI).
Sixty patients with first STEMI undergoing direct percutaneous coronary intervention from February 2008 to October 2008 were randomly assigned to an exercise group (n = 30) and a control group (n = 30). The levels of MMP-9 and TIMP-1 were measured in all patients at 1 d, 10-14 d, 30 d, and 6 mo after admission. Two-dimensional echocardiography and cardiopulmonary exercise testing were done in patients at 10-14 d and 6 mo after admission.
There was no significant difference in CPET at baseline between the exercise group and the control group. At 6 mo, the time of exercise, peak and anaerobic threshold values of O2 uptake, and metabolic equivalents increased in both groups, but markedly increased in the exercise group. At baseline, there were no significant differences in left ventricular ejection fraction (LVEF) between the two groups. At 6 mo, LVEF increased in the exercise group, but not in the control group. At 6 mo, the percentage of patients with positive result of LVRM was 26.6% in the exercise group and 52.6% in the control group (P < 0.05). The levels of plasma MMP-9 and TIMP-1 and the ratio of MMP-9 to TIMP-1 in both groups had no significant difference at 1 d and 10-14 d after AMI, but at 30 d and 6 mo, the levels of plasma MMP-9 and TIMP-1 in the exercise group were significantly lower than those in the control group; the ratio of MMP-9 to TIMP-1 in the exercise group was significantly higher than that in the control group.
ET under supervision based on home condition in early and recovery stage of AMI can improve exercise cardiopulmonary function and prevent the LVRM. Therefore, it may reduce unfavorable remodeling response by decreasing the levels of plasma MMP-9 and TIMP-1 and adjusting the ratio of MMP-9 to TIMP-1 hereafter.
Core Tip: Exercise training under supervision based on home condition in early and recovery stage of acute myocardial infarction can improve exercise cardiopulmonary function and prevent the left ventricular remodeling. And there was no negative effect on left ventricular ejection fraction. Therefore, it may reduce unfavorable remodeling response by decreasing the levels of plasma matrix metallopeptidase 9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) and adjusting the ratio of MMP-9 to TIMP-1 hereafter.
- Citation: Cai M, Wang L, Ren YL. Effect of exercise training on left ventricular remodeling in patients with myocardial infarction and possible mechanisms. World J Clin Cases 2021; 9(22): 6308-6318
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6308.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6308
Cardiac pulmonary function recovery after acute myocardial infraction (AMI) for a long time has been taking the attention of the vast numbers of medical professionals and patients[1], and the occurrence and development of left ventricular remodeling (LVRM) in patients are vital for AMI prognosis. A growing amount of evidence provides support for the hypothesis that AMI patients should go through cardiopulmonary exercise testing (CPET) about 3-5 d after AMI is diagnosed, make reasonable exercising prescription, and conduct exercise training (ET) under supervision[2,3]. Moreover, it can improve left ventricular systolic function, exercise tolerance, and quality of life, with no adverse impact on cardiac structure[4,5]. Furthermore, previous studies indicated[6,7]that ET can also help slow down the development of LVRM.
Many experiments support that matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) play an important role in LVRM[8,9]. However, so far, no study has been done on the relationship between rehabilitation exercise and changes of MMP-9 and TIMP-1 plasma levels in AMI.
In the present study, we investigated the effect of ET on LVRM in patients with acute ST segment elevation myocardial infarction (STEMI) and explored the possible mechanisms by detecting variations of MMP-9 and TIMP-1.
Sixty patients who experienced the first occurrence of STEMI within the period from February to October 2008 at our hospital were recruited. All patients had Killip grade I-II cardiac function and received direct percutaneous coronary intervention (PCI) at the Emergency Department.
Inclusion criteria: The diagnostic criteria for STEMI complied with ACC/AHA Guidelines for the Management of Patients with ST Elevation Myocardial Infarction[10]. The inclusion criteria were: (1) First occurrence of acute STEMI; (2) Age 35-74 years; (3) Grade I-II (Killip’s) cardiac function as revealed by LVEF > 40%; (4) Absence of serious cardiac arrhythmia; (5) Absence of serious post-AMI complications; and (6) Willingness to receive CEPT and ET treatment and provide informed consent.
Exclusion criteria: The exclusion criteria were: (1) Suspected diagnosis of AMI that does not meet the above diagnostic criteria; (2) History of AMI and heart valve diseases and pulmonary embolisms; (3) Serious complications of acute AMI (pulmonary edema, serious cardiac arrhythmia, and cardiac shock); (4) Concomitant atrial fibrillation or paced rhythm or preexcitation syndrome; (5) Concomitant serious diseases of other systems (e.g., human immunodeficiency virus infection, malignant tumor, serious primary hepatic or renal diseases, and chronic lung diseases); (6) Inability to exercise and unwillingness to cooperate; (7) Unwillingness or inability to accept PCI; and (8) Unwillingness to provide informed consent.
Standard secondary prevention and treatment of coronary heart disease were administered in all patients according to Guidelines for the Management of Acute Coronary Syndrome.
Randomization: Sixty patients were randomized in a 1:1 ratio to either a rehabilitation exercise group (rehabilitation exercise guidance was provided) (n = 30) or a control group (no rehabilitation exercise guidance was provided) (n = 30). Routine post-AMI secondary prevention medications were given regularly to patients in both groups.
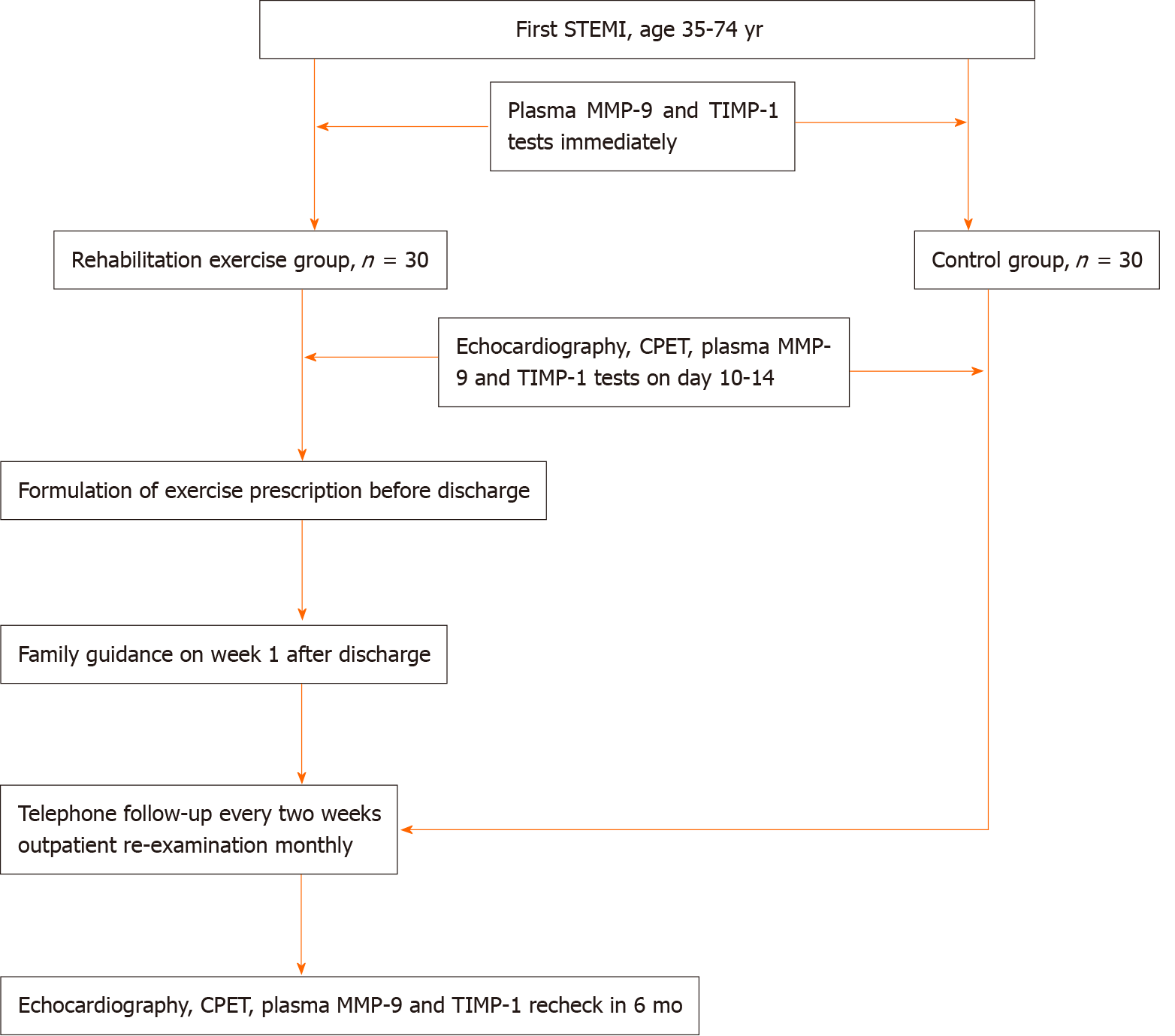
Study protocol: Emergency PCI was performed after admission. Blood samples were drawn immediately, at days 10-14, day 30, and 6 mo after admission, and plasma was separated and stored in a -80 oC freezer for plasma MMP-9 and TIMP-1 tests. Echocardiography was performed at days 10-14 and 6 mo after AMI. CPET was performed at days 10-14 after AMI and again at 6 mo. After discharge, the patients routinely took secondary prevention medications for coronary heart disease. Patients in the rehabilitation exercise group took regular walking exercise. Exercise time, intensity, and frequency depended on the exercise prescription. Family guidance was provided to patients in this group and the exercise prescription was adjusted at week 1. Patients in the control group were not given any exercise prescription and their exercise was not intervened. For patients in both groups, telephone follow-up was performed every 2 wk and outpatient follow-up monthly until the end of 6 mo. Figure 1 and Table 1 show the protocol in detail.
| Item | Immediately | Days 10-14 | Follow-up (mo) | |
| 1 | 6 | |||
| Informed consent | √ | |||
| Medical history | √ | |||
| Physical examination | √ | √ | √ | |
| MMP-9 | √ | √ | √ | √ |
| TIMP-1 | √ | √ | √ | √ |
| Transthoracic echocardiography | √ | √ | ||
| CPET | √ | √ | ||
| Concomitant medication | √ | √ | √ | √ |
Fasting venous blood (3 mL) was drawn in the morning, added into a vacuum blood collection tube containing 10% EDTA, mixed well, and centrifuged at 3000 rpm at 4 °C for 10 min. The supernatant (20 μL) was retained and preserved in a -80 °C freezer. ELISA was carried out by special personnel blinded using kits manufactured by BIOSAURCE.
Evaluation of ventricular remodeling was performed by experienced and professional echocardiography physicians at days 10-14 and month 6 after the AMI onset.
Routine ultrasound and Doppler tissue images were collected in the long and short axis views of the left ventricle, apical four-chamber view, left ventricular two-chamber view, and the apical long axis view using a VIVID-I echocardiography system manufactured by GE (United States).
LVEF was evaluated using Biplane Simpson’s method as follows:
LVEF = (LVEDD - LVESD)/LVEDV × 100% (left ventricular end-diastolic dimen
Biplane Simpson method was performed to measure LVESV and LVEDV. LVESV and LVEDV were measured at days 10-14 (LVESV1 and LVEDV1) and 6 mo (LVESV2 and LVEDV2) after AMI. If both LVESV2/LVESV1 and LVEDV2/LVEDV1 were higher than 1.1 or either one was higher than 1.2 (i.e., increased by 10%-20% as compared to the baseline value), left ventricular dilation would be considered.
The measurement was performed by professional rehabilitation physicians.
CPET was performed using a MAX-II cardiopulmonary exercise device manu
Graded treadmill exercise test, 3 min per grade, was carried out using the modified Bruce protocol. The patient’s movement electrocardiogram, gaseous metabolism changes, and vital signs were monitored, and the spontaneous symptoms were inquired about any time.
The patient’s exercise time, peak oxygen uptake, peak metabolic equivalent, anaerobic threshold, and metabolic equivalent at anaerobic threshold were measured.
The evaluation was terminated in any of the following circumstances: (1) The patient developed instability of gait, weakness of bilateral lower limbs, dizziness, pale complexion, fatigue, short breath, and asthma or the patient asked for rest; (2) The systolic pressure increased by less than 10 mmHg or decreased as compared to the level at rest; the blood pressure increased abnormally, and the systolic pressure was higher than 230 mmHg; (3) Higher than grade II angina (Canadian angina grading); (4) Malignant arrhythmia occurred, including ventricular tachycardia, multifocal premature ventricular contraction or higher than second-degree type II atrio
Individualized exercise prescriptions were prepared for patients in the rehabilitation exercise group by rehabilitation physicians according to CPET results before discharge. The exercise method was walking, and the exercise time, intensity, and frequency were implemented per the exercise prescription. In addition to walking rate prepared by the rehabilitation physician, patient’s self-felt fatigue was considered for controlling the exercise intensity. Patients were asked to exercise at the anaerobic threshold level or a slightly lower level. Patients in the control group were not given the exercise prescription or any exercise guidance and were allowed to arrange their daily activities themselves.
Statistical analyses were performed using SPSS13.0 software. Measurement data are presented as the mean ± SD. For comparison of continuous variables between two groups, t test was carried out. P values < 0.05 were considered statistically significant.
A total of 60 male patients (mean age, 56 ± 8 years) were enrolled in this study, 30 in each group.
Tables 2 presents the patients’ characteristics at baseline. Patients in the two groups were not statistically different in age, myocardial infarction area, history of smoking, or concomitant diseases (P > 0.05). All patients accepted standard secondary prevention and treatment of coronary heart disease using aspirin, clopidogrel, statins, angiotensin-converting enzyme inhibitor (ACEI)/angiotensinreceptorblocker (ARB), β-receptor blocker, etc., with no statistical difference between the two groups (P > 0.05). Data of the two groups were comparable (Table 2).
| Rehabilitation exercise group | Control group | |
| n = 30 | n = 30 | |
| Age (yr) | 55 ± 9 | 58 ± 8 |
| Infarction area, n (%) | ||
| Anterior AMI | 18 (60) | 15 (50) |
| Non-anterior AMI | 12 (40) | 15 (50) |
| History of smoking, n (%) | 28 (93.3) | 24 (80) |
| Concomitant disease, n (%) | ||
| Diabetic mellitus | 2 (6.7) | 3 (10) |
| Hypertension | 5 (16.6) | 4 (13.3) |
| Hypercholesteremia | 3 (10) | 4 (13.3) |
| Drug therapy, n (%) | ||
| Aspirin | 30 (100) | 30 (100) |
| Clopidogrel | 29 (96.7) | 28 (93.3) |
| β-receptor blocker | 25 (83.3) | 28 (93.3) |
| ACEI/ARB | 29 (96.7) | 27 (90) |
| Statins | 28 (93.3) | 29 (96.7) |
No significant difference was found between the two groups with respect to baseline exercise time, anaerobic threshold, metabolic equivalent at anaerobic threshold, peak oxygen uptake, and peak metabolic equivalent at days 10-14 after admission (P > 0.05). The above exercise indicators significantly increased at the follow-up visit at month 6 as compared to baseline values (P < 0.05-0.01) (Table 3), with the increase being significantly more pronounced in the rehabilitation group (P < 0.05-0.01) (Table 4).
| Baseline | 6 mo after onset of myocardial infarction | |||
| Control group (n = 30) | Exercise group (n = 30) | Control group (n = 30) | Exercise group (n = 30) | |
| Exercise time (min) | 8.19 ± 2.55 | 8.11 ± 2.69 | 9.37 ± 2.48a | 11.89 ± 2.34b |
| Anaerobic threshold (mL/min) | 958.64 ± 298.65 | 977.23 ± 288.33 | 1235.88 ± 347.56b | 1388.07 ± 355.67b |
| Metabolic equivalent at anaerobic threshold | 3.76 ± 1.05 | 3.89 ± 1.01 | 4.58 ± 1.06b | 5.20 ± 1.19b |
| Peak oxygen uptake (mL/min) | 1272.37 ± 346.85 | 1267.35 ± 322.84 | 1507.88 ± 398.46b | 1664.67 ± 365.35b |
| Peak metabolic equivalent | 4.98 ± 1.03 | 4.87 ± 0.98 | 5.62 ± 1.01b | 6.38 ± 1.09b |
| Changes of the two tests | ||
| Control group (n = 30) | Exercise group (n = 30) | |
| Exercise time (min) | 1.13 ± 1.69 | 3.41 ± 2.88b |
| Anaerobic threshold (mL/min) | 252.13 ± 233.66 | 405.36 ± 207.89a |
| Metabolic equivalent at anaerobic threshold | 0.82 ± 0.71 | 1.46 ± 0.82b |
| Peak oxygen uptake (mL/min) | 232.73 ± 254.96 | 402.88 ± 258.35a |
| Peak metabolic equivalent | 0.77 ± 0.86 | 1.49 ± 0.93b |
LVRM was considered positive when both LVEDV2/LVEDV1 and LVESV2/LVESV1 were higher than 1.1 or either one was higher than 1.2[4]. The positive rate in the control group was significantly higher than that in the rehabilitation exercise group (52.6% vs 26.6%, P < 0.01).
Baseline values of LVEF of the two groups were not significantly different. LVEF of the rehabilitation exercise group at the follow-up visit at month 6 was higher than but not statistically significantly different from the baseline value (58.4% ± 8.6% vs 57.73% ± 7.89%, P > 0.05), and LVEF of the control group at the follow-up visit at month 6 was lower than but not statistically significantly different from the baseline value (53% ± 8.86% vs 56.1% ± 7.98%, P > 0.05). LVEF of the rehabilitation exercise group at month 6 was significantly higher than that of the control group (58.4% ± 8.6% vs 53% ± 8.86%, P < 0.01; Table 5).
Plasma MMP-9 levels of patients in the two groups were not significantly different immediately after AMI and at days 10-14 (P > 0.05). It decreased in both groups at day 30 and month 6, with the decrease being more pronounced in the rehabilitation exercise group (P < 0.05).
Plasma TIMP-1 levels of patients in the two groups were not significantly different immediately after AMI and at days 10-14 (P > 0.05). It increased in both groups at day 30 and month 6, with the increase being less pronounced in the rehabilitation exercise group (P < 0.05).
MMP-9/TIMP-1 ratio of patients in the two groups was not significantly different immediately after AMI and at days 10-14 (P > 0.05). The MMP-9/TIMP-1 ratio of the rehabilitation exercise group was significantly higher than that of the control group at day 30 and month 6 (P < 0.05; Table 6).
| Items | Time | Rehabilitation exercise group (n = 30) | Control group (n = 30) |
| MMP-9 (ng/mL) | Immediately | 6.7 ± 2.97 | 6.8 ± 3.12 |
| Days 10-14 | 8.2 ± 2.5 | 8.1 ± 2.55 | |
| Day 30 | 4.2 ± 1.88 | 5.0 ± 1.98a | |
| 6 mo | 4.0 ± 1.87 | 5.1 ± 2.02a | |
| TIMP-1 (ng/mL) | Immediately | 2.1 ± 0.52 | 2.1 ± 0.51 |
| Days 10-14 | 2.4 ± 0.71 | 2.5 ± 0.65 | |
| Day 30 | 2.8 ± 0.67 | 3.0 ± 0.55a | |
| 6 mo | 2.7 ± 0.56 | 2.98 ± 0.51a | |
| MMP-9/TIMP-1 | Immediately | 3.05 ± 1.05 | 2.98 ± 1.03 |
| Days 10-14 | 4.21 ± 1.58 | 4.11 ± 1.44 | |
| Day 30 | 1.78 ± 0.88 | 1.25 ± 0.66a | |
| 6 mo | 1.58 ± 0.67 | 1.22 ± 0.58a |
This study examined the effect of exercise training on LVRM in patients with STEMI. Ultrasonic cardiogram (UCG) showed that LVEF of the ET group was significantly higher than that of the control group, indicating that guided rehabilitation exercise in early stage of AMI could maintain patient’s left ventricular systolic function, which is consistent with findings in previous studies[11,12]. In contrast with a decrease of cardiac function in the control group, a slight increase was observed in the ET group, possibly for the following reasons. (1) The patients’ LVRM was mild; (2) Exercise increased myocardial elastic recoil, decreased resting heart rate, and prolonged left ventricular filling time in AMI patients, thereby enlarging cardiac output; and (3) Exercise reduced poor wall motion and increased left ventricular systolic synchrony.
Consistent with previous reports[13-19], ET resulted in a significantly lower LVRM positive rate (26.6% in ET group vs 52.6% in control group) using LVEDV2/LVEDV1 and LVESV2/LVESV1 ratio as LVRM positive indicators, suggesting that LVRM was more apparent in the control group than in the ET group and ET may, to a certain extent, limit LVRM.
The study results showed that plasma levels of MMP-9 and TIMP-1, and MMP-9/TIMP-1 ratio of patients in the ET group and the control group were not significantly different immediately after AMI and at days 10-14. At day 30 and month 6, compared with control group, plasma MMP-9 and TIMP-1 levels were significantly lower and MMP-9/TIMP-1 ratio was significantly higher in the ET group, in which positive LVRM rate was higher, indicating that high plasma MMP-9 and TIMP-1 levels and low MMP-9/TIMP-1 ratio in post-AMI recovery period all had adverse effects on LVRM.
Extracellular matrix (ECM) remodeling was found in recent studies to lead to myocardial fibrosis and progressive ventricular dilatation and to result in heart failure[20-22]. It is currently believed that the ECM plays a very important role in LVRM and that ECM synthesis-degradation imbalance is associated with development and progression of LVRM. Among the ECM secreted by fibroblasts, collagen, mainly type I and type III collagen, accounts for the major part. About 85% of the collagen is type I, which is responsible for myocardial strength and endurance, and about 11% is type III, which is responsible for myocardial elastic recoil. MMP-9 is a gelatinase, which mainly degrades type I and type III collagen. TIMP-1 is a natural inhibitor of MMP-9 and promotes synthesis and deposition of collagen. MMP-9/TIMP-1 imbalance is thought to be able to further enhance remodeling progression[23]. Kelly et al[24] studied variation of post-AMI MMP-9 levels and found that increased MMP-9 level at early stage of AMI was positively correlated with leukocyte and neutrophil levels, suggesting that plaque rupture and increased inflammatory factors promoted the release of MMP-9, which intensified collagen degradation and contributed to the development and progression of early-phase LVRM. At the subsequent 90-d follow-up, MMP-9 curve turned from a high peak in the acute phase into a flat top, which had a protective effect on left ventricular function. According to the analysis, excessively high MMP-9 levels enhanced collagen degradation whereas excessively high TIMP-1 level led to excessive decomposition of collagen. Only when an appropriate ratio is maintained can the normal shape and function of cardiac ventricles be kept.
Significantly more decreased plasma MMP-9 level and less increased plasma TIMP-1 level were observed in ET patients at day 30 and month 6, as compared to control patients, indicating that although collagen synthesis and deposition are inevitable in late stage of AMI, rehabilitation exercise might delay LVRM occurrence and lower positive LVRM rate by inhibiting excessive degradation and reducing excessive synthesis and deposition of collagen.
Although the mechanism of LVRM is still not completely known, MMP-9/TIMP-1 imbalance is suggested as an important reason for ECM remodeling, as also shown in the present study[25]. MMP-9/TIMP-1 ratio of the ET group was significantly higher than that of the control group; namely, the proportion of TIMP-1 in plasma of patients in the ET group was lower. Evidence is available that high concentration of TIMP-1 in plasma may increase the activity of fibroblasts and enhance collagen synthesis and deposition[26]. It is thus deduced that ET has decreased the concentration of TIMP-1 in plasma, adjusted the proportions of MMP-9 and TIMP-1, and reduced excessive collagen synthesis and deposition in late stage of AMI. The effect of ET on post-AMI LVRM of patients is possibly related to its action in adjusting the MMP-9/TIMP-1 ratio.
However, this study has the following limitations: (1) Although the principle of randomization was followed as far as possible in design and implementation of the trial, given the relatively small sample size, some artificial factors such as patients’ individual exercise habits and working time could not be excluded, leading to randomness reduction and selection bias; (2) Rehabilitation exercise was home based. Although telephone follow-up was performed once every 2 wk and outpatient follow-up once per month, the problem of unfixed exercise intensity of the ET group still could not be avoided, leading to errors in study results; (3) The exercise intensity administered in the study was low and the follow-up duration was short. Therefore, individual exercise habits account for an important part of exercise intensity, which might lead to errors in study results; (4) Regarding exercise indicators, the first UCG examination was performed at days 10-14 after AMI and the result was used as the baseline UCG value. The effect of post-AMI early-stage LVRM on ventricular structure and function was neglected, which may have certain influence on the study results; and (5) Due to limited fund and patients’ reasons, cardiac structure and function were not evaluated using heart MRI or ventriculography. UCG evaluation is subject to subjective influence.
Early rehabilitation exercise after AMI can, to some extent, inhibit progression of LVRM and maintain left ventricular function. Moreover, the post-AMI LVRM limitation by ET is likely due to its action in lowering MMP-9 and TIMP-1 levels and adjusting MMP-9/TIMP-1 ratio.
A growing amount of evidence provides support for the hypothesis that acute myocardial infarction (AMI) patients should go through cardiopulmonary exercise testing about 3-5 d after AMI is diagnosed, make reasonable exercising prescription, and conduct exercise training under guidance.
To investigate the effect of exercise training on left ventricular systolic function and left ventricular remodeling and to study the possible mechanisms of left ventricular remodeling (LVRM) by the changes of matrix metallopeptidase 9 (MMP-9) and tissue inhibitor of metalloproteinases 1 (TIMP-1) in patients with acute ST-segment elevation myocardial infarction (STEMI).
To investigate the effect of exercise training on left ventricular systolic function and left ventricular remodeling and to study the possible mechanisms of LVRM.
Sixty patients with first STEMI undergoing direct percutaneous coronary intervention from February 2008 to October 2008 were randomly assigned to an exercise group (n = 30) or a control group (n = 30). The levels of MMP-9 and TIMP-1 were measured in all patients at 1 d, 10-14 d, 30 d, and 6 mo after admission. Two-dimensional echocardiography and cardiopulmonary exercise testing were done in patients at 10-14 d and 6 mo after admission.
In cardiopulmonary exercise testing of the two group, at 6 mo, the time of exercise, peak and anaerobic threshold values of O2 uptake, and metabolic equivalents increased in both groups, but markedly increased in the exercise group than in the control group. There was no significant differences in LVESV, LVEDV, or left ventricular ejection fraction (LVEF) between the two groups. At 6 mo, LVEF increased in the exercise group, but not in the control group. Change in LVEF in the exercise group was significantly higher than that of the control group. At 6 mo, the percentage of patients with positive result of LVRM was 26.6% in the exercise group and 52.6% in the control group (P < 0.05). The levels of plasma MMP-9 and TIMP-1 and the ratio of MMP-9 to TIMP-1 in both groups had no significant difference at 1 d and 10-14 d after AMI, but at 30 d and 6 mo, the levels of plasma MMP-9 and TIMP-1 in the exercise group were significantly lower than those in the control group; the ratio of MMP-9 to TIMP-1 in the exercise group was significantly higher than that in the control group.
Exercise training under supervision based on home condition in early and recovery stage of AMI can improve exercise cardiopulmonary function and prevent the LVRM. Therefore, it may reduce unfavorable remodeling response by decreasing the levels of plasma MMP-9 and TIMP-1 and adjusting the ratio of MMP-9 to TIMP-1 hereafter.
Randomized controlled trials are needed to investigate the effect of exercise training on left ventricular systolic function and LVRM and to study the possible mechanisms of LVRM in patients with acute STEMI.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Snyder J S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Acar RD, Bulut M, Ergün S, Yesin M, Eren H, Akçakoyun M. Does cardiac rehabilitation improve left ventricular diastolic function of patients with acute myocardial infarction? Turk Kardiyol Dern Ars. 2014;42:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Trachsel LD, David LP, Gayda M, Henri C, Hayami D, Thorin-Trescases N, Thorin É, Blain MA, Cossette M, Lalongé J, Juneau M, Nigam A. The impact of high-intensity interval training on ventricular remodeling in patients with a recent acute myocardial infarction-A randomized training intervention pilot study. Clin Cardiol. 2019;42:1222-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Peixoto TC, Begot I, Bolzan DW, Machado L, Reis MS, Papa V, Carvalho AC, Arena R, Gomes WJ, Guizilini S. Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: a randomized controlled trial. Can J Cardiol. 2015;31:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Zhang YM, Lu Y, Tang Y, Yang D, Wu HF, Bian ZP, Xu JD, Gu CR, Wang LS, Chen XJ. The effects of different initiation time of exercise training on left ventricular remodeling and cardiopulmonary rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Disabil Rehabil. 2016;38:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Rivas-Estany E, Sixto-Fernández S, Barrera-Sarduy J, Hernández-García S, González-Guerra R, Stusser-Beltranena R. [Effects of long-term exercise training on left ventricular function and remodeling in patients with anterior wall myocardial infarction]. Arch Cardiol Mex. 2013;83:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Tucker WJ, Beaudry RI, Liang Y, Clark AM, Tomczak CR, Nelson MD, Ellingsen O, Haykowsky MJ. Meta-analysis of Exercise Training on Left Ventricular Ejection Fraction in Heart Failure with Reduced Ejection Fraction: A 10-year Update. Prog Cardiovasc Dis. 2019;62:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | McGREGOR G, Gaze D, Oxborough D, O'Driscoll J, Shave R. Reverse left ventricular remodeling: effect of cardiac rehabilitation exercise training in myocardial infarction patients with preserved ejection fraction. Eur J Phys Rehabil Med. 2016;52:370-378. [PubMed] |
| 8. | Iyer RP, Jung M, Lindsey ML. MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;311:H190-H198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Kwon JS, Kim YS, Cho AS, Cho HH, Kim JS, Hong MH, Jeong HY, Kang WS, Hwang KK, Bae JW, Jeong MH, Cho MC, Ahn Y. Regulation of MMP/TIMP by HUVEC transplantation attenuates ventricular remodeling in response to myocardial infarction. Life Sci. 2014;101:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 1136] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Cao H, Jiang P, Tang H. Cardiac rehabilitation in acute myocardial infarction patients after percutaneous coronary intervention: A community-based study. Medicine (Baltimore). 2018;97:e9785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Vilela EM, Ladeiras-Lopes R, Ruivo C, Torres S, Braga J, Fonseca M, Ribeiro J, Primo J, Fontes-Carvalho R, Campos L, Miranda F, Nunes JPL, Gama V, Teixeira M, Braga P. Different outcomes of a cardiac rehabilitation programme in functional parameters among myocardial infarction survivors according to ejection fraction. Neth Heart J. 2019;27:347-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Santos-Hiss MD, Melo RC, Neves VR, Hiss FC, Verzola RM, Silva E, Borghi-Silva A, Porta A, Montano N, Catai AM. Effects of progressive exercise during phase I cardiac rehabilitation on the heart rate variability of patients with acute myocardial infarction. Disabil Rehabil. 2011;33:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Xu L, Cai Z, Xiong M, Li Y, Li G, Deng Y, Hau WK, Li S, Huang W, Qiu J. Efficacy of an early home-based cardiac rehabilitation program for patients after acute myocardial infarction: A three-dimensional speckle tracking echocardiography randomized trial. Medicine (Baltimore). 2016;95:e5638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Cha S, Park JJ, Kim S, Ahn HY, Han K, Lee Y, Kim WS, Paik NJ. Need for Systematic Efforts to Modify Health-Related Behaviors After Acute Myocardial Infarction in Korea. Circ J. 2018;82:2523-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Blumenthal JA, Babyak MA, Carney RM, Huber M, Saab PG, Burg MM, Sheps D, Powell L, Taylor CB, Kaufmann PG. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc. 2004;36:746-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Goto Y. Current state of cardiac rehabilitation in Japan. Prog Cardiovasc Dis. 2014;56:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Kim C, Kim DY, Moon CJ. Prognostic influences of cardiac rehabilitation in korean acute myocardial infarction patients. Ann Rehabil Med. 2011;35:375-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Okutucu S, Aytemir K, Evranos B, Aksoy H, Sabanov C, Karakulak UN, Kaya EB, Kabakci G, Tokgozoglu L, Ozkutlu H, Oto A. Cardiac resynchronization therapy improves exercise heart rate recovery in patients with heart failure. Europace. 2011;13:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Frangogiannis NG. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ Res. 2019;125:117-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 21. | Radosinska J, Barancik M, Vrbjar N. Heart failure and role of circulating MMP-2 and MMP-9. Panminerva Med. 2017;59:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Li L, Zhao Q, Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018;68-69:490-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 23. | Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B, Boichot E, Lagente V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep. 2016;36:e00360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007;28:711-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Opstad TB, Seljeflot I, Bøhmer E, Arnesen H, Halvorsen S. MMP-9 and Its Regulators TIMP-1 and EMMPRIN in Patients with Acute ST-Elevation Myocardial Infarction: A NORDISTEMI Substudy. Cardiology. 2018;139:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Halapas A, Zacharoulis A, Theocharis S, Karavidas A, Korres D, Papadopoulos K, Katopodis H, Stavropoulou A, Lembessis P, Xiromeritis C, Koutsilieris M. Serum levels of the osteoprotegerin, receptor activator of nuclear factor kappa-B ligand, metalloproteinase-1 (MMP-1) and tissue inhibitors of MMP-1 levels are increased in men 6 months after acute myocardial infarction. Clin Chem Lab Med. 2008;46:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |