Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6155
Peer-review started: March 29, 2021
First decision: April 28, 2021
Revised: April 30, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 26, 2021
Processing time: 113 Days and 23.7 Hours
The inflammatory myofibroblastic tumor (IMT) is a rare, idiopathic, usually benign, mass-forming disease with myofibroblastic proliferation and a varying amount of inflammatory cells. Although it can affect various organs, the biliary tract is a rare localization of primary IMT, clinically, endoscopically and radiologically imitating cholangiocarcinoma. The treatment options are based only on clinical practice experience.
A 70-year-old woman was referred to our center due to progressive fatigue, weight loss, abdominal pain, night sweats, and elevated liver enzymes. Magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography (ERCP) revealed proximal common hepatic duct and hilar biliary strictures extending bilaterally to lobular bile ducts. Although initial clinical, endoscopic and radiological signs were typical for hilar cholangiocarcinoma, histological examination showed no signs of malignancy. In total, 8 biopsies using different approaches were performed (several biopsies from dominant stricture during ERCP and direct cholangioscopy; ultrasound-guided liver biopsy; diag
IMT presenting with hilar biliary strictures is a unique diagnostic and clinical challenge as it is indistinguishable from cholangiocarcinoma, and there are no evidence-based treatment options. Our goal is to increase the understanding of this rare disease and its possible course.
Core Tip: Biliary inflammatory myofibroblastic tumor (IMT) is a rare, idiopathic, usually benign, mass-forming disease with myofibroblastic proliferation and the varying amount of inflammatory cells that can occur in almost every organ. IMTs of the biliary ducts are an uncommon cause of obstructive jaundice. The clinical and radiological presentation usually mimics cholangiocarcinoma (Klatskin tumor). However, histological examination shows no malignancy. We present a rare, difficult to diagnose and treat biliary IMT, which was unresponsive to the pharmacological treatment and complicated by recurrent infections and disease progression. The global experience towards diagnosing and treating this disease is limited and based mostly on clinical practice experience.
- Citation: Strainiene S, Sedleckaite K, Jarasunas J, Savlan I, Stanaitis J, Stundiene I, Strainys T, Liakina V, Valantinas J. Complicated course of biliary inflammatory myofibroblastic tumor mimicking hilar cholangiocarcinoma: A case report and literature review. World J Clin Cases 2021; 9(21): 6155-6169
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6155.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6155
An inflammatory myofibroblastic tumor (IMT) is a rare, usually benign mass or multiple masses forming disease with myofibroblastic proliferation along with the varying amount of inflammatory infiltrate. These lesions can be found in every organ throughout the body[1-3]. Although their appearance clinically and radiologically mimics various aggressive malignancies, IMTs are usually considered as benign neoplasms. However, these tumors might also have a diverse spectrum of biological behavior[4]. According to the World Health Organization (WHO) classification, IMT is an intermediate-grade tumor[3]. Several terms have been applied to this condition, such as inflammatory pseudotumor (IPT), fibrous xanthoma, plasma cell granuloma, pseudosarcoma, lymphoid hamartoma, myxoid hamartoma, inflammatory myofibrohistiocytic proliferation, benign myofibrosblastoma, and most recently, IMT. The diverse nomenclature is mainly descriptive and reflects the uncertainty regarding the true biologic nature of these lesions[5,6].
The biliary tract is rarely involved by IMT[1,2]. It should be emphasized that since the first description of liver IMT by Pack and Baker[7] in 1953, over 300 Liver IMT cases have been reported, and very few IMTs were located in a biliary tract[8]. Biliary IMT is a great challenge for clinicians to diagnose and treat, as there are no confirmed recommendations or systemic trials towards treating this condition. We present a unique case of unknown origin, difficult to diagnose and treat biliary IMT presenting with significant biliary strictures, clinically, endoscopically, and radiologically imita
A 70-year-old woman was referred to our tertiary Center of Hepatology, Gastroenterology and Dietetics due to progressive fatigue, weight loss (about 10 kg per year without special diets), right upper abdominal quadrant pain, heatwaves, and night sweats.
The patient was observed by a family physician due to hepatosteatosis and slightly elevated liver enzymes since 2017. Two years later, during the routine yearly check-up, laboratory tests showed a more significant elevation of liver enzymes (aspartate aminotransferase (AST) by 76 U/L, alanine aminotransferase (ALT)) by 80 U/L, alkaline phosphatase by 296 U/L, and gamma-glutamyltransferase by 455 U/L)). She was then further investigated for possible liver diseases. Viral hepatitis B and C, autoimmune hepatic diseases were excluded: The patient was negative for viral hepatitis B and C markers, antimitochondrial (AMA), and antinuclear (ANA) antibody titers were also negative. Abdominal ultrasound showed dilatation of intrahepatic ducts in the right lobe and segment IV, without apparent perihilar tumor signs. There were no gallstones in the gallbladder and common bile duct. The patient was referred to our center for further investigation.
The patient was diagnosed with dyslipidemia, hepatosteatosis, arterial hypertension, nontoxic multinodular goiter. There was no history of jaundice, previous hepatobiliary diseases, infections, or abdominal operations.
The patient denied alcohol consumption, allergies to food or medicines.
On admission, the patient was asthenic (normal body mass index 22 kg/m2), afebrile (body temperature 36.8ºC) with normal vital signs (blood pressure 120/70 mmHg, pulse 88 bpm). There was no visible jaundice and no palpable abdominal pain. The patients’ liver, spleen and superficial lymph nodes were not enlarged.
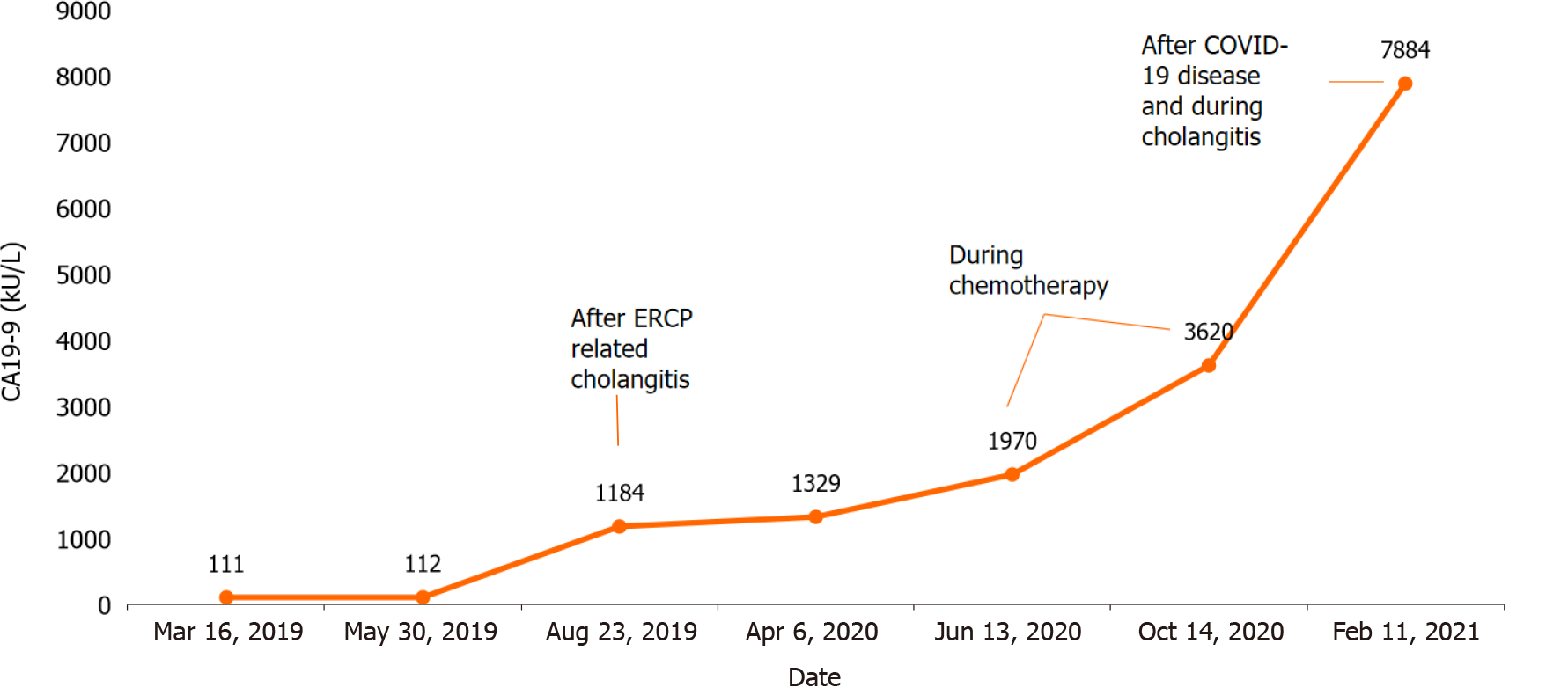
Laboratory tests revealed signs of inflammation, elevated liver enzymes, mild hyperbilirubinemia, and slightly elevated carbohydrate antigen (CA) 19-9 of 111.98 kU/L (Table 1).
| Value | Normal range | |
| White blood cell count | 10.04 | 4.0 x 109/L-9.8 x 109/L |
| Hemoglobin | 125 | 128-160 g/L |
| Platelet count | 328 | 130 x 109/L-400 x 109/L |
| C reactive protein | 55.4 | ≤ 5 mg/L |
| Aspartate aminotransferase | 76 | < 40 U/L |
| Alanine aminotransferase | 135 | < 40 U/L |
| γ- glutamyl transferase | 344 | ≤ 36 U/L |
| Alkaline phosphatase | 376 | 40-150 U/L |
| Total bilirubin | 30 | < 21 μmol |
| Albumin | 36.2 | 36-52 g/L |
| K+ | 4.3 | 3.8-5.3 mmol/L |
| Na+ | 132 | 134-145 mmol/L |
| SPA | 95 | 70%-130% |
| INR by Owren | 1.02 | 0.90-1.19 |
| Creatinine | 51 | 62-11 µmol/L |
| Urea | 3.7 | 2.5-7.5 mmol/L |
| CA 19-9 | 111.98 | < 37 kU/L |
| CEA | 1.0 | < 5 mkg/L |
| AFP | 1.30 | 0.5-5.5 kU/L |
| HBsAg | 0.11 negative | Negative s/co |
| Anti-HCV | 0.11 negative | Negative |
| AMA: M2 | Negative | Negative |
| ANA | Negative (1:40) | < 1:40 |
| Immunoglobulin G4 | 0.653 | 0.08-1.40 g/L |
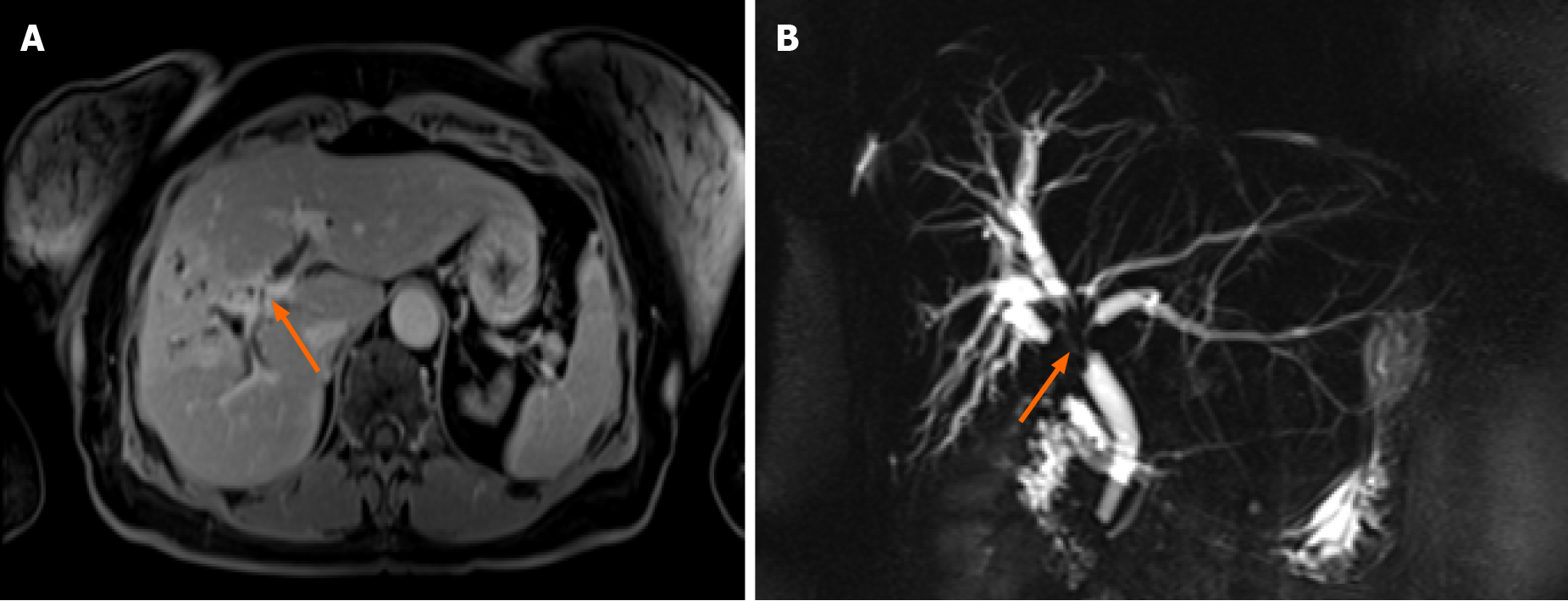
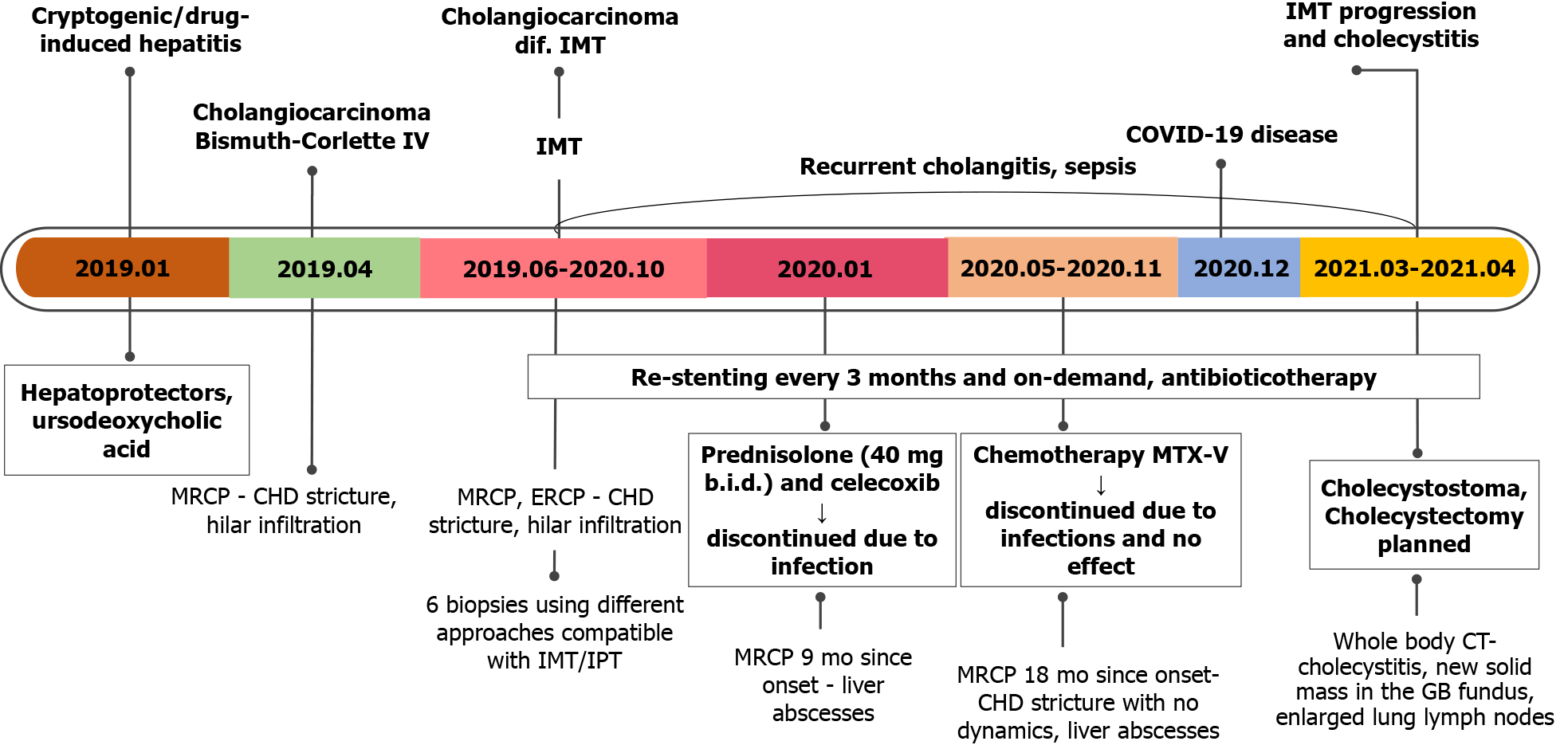
Abdominal ultrasound showed dilated intrahepatic ducts with a normal common bile duct. Contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) was performed to clarify the diagnosis, revealing a radiological image resembling perihilar cholangiocarcinoma, Bismuth-Corlette type IV, also known as Klatskin tumor (Figure 1).
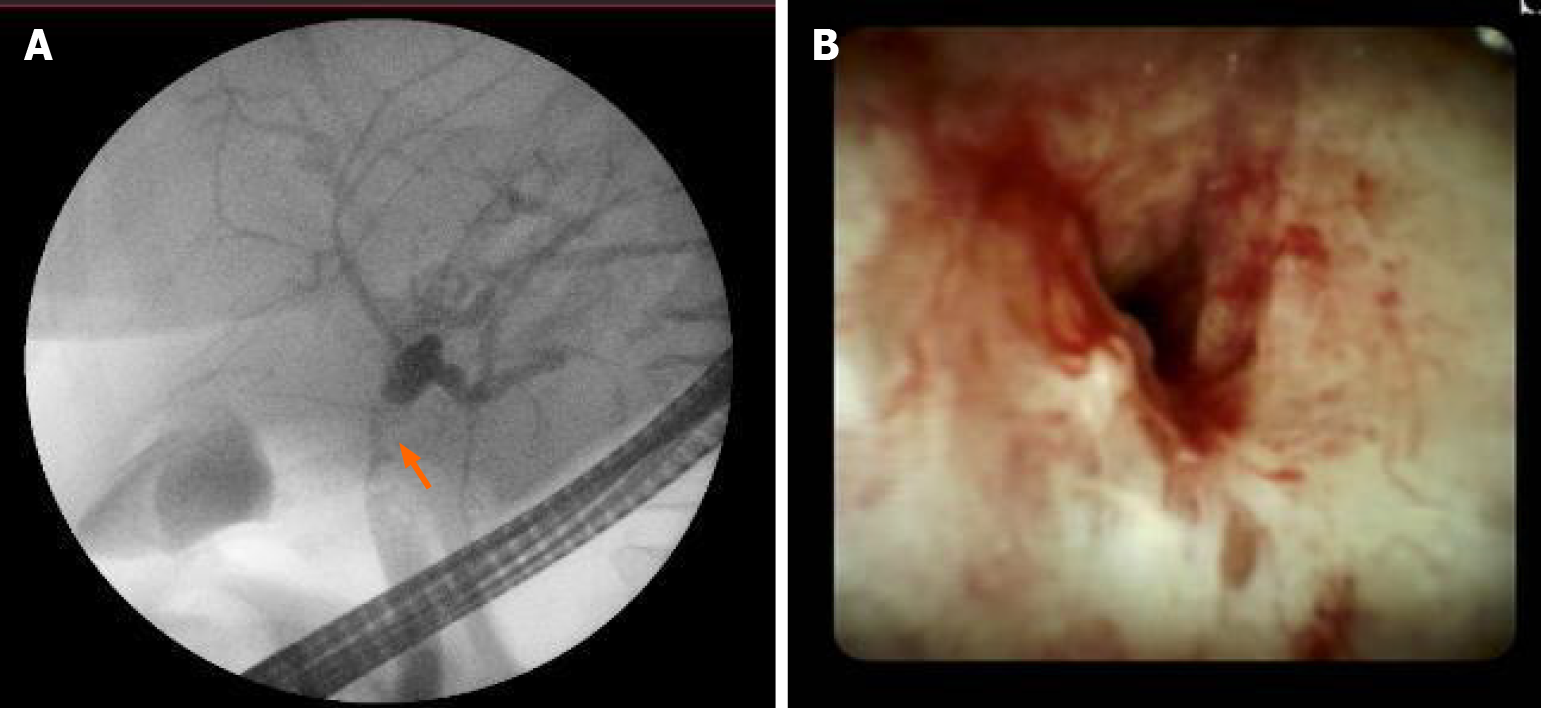
Further, we proceeded with endoscopic retrograde cholangiopancreatography (ERCP). ERCP revealed tight stricture in the proximal part of the common hepatic duct (CHD) and the confluence extending to the left and right hepatic ducts (Figure 2A). Right and left hepatic ducts were not visualized due to complete obstruction. The radiological image was typical for cholangiocarcinoma, Bismuth Corlette IV. The biopsy was taken from the dominant CHD stricture, and a 7 Fr 12 cm pigtail stent was placed into the left hepatic duct to manage cholestasis. The preventive pancreatic 5 Fr 5 cm stent was placed in the pancreatic duct during the first ERCP procedure after nonintentional cannulation of the pancreatic duct.
Histological examination of the first biopsy revealed no signs of malignant disease. Therefore, direct cholangioscopy with biopsies was performed (Figure 2B). However, histological examination repeatedly showed no atypical changes.
Based on clinical, radiological and endoscopic findings, the patient was diagnosed with perihilar cholangiocarcinoma during the multidisciplinary team meeting (MDT). The treatment with re-stenting every 3 mo and stereotaxic radiotherapy was recom
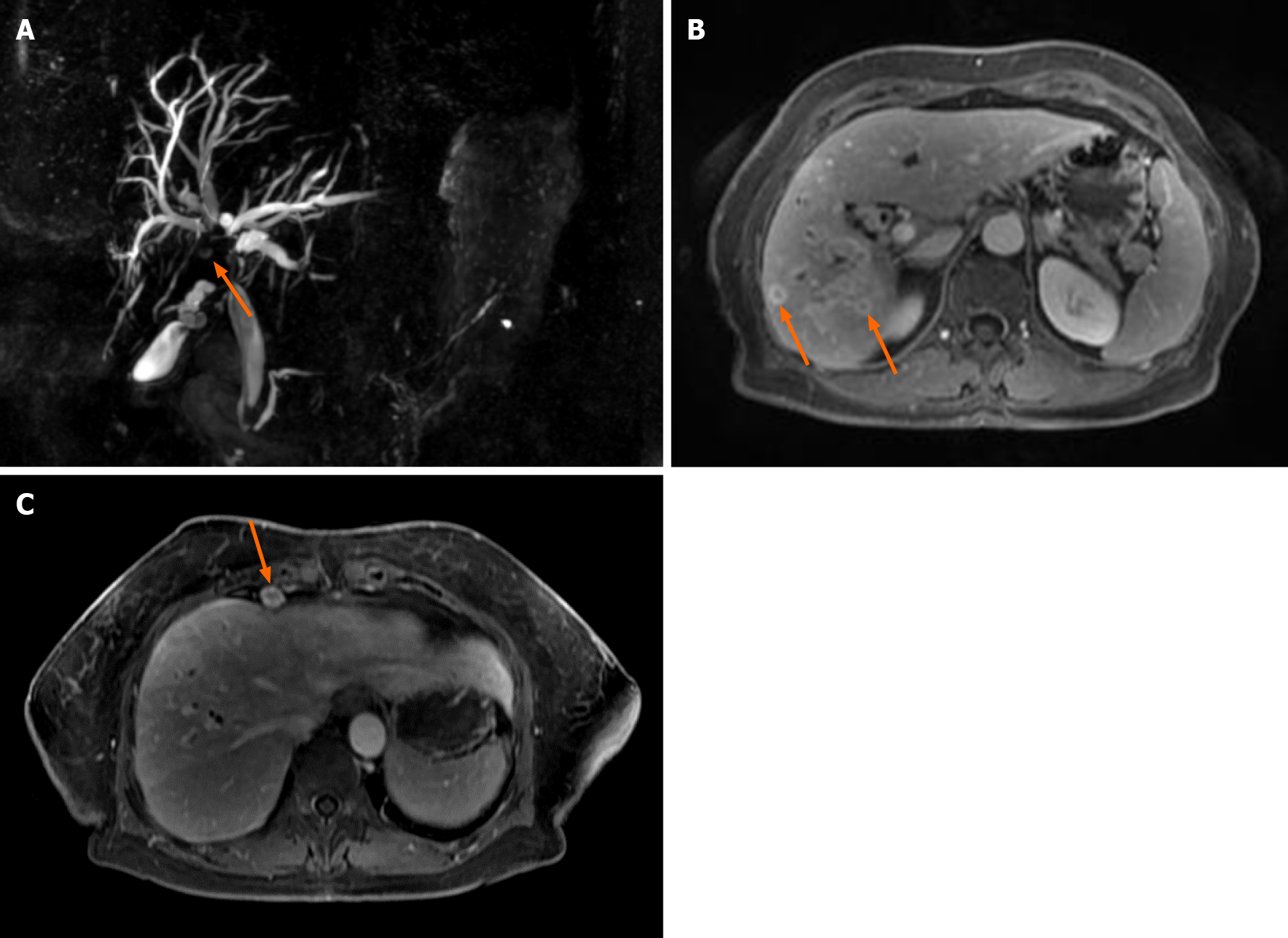
The second MRCP 3 mo later showed a periductal mass in the liver hilum obstruc
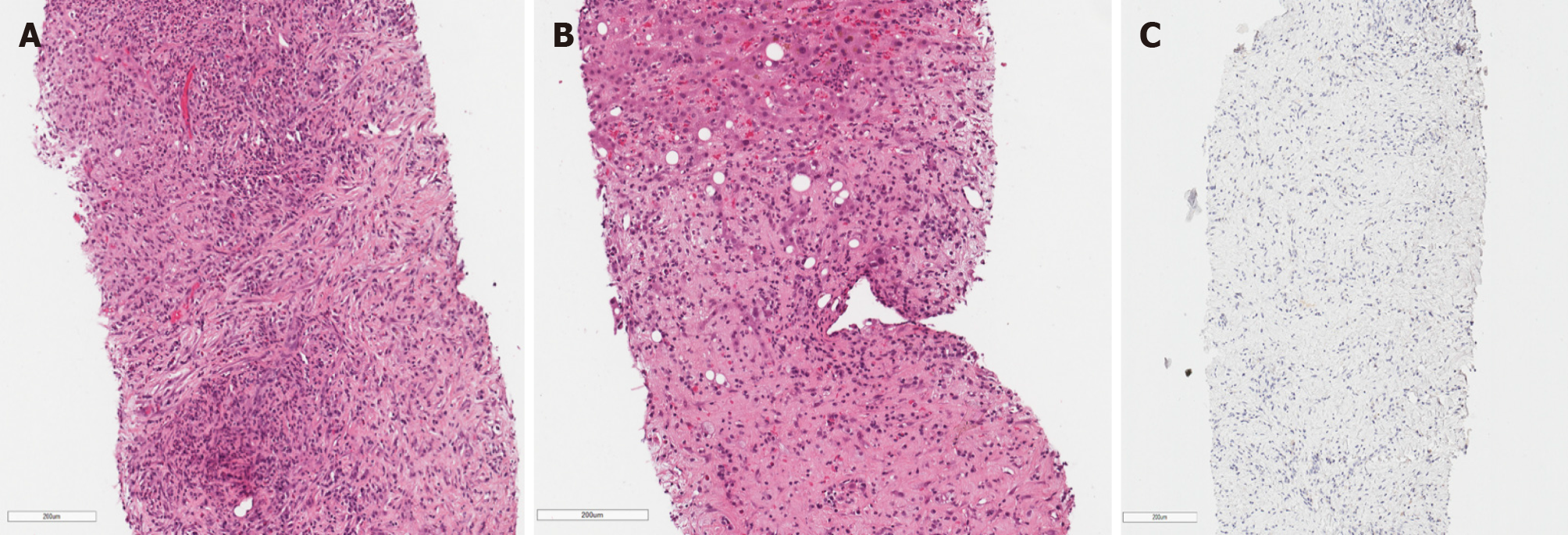
During the second MDT meeting, it was decided to perform diagnostic laparoscopy to specify the lesions and finally withdraw cholangiocarcinoma. The radical surgical treatment was contraindicated as the mass was extending into both hepatic and some segmental ducts. Liver transplantation was not an option because of the patient’s age. Histological examination of the CBD, liver mass and lymph node biopsies was compatible with the previous dominant stricture biopsies. Histopathology of the lesions showed no evidence of malignancy, atypical mitosis or necrosis. The non-specific changes were noted: Widespread myofibroblastic and fibroblastic spindle cells with inflammatory infiltrate of lymphocytes, plasma cells, eosinophils, histiocytes. The immunohistochemical reaction for anaplastic lymphoma kinase (ALK)-1 and ALK (D5F3) was negative (Figure 4). Histologically, the tumor proved an IMT arising from the bile duct epithelium and confirmed the diagnosis of biliary IMT.
To specify the possible etiology of the tumor, several additional laboratory tests were performed. The patient was tested negative for cytomegalovirus, Epstein-Barr virus, HIV 1 and 2, and immunoglobulin (Ig) G4. Tuberculosis was also withdrawn. The biochemical markers for common oncological diseases were also negative (Table 1). The patient did not have a history of cholangitis before the first ERCP.
Although imaging examinations showed signs of perihilar cholangiocarcinoma, the pathological investigations revealed only inflammatory changes, and the other possible diseases were excluded. Accordingly, the patient was diagnosed with biliary IMT and dominant CHD stricture.
The primary symptomatic treatment with permanent CBD stenting every 3 mo was applied and continued throughout patients’ treatment and follow-up in our hospital. After confirming the diagnosis of biliary IMT, it was decided to start the treatment with a 1-month course of steroids (40 mg/d of prednisolone tapering by 5 mg) and non-steroidal anti-inflammatory drugs (NSAIDs) (celecoxib 100 mg/d) based on the cohort study by Casanova et al[5]. However, prednisolone was discontinued 10 d after due to significantly increased C-reactive protein (to 100 mg/L).
We continued restenting every 3 mo. However, the majority of procedures have been followed by cholangitis and septic complications. Therefore, the culture from the bile was taken after elective ERCP. Six pathogens were cultured: Pseudomonas aeru
Further, the patient was consulted by the oncochemotherapist. As steroids and celecoxib therapy failed due to infectious complications, it was decided to start the second-line treatment with vinblastine and methotrexate. MRCP after 3 cycles of chemotherapy (18 mo since diagnosis) showed no positive results. There were no liver abscesses seen on subsequent MRI. However, the stricture length of the bile ducts remained the same, and cardiophrenic and liver hilum lymph nodes did increase by few millimeters in size. It is hard to attribute lymph node enlargement for tumor progression as they can also enlarge due to recurrent infections. The patient’s clinical condition and well-being did improve at that time, but this is most likely due to treated infectious complications.
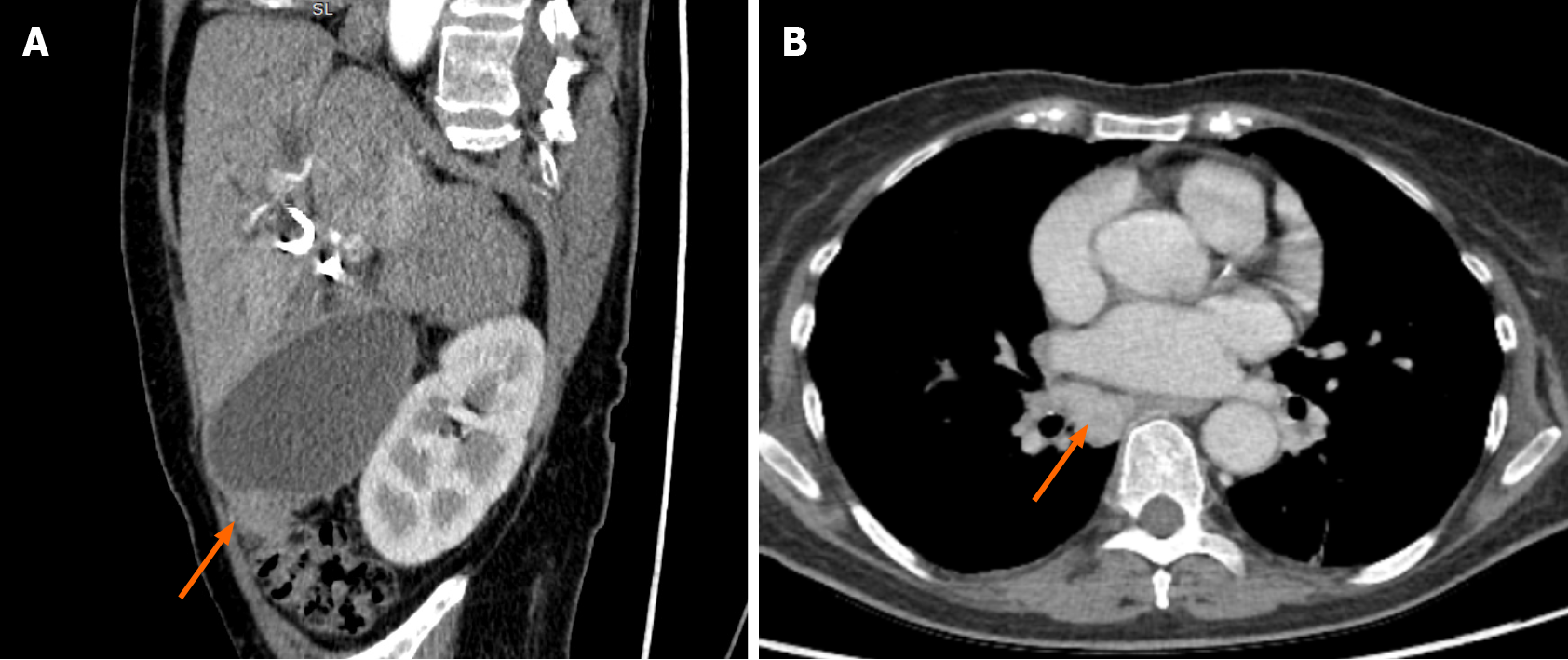
The patient continued chemotherapy (5 cycles). Unfortunately, this treatment was discontinued, as the patient was repeatedly hospitalized due to recurrent cholangitis and sepsis. What is more, she gained a COVID-19 infection. After fighting COVID-19 disease, the patient’s condition significantly worsened as she developed cholecystitis
IMTs are rare, usually benign mass-forming lesions of unknown origin. It encompasses a spectrum of myofibroblastic proliferation and varying amounts of polymorphous inflammatory cell infiltrate consisting of spindle cells, plasma cells, lymphocytes, eosinophils and macrophages. Also, there are variable amounts of fibrosis, necrosis, granulomatous reactions[3,9]. These lesions can be found in every organ throughout the body, most commonly in the lung, orbit, peritoneum, omentum and mesentery, followed by soft tissue, mediastinum, gastrointestinal tract, pancreas, genitourinary tract, oral cavity, skin, breast, nerve, bone, and central nervous system[1-3].
IMTs of the biliary ducts are an uncommon cause of obstructive jaundice. The cases found during the literature search using PubMed are presented in Table 2 (used keywords: biliary IMT, inflammatory myofibroblastic tumor of the biliary tract, perihilar IMT, hepatic bifurcation IMT).
| Ref. | Age /gender | Location/extension | Treatment | Follow- up | Outcome |
| Haith et al[46] | 6/M | Distal CBD | PD and celecoxib | 5 mo | NR |
| Stamatakis et al[47] | 13/F | Proximal CBD, cystic duct, CHD | Extrahepatic BD excision | 21 mo | NR |
| Ikeda et al[48] | 43/M | Proximal CBD/ intrahepatic ducts, CHD, gall bladder, lymph nodes | Surgery | 7 mo | Lung metastasis |
| Fukushima et al[49] | 58/F | Mid-CBD/pancreas and lymph nodes | PD | - | NR |
| Walsh et al[50] | 50/M | Proximal CBD | PD | 19 yr | Metastasis |
| Sobesky et al[51] | 51/F | Distal CBD | PD | 2 yr | NR |
| Venkataraman et al[52] | 17–52/3 M, 1 F | 1 liver mass; 1 liver mass and periportal infiltration; 2 periportal infiltration | - | - | - |
| Büyükyavuz et al[53] | 8/F | Hepatic hilar | Surgery, antibiotics | - | - |
| Lopez-Tomassetti Fernandez et al[54] | 55/F | Distal CBD | PD | 4 yr | R |
| Martín Malagón et al[55] | 51/F | Distal CBD | PD | - | - |
| Kim et al[56] | 63/F | Hilar bile duct | Hepatectomy, caudate lobectomy | 5 mo | R |
| Sekaran et al[57] | 17/F | Left hepatic, CHD, proximal CBD | Left hepatectomy | 6 wk | NR |
| Abu-Wasel et al[58] | 55/F | Distal CBD | Extrahepatic BD excision | 14 mo | NR |
| Vasiliadis et al[2] | 70/F | Mid-CBD | Extrahepatic BD excision | 8 mo | NR |
| D’Cunha et al[59] | 12/F | Distal CBD | Debulking, corticosteroids | - | NR |
| Verma et al[1] | 24/F | Mid-CBD | Extrahepatic BD excision | 12 mo | NR |
| Karimi et al[36] | 12/F | Hepatic duct bifurcation | CBD resection | - | R |
| Present case | 70/F | Proximal CBD, CHD | Corticosteroids, chemotherapy | 24 mo | Disease progression |
The exact origin and pathogenesis of the disease are poorly understood. The tumor can affect both sexes and all ethnic groups equally, and can occur in any age group[10,11]. The most popular and widely accepted theories describe IMTs as either primary reactive and inflammatory processes or low-intermediate grade neoplastic processes with secondary inflammation. Infection, minor trauma, previous abdominal surgery or procedure, radiotherapy, chemotherapy, and steroid use - all might play a role in the pathogenesis of the heightened inflammatory reaction and result in IMT/IPT. An immune-autoimmune mechanism has also been implicated[9]. The unusual inflammatory or immune responses such as sclerosing cholangitis, phlebitis, and retroperitoneal fibrosis also can be found in association with IPT.
Some organisms like parasitic fragments, Escherichia coli, gram-positive cocci, Klebsiella pneumonia, Epstein-Barr virus were found in splenic and nodal pseudotumors. Actinomycetes and nocardiae were found in hepatic and pulmonary pseudotumors, respectively; mycobacteria associated with spindle cell tumor and mycoplasma found in pulmonary pseudotumors[9,12]. There have been reports of IMTs/IPTs related to Mycobacterium avium–intracellulare complex, Escherichia coli, Klebsiella, Bacillus sphaeri
In our case, the etiology of the lesion remained unclear. The patient didn’t have any predisposing infections, no history of cholangitis or any abdominal surgery proce
Painless obstructive jaundice, abdominal pain, weight loss and fever are the most common clinical findings in patients with biliary IMTs. Their clinical presentation and imaging features are non-specific and are indistinguishable from cholangiocarcinoma, especially when hepatic hilum or bile ducts are involved[18].
Laboratory tests usually show leukocytosis, elevated C-reactive protein (CRP), anemia, thrombocytosis, polyclonal hypergammaglobulinemia, and slightly elevated liver enzymes. Tumor markers, such as carcinoembryonic antigen (CEA) and serum alpha-fetoprotein (AFP), are usually normal, although in some patients, marginally elevated CA 19-9 can be found[8,19].
Highly increased CA 19-9 is characteristic of cholangiocarcinoma and other malig
Imaging findings of IMT are not specific, and their radiological identification is not always possible. Typical radiological features of the biliary IMT are infiltrating hilar lesions and intrahepatic ductal dilatation[24]. Tublin et al[18] studied biliary pseudo
Immunohistochemical studies of T and B-cell subpopulations may help to distin
About half of recently analyzed cases of IMT harbors an anaplastic lymphoma kinase (ALK-1) gene rearrangement locus on chromosome 2p23, causing aberrant ALK-1 expression[26]. Although there is no statistically significant correlation between ALK-1 expression, tumor type, recurrence and metastasis, it is a useful diagnostic aid, based on which decision on chemotherapeutic treatment might be made[27]. It has been suggested that predominating histiocytic cells in IPT are associated with in
Although poorly understood, the etiology of IMT seems to relate closely to an altered immune response to injury. Infection, trauma, previous abdominal surgery, radio
Conservative management of IMT includes NSAIDs, corticosteroids and chemo
However, in our case, treatment with NSAIDs was not effective, and steroids were discontinued due to increased inflammatory markers. Therefore, an alternative treatment plan was applied. Cytotoxic chemotherapy with methotrexate and/or vinorelbine/vinblastine (MTX-V) has been shown to be very effective, and long-term disease control could be achieved[34]. Casanova et al[5] reported the clinical findings and treatment results in the cohort of patients with IMT managed according to the European pediatric Soft Tissue Sarcoma Study Group protocol from 2005 to 2016. However, data is inconclusive on long-term remission, as studies have also shown recurrence of IMT combining surgery and chemotherapy[35]. Possible treatment options for IMT are summarised in Table 3.
| Possible treatment options for IMTs |
| High-dose steroids |
| Low dose steroids |
| Non-steroidal anti-inflammatory drugs (e.g., celecoxib) |
| Vinblastine and methotrexate |
| Anaplastic lymphoma kinase inhibitors |
| Ifosfamide-based chemotherapy |
| Vinorelbine and low-dose cyclophosphamide |
| Vincristine and actinomycin-D |
| Cyclosporine, azathioprine |
| Radical surgical treatment (when anatomically and physiologically feasible) |
Despite reported successful treatment, complete reduction and tumor regression using a conservative approach (corticosteroids, NSAIDs, antibiotics, chemotherapy), tumors identified as IMTs by histopathology are locally progressive and often need surgical resection, especially if medical therapy is not effective[36,37]. Although there are no approved recommendations for operative hepatobiliary IMTs treatment, the literature suggests that patients with resectable IMTs should be managed with radical surgical resection when it is anatomically and physiologically feasible[5,35-37]. The selection of liver resection candidates must be based on the patient’s condition, tumor location, size and extension, the functional reserve capacity, and a sufficient liver remnant assessed by clinical and biochemical measures and by hepatic volume in cases of major hepatectomy[38,39]. Complete resection of hilar tumors requires a partial hepatectomy or extended hemi-hepatectomy[39,40]. There have also been some reports of a successful liver transplant, pancreaticoduodenectomy and combined liver transplant with pancreaticoduodenectomy in patients with hilar IMTs (Table 3)[1,37].
Cases with hilar tumors are always challenging (particularly those in perihilar cholangiocarcinoma) as anatomically these tumors in the hepatic hilum are in intimate relation with the portal vein and hepatic artery. Only about 1 in 5 patients with perihilar cholangiocarcinoma is eligible for surgery at the time of presentation[41]. The biliary extent of the tumor towards the segmental bile ducts is often more extensive than seemed on preoperative imaging[40].
The outcome of liver surgery during the past few decades improved. However, postoperative liver failure remains the leading cause of postoperative mortality. The assessment of hepatic functional reserve remains one of the most important issues in liver surgery[42]. The limit for “safe” liver resection is leaving a future liver remnant of at least 25% of the preoperative liver volume in patients with normal liver parenchyma or at least 30% to 40% in livers that are compromised by steatosis, chronic cholestasis, cirrhosis or chemotherapy[40,43,44]. The potential risks and benefits of the surgery must be considered, especially when there are no established recommendations in the surgical treatment of biliary IMT.
Contraindications for liver resection are similar to those of cholangiocarcinoma and include: (1) Patient factors (medically unfit for operation, cirrhosis/portal hyperten
Usually, IMTs are considered as benign neoplasms. According to the WHO classification, IMT is an intermediate-grade tumor with the potential for recurrence and rare metastasis[3]. In rare cases, IMT takes a malignant course and is hard to treat. There are no definite histopathologic, molecular, or cytogenetic features to predict malignant transformation, recurrence or metastasis[5]. Some IMTs are classified as neoplastic lesions, or reactive lesions, that have undergone a malignant transformation because a subset of IMT grows aggressively and shows a malignant behavior, sometimes with the formation of metastases. Whether a pseudotumor is a neoplastic or reactive process remains a debatable question. Some features, such as progressive growth, local recurrence, the development of multifocal masses, the destruction of liver substance and bile ducts, and the vascular (portal-venous) invasion, deny the purely reacti
The patient’s clinical course of the disease and instrumental examinations were compatible with malignant disease, most likely perihilar cholangiocarcinoma. There
This case’s management included repeated biliary tract stenting, glucocorticoids, NSAIDs, and, lastly, chemotherapy. Radical surgical treatment was impossible as the mass was locally advanced - extending into both hepatic and some segmental ducts. Liver transplantation was also not an option due to the patient’s age and recurrent infections. The infectious complications almost after each ERCP and re-stenting procedure limited the ability to prescribe and continue steroids. Based on the literature, chemotherapy is one of the possible treatment methods for IMT as it is defined as intermediate-grade sarcoma. Therefore, after rigorous discussion with oncochemotherapists, we decided to start cytotoxic chemotherapy, as other treatment methods showed no effect. Although the patient’s overall condition improved after the MTX-V scheme, it is probably misleading as the stricture length remained almost the same, and the MRCP images were performed during the time with and without cholangitis. Later on, it became impossible to continue chemotherapy due to constant infections. The patient developed COVID-19 disease, after which cholangitis became more severe: the patient developed cholecystitis, and some more solid masses were observed in the CT, indicating disease progression.
The reported outcomes of IMTs is ranging from completely benign to malignant and even fatal outcomes. In our case, IMT took a complicated course with constant infections leaving us with no effective treatment options.
IMTs can occur in various sites of the body. However biliary tract as the tumor’s primary location is reported rarely. Differentiation between benign and malignant strictures is a challenge for radiologists, endoscopists and gastroenterologists, as lesions are often indistinguishable from cholangiocarcinoma. Although IMTs are generally benign, these lesions can lead to hardly controlled recurrent infections or malignant course. Data about disease characteristics and effective treatment methods is lacking. Therefore, we report a case of an unusual localization, non-operable hard to diagnose and treat IMT of the biliary tract, and we hope this article will contribute to increasing the understanding of this rare condition.
We wish to thank the patient and Vilnius University Hospital Santaros Clinics for giving the consent and providing the data to report this case.
Manuscript source: Unsolicited Manuscript
Corresponding Author's Membership in Professional Societies: United European Gastroenterology; European Association for the Study of the Liver (EASL), 62635; Lithuanian Society of Gastroenterology.
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Moura EGH S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
| 1. | Verma R, Saha A, Saha K. Inflammatory Myofibroblastic Tumor of the Mid Common Bile Duct Masquerading as Cholangiocarcinoma. J Gastrointest Cancer. 2019;50:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Vasiliadis K, Fortounis K, Papavasiliou C, Kokarhidas A, Al Nimer A, Fachiridis D, Pervana S, Makridis C. Mid common bile duct inflammatory pseudotumor mimicking cholangiocarcinoma. A case report and literature review. Int J Surg Case Rep. 2014;5:12-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumor. In: Fletcher CDM, Bridge JA, Hogendooorn PCW, Mertens F, editors. WHO classification of tumors of soft tissue and bone. 4th edition. Lyon, France: IARC Press; 2013: 83-84. |
| 4. | Biniraj KR, Janardhanan M. Inflammatory myofibroblastic tumor of maxilla showing sarcomatous change in an edentulous site with a history of tooth extraction following periodontitis: A case report with discussion. J Indian Soc Periodontol. 2014;18:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, van Noesel MM, Corradini N, Minard-Colin V, Zanetti I, Bisogno G, Gallego S, Merks JHM, De Salvo GL, Ferrari A. Inflammatory myofibroblastic tumor: The experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur J Cancer. 2020;127:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 6. | Poh CF, Priddy RW, Dahlman DM. Intramandibular inflammatory myofibroblastic tumor--a true neoplasm or reactive lesion? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Pack GT, Baker HW. Total right hepatic lobectomy; report of a case. Ann Surg. 1953;138:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 237] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Elpek GÖ. Inflammatory Myofibroblastic Tumor of the Liver: A Diagnostic Challenge. J Clin Transl Hepatol. 2014;2:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Lai LM, McCarville MB, Kirby P, Kao SC, Moritani T, Clark E, Ishigami K, Bahrami A, Sato Y. Shedding light on inflammatory pseudotumor in children: spotlight on inflammatory myofibroblastic tumor. Pediatr Radiol. 2015;45:1738-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kim JH, Cho JH, Park MS, Chung JH, Lee JG, Kim YS, Kim SK, Shin DH, Choi BW, Choe KO, Chang J. Pulmonary inflammatory pseudotumor--a report of 28 cases. Korean J Intern Med. 2002;17:252-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Dehner LP. The enigmatic inflammatory pseudotumours: the current state of our understanding, or misunderstanding. J Pathol. 2000;192:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Sanders BM, West KW, Gingalewski C, Engum S, Davis M, Grosfeld JL. Inflammatory pseudotumor of the alimentary tract: clinical and surgical experience. J Pediatr Surg. 2001;36:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Lu H, Ji H, Li Y. Inflammatory pseudotumor of the liver: A case report and literature review. Intractable Rare Dis Res. 2015;4:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Nonomura A, Minato H, Shimizu K, Kadoya M, Matsui O. Hepatic hilar inflammatory pseudotumor mimicking cholangiocarcinoma with cholangitis and phlebitis--a variant of primary sclerosing cholangitis? Pathol Res Pract. 1997;193:519-25; discussion 526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Toda K, Yasuda I, Nishigaki Y, Enya M, Yamada T, Nagura K, Sugihara J, Wakahara T, Tomita E, Moriwaki H. Inflammatory pseudotumor of the liver with primary sclerosing cholangitis. J Gastroenterol. 2000;35:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Koide H, Sato K, Fukusato T, Kashiwabara K, Sunaga N, Tsuchiya T, Morino S, Sohara N, Kakizaki S, Takagi H, Mori M. Spontaneous regression of hepatic inflammatory pseudotumor with primary biliary cirrhosis: case report and literature review. World J Gastroenterol. 2006;12:1645-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Tublin ME, Moser AJ, Marsh JW, Gamblin TC. Biliary inflammatory pseudotumor: imaging features in seven patients. AJR Am J Roentgenol. 2007;188:W44-W48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Lin MS, Huang JX, Yu H. Elevated serum level of carbohydrate antigen 19-9 in benign biliary stricture diseases can reduce its value as a tumor marker. Int J Clin Exp Med. 2014;7:744-750. [PubMed] |
| 20. | Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Howaizi M, Abboura M, Krespine C, Sbai-Idrissi MS, Marty O, Djabbari-Sobhani M. A new cause for CA19.9 elevation: heavy tea consumption. Gut. 2003;52:913-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ogawa T, Yokoi H, Kawarada Y. A case of inflammatory pseudotumor of the liver causing elevated serum CA19-9 Levels. Am J Gastroenterol. 1998;93:2551-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Kim HJ, Lee KW, Kim YJ, Oh DY, Kim JH, Im SA, Lee JS. Chemotherapy-induced transient CEA and CA19-9 surges in patients with metastatic or recurrent gastric cancer. Acta Oncol. 2009;48:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012;198:W217-W227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Chougule A, Bal A, Das A, Agarwal R, Singh N, Rao KL. A Comparative Study of Inflammatory Myofibroblastic Tumors and Tumefactive IgG4-related Inflammatory Lesions: the Relevance of IgG4 Plasma Cells. Appl Immunohistochem Mol Morphol. 2016;24:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Saab ST, Hornick JL, Fletcher CD, Olson SJ, Coffin CM. IgG4 plasma cells in inflammatory myofibroblastic tumor: inflammatory marker or pathogenic link? Mod Pathol. 2011;24:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Telugu RB, Prabhu AJ, Kalappurayil NB, Mathai J, Gnanamuthu BR, Manipadam MT. Clinicopathological Study of 18 Cases of Inflammatory Myofibroblastic Tumors with Reference to ALK-1 Expression: 5-Year Experience in a Tertiary Care Center. J Pathol Transl Med. 2017;51:255-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Su W, Ko A, O'Connell T, Applebaum H. Treatment of pseudotumors with nonsteroidal antiinflammatory drugs. J Pediatr Surg. 2000;35:1635-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Al-Hussaini H, Azouz H, Abu-Zaid A. Hepatic inflammatory pseudotumor presenting in an 8-year-old boy: A case report and review of literature. World J Gastroenterol. 2015;21:8730-8738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Zhao J, Olino K, Low LE, Qiu S, Stevenson HL. Hepatic Inflammatory Pseudotumor: An Important Differential Diagnosis in Patients With a History of Previous Biliary Procedures. ACG Case Rep J. 2019;6:e00015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Ballester-Pla N, García-Domínguez R, Pérez-Girbes A, Orbis-Castellanos JF, Pareja E. Conservative treatment of hepatic inflammatory pseudotumor. Cir Esp. 2016;94:422-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Pfeifer L, Agaimy A, Janka R, Boxberger F, Wein A, Neurath MF, Siebler J. Complete Long-Term Remission of an Inflammatory Pseudotumor under Corticosteroid Therapy. Case Rep Oncol. 2011;4:304-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Tao YL, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol. 2012;18:7100-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Baldi GG, Brahmi M, Lo Vullo S, Cojocaru E, Mir O, Casanova M, Vincenzi B, De Pas TM, Grignani G, Pantaleo MA, Blay JY, Jones RL, Le Cesne A, Frezza AM, Gronchi A, Collini P, Dei Tos AP, Morosi C, Mariani L, Casali PG, Stacchiotti S. The Activity of Chemotherapy in Inflammatory Myofibroblastic Tumors: A Multicenter, European Retrospective Case Series Analysis. Oncologist. 2020;25:e1777-e1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Trahair T, Gifford AJ, Fordham A, Mayoh C, Fadia M, Lukeis R, Wood AC, Valvi S, Walker RD, Blackburn J, Heyer EE, Mercer TR, Barbaric D, Marshall GM, MacKenzie KL. Crizotinib and Surgery for Long-Term Disease Control in Children and Adolescents With ALK-Positive Inflammatory Myofibroblastic Tumors. JCO Precis Oncol. 2019;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Karimi M, Tizmaghz A, Shabestanipour G. An interesting case of inflammatory myofibroblastic tumor presenting as cholangiocarcinoma. Int J Surg Case Rep. 2018;47:38-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Berumen J, McCarty P, Mo J, Newton K, Fairbanks T, Mekeel K, Hemming A. Combined liver transplant and pancreaticoduodenectomy for inflammatory hilar myofibroblastic tumor: Case report and review of the literature. Pediatr Transplant. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol. 2008;14:3452-3460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 39. | Yamada S, Shimada M, Morine Y, Imura S, Ikemoto T, Saito Y, Takasu C, Yoshikawa M, Teraoku H, Yoshimoto T. A new formula to calculate the resection limit in hepatectomy based on Gd-EOB-DTPA-enhanced magnetic resonance imaging. PLoS One. 2019;14:e0210579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 41. | Chaiteerakij R, Harmsen WS, Marrero CR, Aboelsoud MM, Ndzengue A, Kaiya J, Therneau TM, Sanchez W, Gores GJ, Roberts LR. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Clary BM, Jarnagin WR, Blumgart LH. Cholangiocarcinoma [Internet]. In: Holzheimer RG, Mannick JA, editors. Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: Zuckschwerdt; 2001. [cited May 2, 2020]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7001/. |
| 43. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 45. | Zhang X, Liu H. Klatskin Tumor: A Population-Based Study of Incidence and Survival. Med Sci Monit. 2019;25:4503-4512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Haith EE, Kepes JJ, Holder TM. Inflammatory pseudotumor involving the common bile duct of a six-year-old boy: successful pancreaticoduodenectomy. Surgery. 1964;56:436-441. [PubMed] |
| 47. | Stamatakis JD, Howard ER, Williams R. Benign inflammatory tumour of the common bile duct. Br J Surg. 1979;66:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Ikeda H, Oka T, Imafuku I, Yamada S, Yamada H, Fujiwara K, Hirata M, Idezuki Y, Oka H. A case of inflammatory pseudotumor of the gallbladder and bile duct. Am J Gastroenterol. 1990;85:203-206. [PubMed] |
| 49. | Fukushima N, Suzuki M, Abe T, Fukayama M. A case of inflammatory pseudotumour of the common bile duct. Virchows Arch. 1997;431:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Walsh SV, Evangelista F, Khettry U. Inflammatory myofibroblastic tumor of the pancreaticobiliary region: morphologic and immunocytochemical study of three cases. Am J Surg Pathol. 1998;22:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Sobesky R, Chollet JM, Prat F, Karkouche B, Pelletier G, Fritsch J, Choury AD, Allonier C, Bedossa P, Buffet C. Inflammatory pseudotumor of the common bile duct. Endoscopy. 2003;35:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Venkataraman S, Semelka RC, Braga L, Danet IM, Woosley JT. Inflammatory myofibroblastic tumor of the hepatobiliary system: report of MR imaging appearance in four patients. Radiology. 2003;227:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Büyükyavuz I, Karnak I, Haliloglu M, Senocak ME. Inflammatory myofibroblastic tumour of the extrahepatic bile ducts: an unusual cause of obstructive jaundice in children. Eur J Pediatr Surg. 2003;13:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Lopez-Tomassetti Fernandez EM, Luis HD, Malagon AM, Gonzalez IA, Pallares AC. Recurrence of inflammatory pseudotumor in the distal bile duct: lessons learned from a single case and reported cases. World J Gastroenterol. 2006;12:3938-3943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Martín Malagón A, López-Tomassetti Fernández E, Arteaga González I, Carrillo Pallarés A, Díaz Luis H. Inflammatory myofibroblastic tumor of the distal bile duct associated with lymphoplasmacytic sclerosing pancreatitis. Case report and review of the literature. Pancreatology. 2006;6:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Kim BS, Joo SH, Kim GY, Joo KR. Aggressive hilar inflammatory myofibroblastic tumor with hilar bile duct carcinoma in situ. J Korean Surg Soc. 2011;81 Suppl 1:S59-S63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Sekaran A, Lakhtakia S, Pradeep R, Santosh D, Gupta R, Tandan M, Reddy DB, Rao GV, Reddy DN. Inflammatory myofibroblastic tumor of biliary tract presenting as recurrent GI bleed (with video). Gastrointest Endosc. 2006;63:1077-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Abu-Wasel B, Eltawil KM, Molinari M. Benign inflammatory pseudotumour mimicking extrahepatic bile duct cholangiocarcinoma in an adult man presenting with painless obstructive jaundice. BMJ Case Rep. 2012;2012:006514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | D'Cunha A, Jehangir S, Thomas R. Inflammatory Myofibroblastic Tumor of Common Bile Duct in a Girl. APSP J Case Rep. 2016;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |