Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6073
Peer-review started: March 18, 2021
First decision: April 23, 2021
Revised: May 6, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: July 26, 2021
Processing time: 124 Days and 23.7 Hours
Syncope presents with diagnostic challenges and is associated with high heal
We describe a case of syncope caused by nOH in Parkinson's disease and review the literature. A 70-year-old man with Parkinson's disease had uncontrolled blood pressure for 1 mo, with blood pressure ranging from 70/40 to 220/112 mmHg, and once lost consciousness lasting for several minutes after getting up. Ambulatory blood pressure monitoring indicated nocturnal hypertension (up to 217/110 mmHg) and morning orthostatic hypotension (as low as 73/45 mmHg). Seated-to-standing blood pressure measurement showed that the blood pressure dropped from 173/96 mmHg to 95/68 mmHg after standing for 3 min from supine position. A diagnosis of nOH with supine hypertension was made. During the course of treatment, Midodrine could not improve the symptoms. Finally, the patient's blood pressure stabilized with simple strategies by strengthening exercises, reducing the duration of lying in bed in the daytime, and consuming water intake before getting up.
nOH is one of the causes of syncope. Ambulatory blood pressure monitoring is a cost-effective method for its diagnosis, and non-pharmacological measures are still the primary management methods.
Core Tip: Syncope presents with diagnostic challenges and is associated with high healthcare costs. For syncope caused by a change in position, neurogenic orthostatic hypotension (nOH) should be considered to reduce missed diagnosis and misdiagnosis. Paying attention to comorbidities, such as Parkinson's disease and diabetes which could cause can autonomic dysfunction, also helps in the diagnosis of the cause of syncope. Ambulatory blood pressure monitoring can assist in diagnosing nOH. It is very challenging for clinicians to manage patients with nOH and supine hypertension. Increasing physical activity and reducing the amount of time in bed are still the primary management methods.
- Citation: Li Y, Wang M, Liu XL, Ren YF, Zhang WB. Neurogenic orthostatic hypotension with Parkinson's disease as a cause of syncope: A case report. World J Clin Cases 2021; 9(21): 6073-6080
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6073.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6073
Syncope is a common presentation that cardiologists encounter. It presents with diagnostic challenges. Up to 25% of cases of syncope are caused by neurogenic orthostatic hypotension (nOH), which is associated with an increased risk of mortality[1,2]. Orthostatic hypotension may develop in patients with autonomic dysfunction, such as diabetes, Parkinson's disease (PD), and multiple atrophy[3,4]. There are challenges associated with the clinical management of blood pressure in patients with nOH and supine hypertension. In this case study, we will review the diagnosis and treatment of syncope caused by nOH in a patient with PD.
A 70-year-old Chinese man had unstable blood pressure for 1 mo and lost consciousness lasting for several minutes 7 d prior to admission. He was presented to our emergency department with uncontrolled high blood pressure.
One month prior, the patient experienced fluctuating blood pressure levels, which ranged from 70/40 to 220/112 mmHg, with dizziness and amaurosis for a few minutes (Table 1). The blood pressure was not controlled following the adjustment of his antihypertensive drugs. The patient’s symptoms were worse 1 wk before admission. The patient experienced dizziness and profuse sweating and started talking gibberish after getting up from bed and going down the stairs, which was followed by loss of consciousness lasting for a few minutes. He was admitted to hospital as the high blood pressure was out of control.
| Time | Event |
| Jan 1, 2010 | Diagnosed with hypertension |
| Sep 25, 2019 | Presented with trembling right hand |
| Mar 15, 2020 | Presented with trembling hands, diagnosed with Parkinson's disease, and treated with Madopar (125 mg three times a day) and Selegiline (5 mg once a day) |
| Jun 22, 2020 | Presented with a forward gait |
| Aug 2, 2020 | Unstable blood pressure, up to 220/112 mmHg, and as low as 70/40 mmHg, antihypertensive drugs did not work |
| Sep 3, 2020 | Felt dizziness, profuse sweating and started talking gibberish, and then lost consciousness for several minutes after getting up and going down the stairs in the morning |
| Sep 11, 2020 | Ambulatory blood pressure monitoring indicated nOH and supine hypertension, taking Midodrine (2.5 mg two times a day, but not within 5 h of bedtime), basic treatment and stopping taking Selegiline |
| Dec 13, 2020 | Much more stable blood pressure and no recurrence syncope |
| May 2, 2021 | No recurrence syncope, and the 24-h ambulatory blood pressure monitoring indicated much more stable blood pressure |
The patient had a history of hypertension for 10 years and the maximum recorded blood pressure was 160/90 mmHg. He experienced right-hand tremors a year ago, and left-hand tremors 6 mo ago. He was subsequently diagnosed with PD and was managed with Madopar (125 mg three times a day) and Selegiline (5 mg once a day).
The personal history was described above in history of past illness. There was no significant or family history to note.
During the patient’s hospital stay, the blood pressure showed fluctuations ranging from 73/45 to 220/117 mmHg, and heart rate ranging from 74 to 110 beats per minute. His body weight was 61 kg and height was 167 cm (body mass index, 21.8 kg/m2). The physical examination revealed hand tremors, a forward gait, and mask-like face.
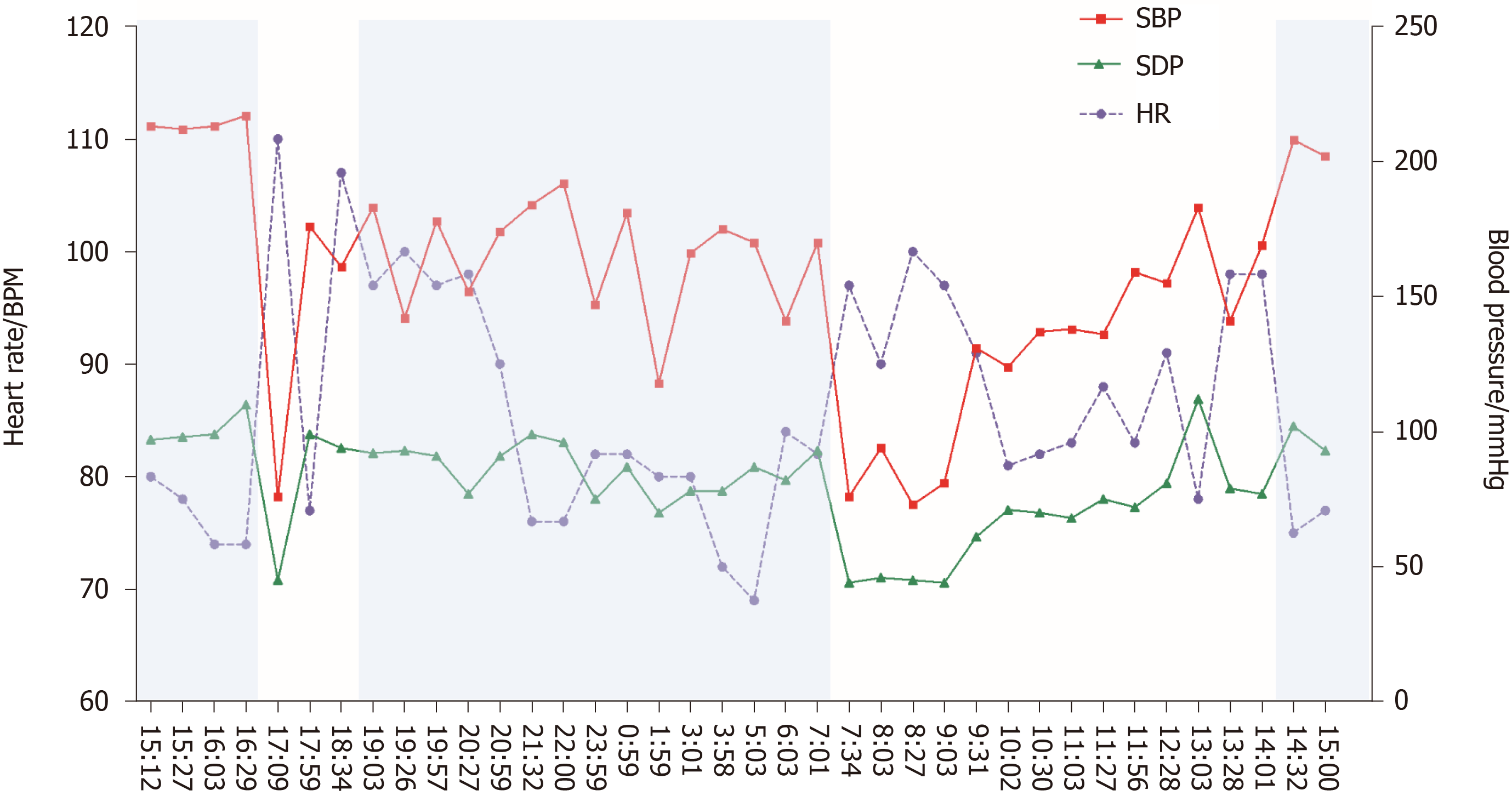
The 24-h ambulatory blood pressure monitoring indicated that the blood pressure fluctuated greatly. The blood pressure in the lying position remained high at night (up to 217/110 mmHg), and the blood pressure in the standing position dropped sharply in the next morning (73/45 mmHg), accompanied by dizziness, blackout, and sweating (Figure 1). Seated-to-standing blood pressure measurement was performed, and the blood pressure dropped from 173/96 mmHg to 95/68 mmHg after standing for 3 min from supine position.
Creatinine clearance and urine albumin were in the reference range, which indicated normal renal function. Plasma and urinary epinephrine, noradrenaline, catecholamines, and 24-h urine vanillylmandelic acid were normal. Plasma dopamine was elevated to 3431.7 pg/mL. Cortisone rhythm, 24-h urinary kalium, and the ratio of plasma aldosterone concentration to plasma renin activity were normal.
Aortic ultrasound and kidney computed tomography (CT) angiography found no aortic coarctation or renal arterial stenosis. There were no adrenal masses by cross sectional imaging with CT of the abdomen. Electroencephalogram was normal.
The final diagnosis was nOH (diagnosed by measuring a at least 20-mmHg drop in systolic blood pressure or a at least 10-mmHg drop in diastolic blood pressure within 3 min of standing[5]), supine hypertension (diagnosed by a systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg when supine position[6]), and Parkinson's disease.
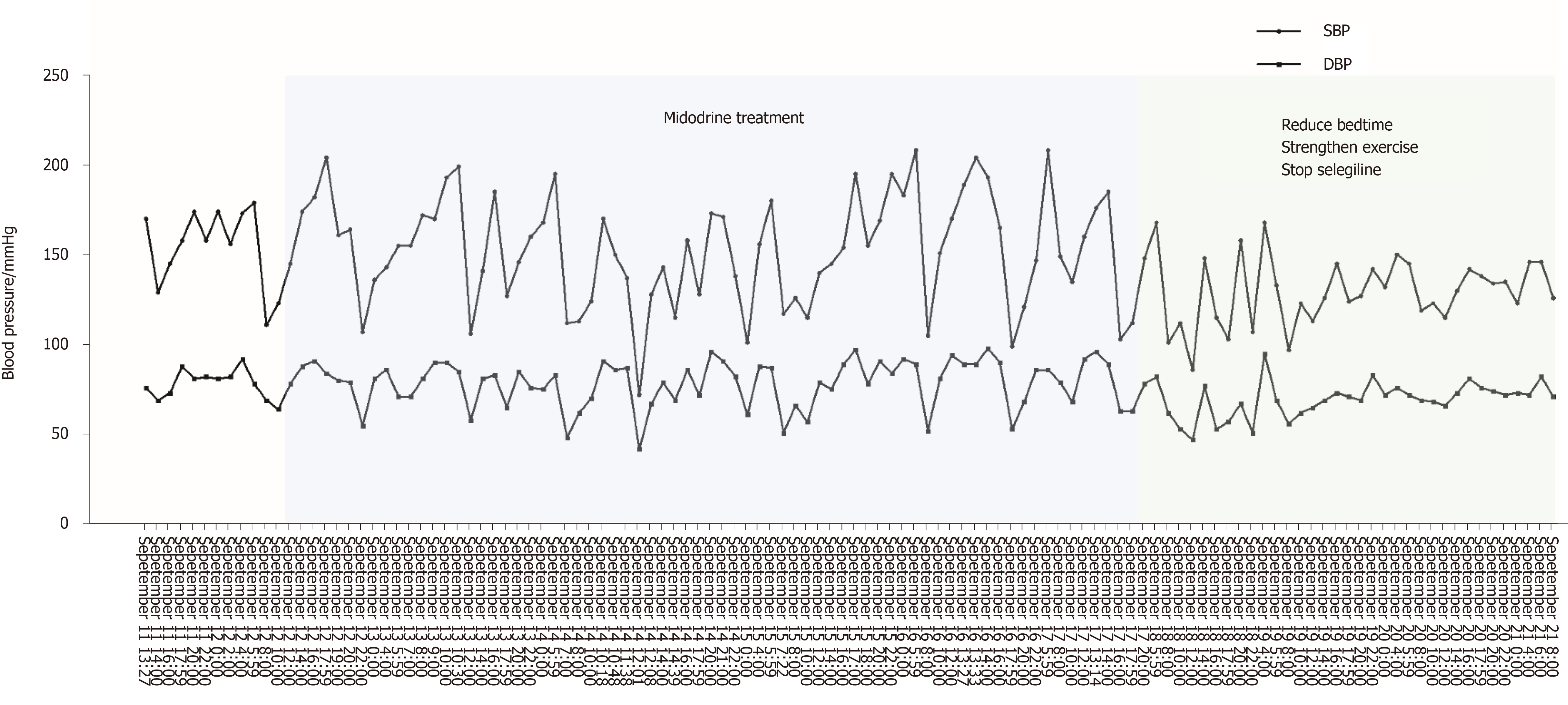
The patient was medically stabilized with Midodrine (2.5 mg twice a day, but not within 5 h before bedtime). After taking Midodrine, the symptoms of nOH and supine hypertension did not improve (Figure 2). After strengthening exercises, reducing the time of lying down during the day, and consuming water before getting up, the variability of blood pressure was gradually getting better. At the same time, in view of the fact that anti-PD drugs (levodopa, dopamine agonists, and MAO-B inhibitors) can aggravate or induce orthostatic hypotension and as Madopar is essential for the treatment of Parkinson's disease, we continued to use Madopar and discontinued Selegiline. Subsequently, blood pressure fluctuations gradually stabilized, and blood pressure at night decreased significantly compared with that before (Figure 2).
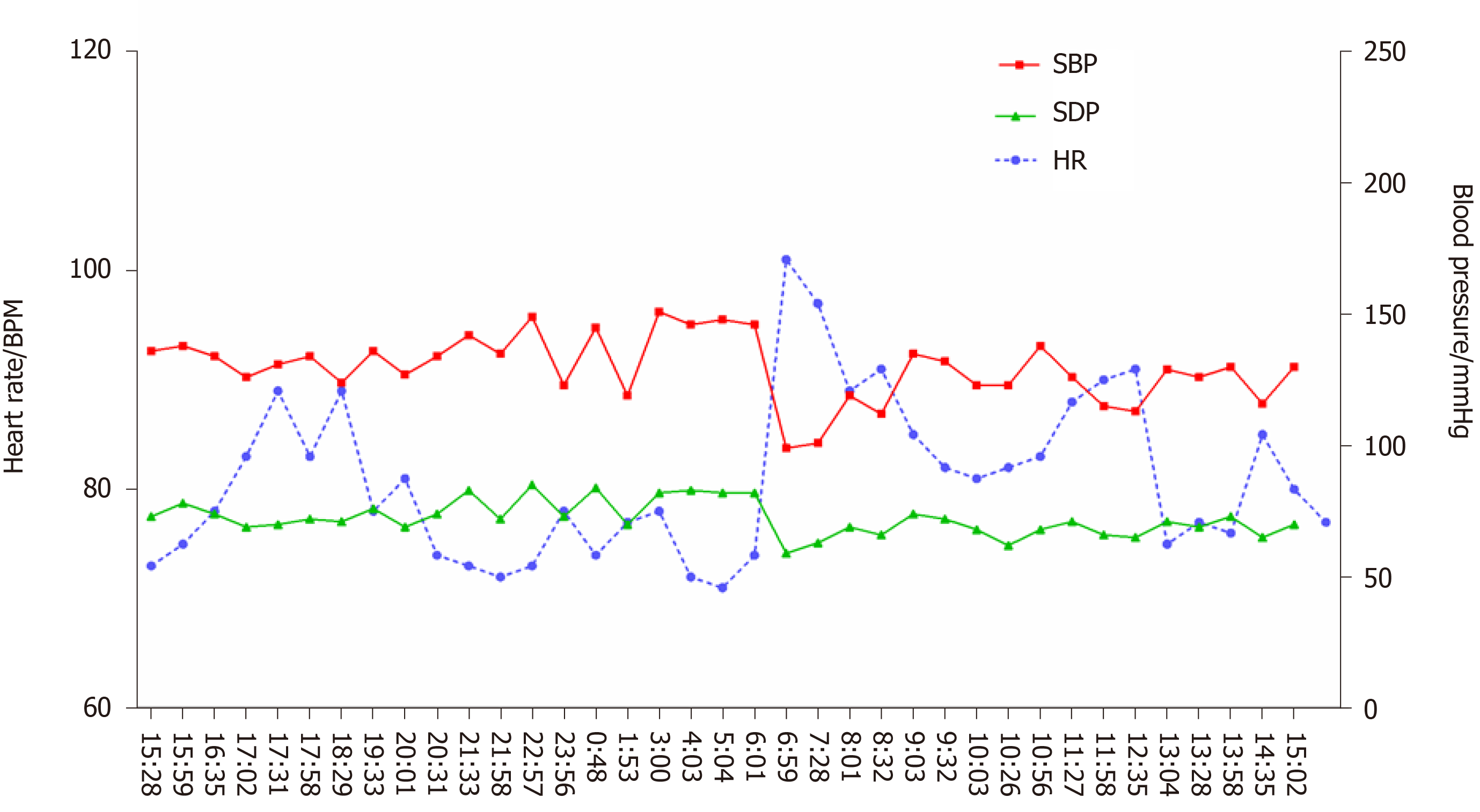
After treatment, dizziness and amaurosis gradually relieved, and blood pressure became more stable. At the 3-mo and 8-mo follow-up, there was no recurrence syncope. At the 8-mo follow-up, the patient rechecked 24-h ambulatory blood pressure monitoring and found that the blood pressure was much more stable compared with that 8 mo before (Figure 3).
Syncope is a transient loss of consciousness caused by insufficient global cerebral blood perfusion. It is a common condition with a recurrence rate up to 41%[7]. It is not a disease but a symptom of other underlying diseases[8]. There are many causes of syncope, among which autonomic dysfunction (nOH) is one of the most important causes (up to 25%)[7]. In our case, the patient was diagnosed with nOH presenting with syncope and uncontrolled blood pressure.
Syncope presents with diagnostic challenges and is associated with high healthcare costs. The United States spends about 410 million dollars every year for the diagnostic evaluation of syncope, and the average cost of hospitalization per patient is about 9400 dollars[9]. Many complex assessments increase the burden of medical insurance, but simple yet effective methods such as the basic medical history and monitoring methods are most often ignored. A detailed medical history and clinical examination are the core steps in finding the causes of syncope. For orthostatic hypotension, syncope occurs while standing or after standing, which is the most prominent clinical manifestation, with its triggers and symptoms having distinct features. Furthermore, simple methods such as ambulatory blood pressure monitoring and seated-to-standing blood pressure measurement are recommended gold standard measurements in diagnosing nOH[10]. Ambulatory blood pressure monitoring in our case indicated that the patient's blood pressure increased at night or in the supine position, with a sharp drop in the morning or after a meal, suggestive of autonomic nerve dysfunction.
Paying attention to comorbidities can also help in the diagnosis of the cause of syncope. nOH is often accompanied by central or peripheral autonomic dysfunction. nOH develops in up to 30% of patients suffering from PD, 80% of patients had multiple atrophy[11,12], and 33% of patients had diabetes[13], amyloidosis[14], and other peripheral diseases. In our case, position-related syncope occurred 1 year after the onset of PD.
The syncope in our case was caused by PD-related nOH, accompanied by supine hypertension. One half of patients with nOH have supine hypertension[15,16], which makes it difficult to manage the blood pressure. It is critical for physicians to understand the underlying mechanism of disease development. Central and peripheral norepinephrine deficiency affects peripheral sympathetic neurotization and vasoconstriction function, and the blood pressure drops due to reduction in venous return on standing. Repeated orthostatic hypotension can chronically activate the renin-angiotensin system and cause the supine blood pressure to rise; nocturnal stress diuresis can also worsen blood pressure in the early morning of the next day[17]. Therefore, such patients experience large fluctuations and abnormal circadian rhythms in blood pressure.
nOH and supine hypertension are two conditions with opposite hemodynamics, making blood pressure control in such patients very challenging. Many medications may improve one at the expense of exacerbating the other. As the first FDA-approved drug for nOH treatment, Midodrine, an α 1-adrenoreceptor agonist, can constrict blood vessels and improve orthostatic symptoms[18,19], but it also carries a high risk of aggravating supine hypertension at the same time[20]. Then nocturnal stress diuresis and reduced blood volume can aggravate orthostatic hypotension in the morning. Midodrine in our case study was not effective for nOH as reported in previous studies[18,19] and meta-analysis[21]; the early morning hypotension did not improve and the night blood pressure remained high following the use of Midodrine for a week (Figure 2). This suggests that Midodrine may not be a suitable choice for patients with nOH accompanied with supine hypertension.
In our case, the patient's blood pressure stabilized with simple strategies such as strengthening exercises, reducing bedtimes, consuming water intake before getting up, and discontinuing the medications that caused hypotension (Figure 2). Therefore, it appears that these strategies remain the primary management therapies for nOH in patients with PD. At the same time, ambulatory blood pressure monitoring also plays a valuable role in evaluating the response to drug treatment. Current treatment for nOH with PD was based on expert's opinion, and more clinical evidence is needed to support the guidelines.
This study has several limitations. First, specialized autonomic reflex tests for the diagnosis of nOH such as the blood pressure response to Valsalva maneuver was lack in our case study. Second, the treatment process of the case was not standardized as pharmacologic treatment came first for primary management methods.
Autonomic neurological dysfunction with nOH is one of the causes of syncope. It is necessary to perform ambulatory blood pressure monitoring in patients with unstable blood pressure to observe fluctuations in blood pressure. At the same time, attention should be paid to patients with comorbid conditions, such as PD, multiple system atrophy, diabetes, and other diseases that can cause autonomic dysfunction, as well as medications that cause hypotension. Increasing physical activity and reducing the amount of time in bed are still the primary management methods for patients with nOH.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anghelescu A S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Li JH
| 1. | Lamb LE, Green HC, Combs JJ, Cheeseman SA, Hammond J. Incidence of loss of consciousness in 1,980 Air Force personnel. Aerosp Med. 1960;31:973-988. [PubMed] |
| 2. | Tilvis RS, Hakala SM, Valvanne J, Erkinjuntti T. Postural hypotension and dizziness in a general aged population: a four-year follow-up of the Helsinki Aging Study. J Am Geriatr Soc. 1996;44:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Eschlböck S, Wenning G, Fanciulli A. Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J Neural Transm (Vienna). 2017;124:1567-1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 6. | Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, Lahrmann H, Mancia G, Mayer G, Norcliffe-Kaufmann L, Pavy-Le Traon A, Raj SR, Robertson D, Rocha I, Struhal W, Thijs R, Tsioufis KP, van Dijk JG, Wenning GK. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018;28:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG; ESC Scientific Document Group . 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1124] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 8. | Singhi P, Saini AG. Syncope in Pediatric Practice. Indian J Pediatr. 2018;85:636-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Sun BC, Emond JA, Camargo CA Jr. Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95:668-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Shibao C, Lipsitz LA, Biaggioni I; American Society of Hypertension Writing Group. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens. 2013;7:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Köllensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M, Klockgether T, Yekhlef F, Tison F, Daniels C, Deuschl G, Coelho M, Sampaio C, Bozi M, Quinn N, Schrag A, Mathias CJ, Fowler C, Nilsson CF, Widner H, Schimke N, Oertel W, Del Sorbo F, Albanese A, Pellecchia MT, Barone P, Djaldetti R, Colosimo C, Meco G, Gonzalez-Mandly A, Berciano J, Gurevich T, Giladi N, Galitzky M, Rascol O, Kamm C, Gasser T, Siebert U, Poewe W, Wenning GK; EMSA-SG. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord. 2010;25:2604-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson's disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15:954-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27:2942-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Wang AK, Fealey RD, Gehrking TL, Low PA. Patterns of neuropathy and autonomic failure in patients with amyloidosis. Mayo Clin Proc. 2008;83:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Fanciulli A, Göbel G, Ndayisaba JP, Granata R, Duerr S, Strano S, Colosimo C, Poewe W, Pontieri FE, Wenning GK. Supine hypertension in Parkinson's disease and multiple system atrophy. Clin Auton Res. 2016;26:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Isaacson SH. Managed care approach to the treatment of neurogenic orthostatic hypotension. Am J Manag Care. 2015;21:s258-s268. [PubMed] |
| 18. | Jankovic J, Gilden JL, Hiner BC, Kaufmann H, Brown DC, Coghlan CH, Rubin M, Fouad-Tarazi FM. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 240] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 210] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Chen JJ, Han Y, Tang J, Portillo I, Hauser RA, Dashtipour K. Standing and Supine Blood Pressure Outcomes Associated With Droxidopa and Midodrine in Patients With Neurogenic Orthostatic Hypotension: A Bayesian Meta-analysis and Mixed Treatment Comparison of Randomized Trials. Ann Pharmacother. 2018;52:1182-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Loavenbruck A, Sandroni P. Neurogenic orthostatic hypotension: roles of norepinephrine deficiency in its causes, its treatment, and future research directions. Curr Med Res Opin. 2015;31:2095-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |