Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5932
Peer-review started: January 22, 2021
First decision: April 29, 2021
Revised: May 8, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: July 26, 2021
Processing time: 179 Days and 16.6 Hours
The side effects of prostate cancer (PCa) treatment are very prominent, with cancer-related fatigue (CRF) being the most common. Fatigue is a distressing symptom that interferes with daily functioning and seriously affects patient quality of life during, and for many years after, treatment. However, compared with other types of cancer, such as breast cancer, little is known about the prevalence of PCa-related fatigue.
To determine the prevalence of CRF in patients with PCa.
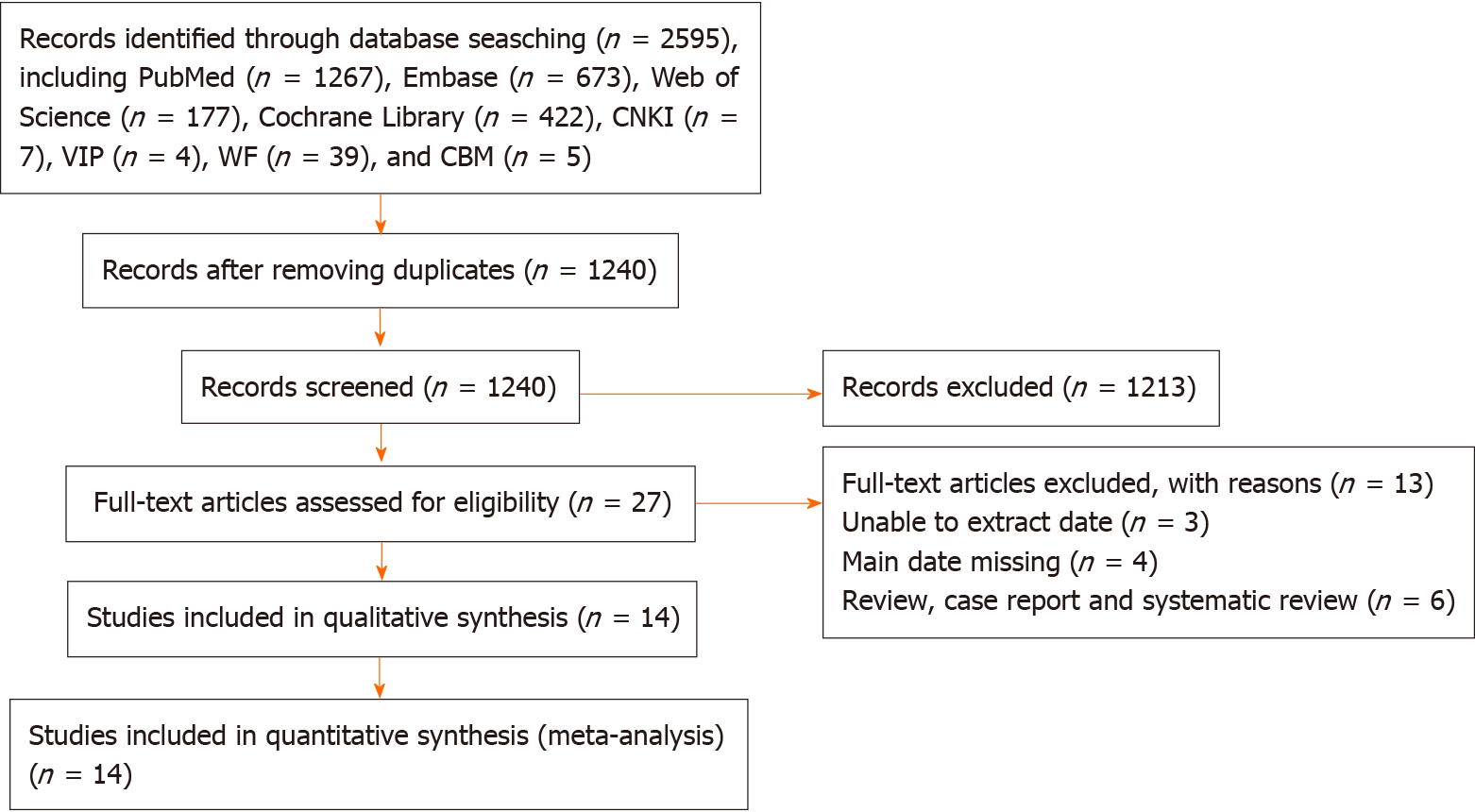
A systematic search of EMBASE, PubMed, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, WANFANG DATA, Technology Journal Database and the Chinese Biological Medical Database was conducted up to July 28, 2020. Included studies measured the incidence of PCa-related fatigue and differentiated fatigue outcomes (incidence) between treatment modalities and fatigue assessment times. In our meta-analysis, both fixed and random-effects models were used to estimate the pooled prevalence of PCa-related fatigue. Subgroup analyses were performed using treatment modalities and fatigue assessment times. Publication and sensitivity bias analyses were performed to test the robustness of the associations.
Fourteen studies, involving 4736 patients, were eligible for the review. The pooled CRF prevalence was 40% in a total sample of 4736 PCa patients [95% confidence interval (CI): 29-52; P < 0.01; I2 = 98%]. The results of the subgroup analyses showed the prevalence of CRF after androgen deprivation therapy treatment, radical prostatectomy and radiotherapy to be 42% (95%CI: 20-67, P < 0.01, I2 = 91%), 21% (95%CI: 16-26, P = 0.87, I2 = 0%) and 40% (95%CI: 22-58, P < 0.01, I2 = 90%), respectively. The prevalence of acute and persistent fatigue was 44% (95%CI: 25-64; P < 0.01; I2 = 93%) and 29% (95%CI: 25-32; P = 0.30; I2 = 17%), respectively.
Our meta-analysis showed that fatigue is a common symptom in men with PCa, especially those using hormone therapy.
Core Tip: This study was a systematic review conducted to determine the prevalence of cancer-related fatigue in patients with prostate cancer. Compared with other types of cancer, little is known about the prevalence of prostate cancer treatment-related fatigue. In this study, we reviewed the data in 14 papers (4736 patients) and found that the pooled prevalence of cancer treatment-related fatigue was 40%. Interestingly, the prevalence of cancer-related fatigue was associated with the type of treatment that the patients received; those undergoing radical prostatectomy had the lowest prevalence of fatigue.
- Citation: Luo YH, Yang YW, Wu CF, Wang C, Li WJ, Zhang HC. Fatigue prevalence in men treated for prostate cancer: A systematic review and meta-analysis. World J Clin Cases 2021; 9(21): 5932-5942
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5932.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5932
Prostate cancer (PCa) is the second most common cancer in men after lung cancer, with an estimated 1.28 million newly diagnosed cases worldwide in 2018[1]. Treatment advances have improved PCa-specific survival, with 5-10-year disease-free survival rates in Western countries reported to be 75%-94%[2-5]. Androgen deprivation therapy (ADT), radiotherapy (RT), chemotherapy and surgery [radical prostatectomy (RP)] are the current mainstream treatment options due to their efficacy in reducing prostate-specific disease progression[6]. However, the side effects of PCa treatment are very prominent, and the clinical focus has shifted to controlling or reducing treatment-related side effects[7-10]. Fatigue is the most common treatment-related side effect of PCa, which seriously affects patient quality of life during treatment and for many years later[11-13].
Cancer-related fatigue (CRF) is defined as a sense of tiredness that persists over time, interferes with activities of daily living and is not relieved by adequate rest[14]. The prevalence of CRF is as high as 59%-100%[15]. Cancer patients who have partially completed treatment still feel tired for one or more years after treatment, and this symptom is listed by patients as the one with the longest duration and the most impact on daily life[16,17]. However, the exact statistics on the prevalence of CRF in patients with prostate cancer remain unknown.
Recently, there has been an increased interest in investigating the impacts of fatigue in men with PCa. Although these studies provide useful information, they are characterized by a number of methodologic limitations, such as small sample sizes and limited follow-up periods. Hence, they do not adequately reflect the current prevalence of fatigue in PCa patients. Therefore, we performed a meta-analysis with two main aims. The first aim was to compute a robust estimate of the prevalence of PCa-related fatigue based on high-quality studies with sufficiently large sample sizes. The second aim was to evaluate the effects of different treatment methods and the fatigue assessment times on the prevalence of CRF in patients.
The PRISMA statement guidelines were followed for the calculation and reporting of meta-analysis data[18]. Literature searches were conducted using EMBASE, PubMed, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, WANFANG DATA, Technology Journal Database and the Chinese Biological Medical Database; the search period was from database inception through July 2020. The following search terms were used: “prostatic neoplasms,” “prostat* neoplasms,” “prostate cancer,” “prostat* cancer,” “prostat* tumor*,” “prostat* tumour*,” “prostat* carcino*,” “fatigue,” “tired*,” “cancer-related fatigue” and “CRF”. The references identified in the relevant publications were also reviewed to identify additional studies.
Studies that met the following criteria were included: investigated fatigue in men with prostate cancer, measured the prevalence of prostate CRF using structured questionnaires with established psychometric properties, differentiated fatigue outcomes (incidence) between treatment options or fatigue assessment time; there were no limitations on the language of publication, year of publication or publication status. Reviews, lectures, case reports and articles in which the data were obviously abnormal or missing (and the author could not be contacted) were excluded from the analysis.
The identified studies were stored in reference management software (EndNote, Clarivate, Philadelphia, PA, United States). Literature screening and data extraction were independently performed by two reviewers. Any disagreements between the reviewers were resolved by discussion with a third reviewer. We extracted the first author’s name, year of publication, study name, country in which the study was conducted, sample size, follow-up period, fatigue assessment scale, study design, fatigue assessment time (clinical fatigue diagnosed during treatment was defined as acute fatigue; fatigue continuing for ≥ 1 year after treatment was defined as persistent fatigue), treatment method and primary outcomes.
Papers that had small sample sizes, did not appropriately justify the questionnaires used, failed to properly control for confounding variables and did not fully explain the statistical methods of analysis were considered to be of low quality; fair- to high-quality papers met some or all of these criteria[19,20]. Publication bias was tested using Egger’s Funnel plots.
We used R software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria) for all statistical analyses. The combined prevalence and 95% confidence interval (95%CI) of CRF in patients with PCa was calculated. Heterogeneity among the studies was assessed using Q and I2 statistic indices. A significant Q value (P < 0.1) indicated a lack of finding homogeneity among the studies; I2 = 0 indicated that an inconsistency among the results makes no statistical difference (I2 < 50% indicated low inconsistency, I2≥ 50% indicated high inconsistency). If the heterogeneity test results are P > 0.1 and I2 < 50%, the homogeneity of the study was considered to be good, and a fixed-effects model was adopted; otherwise, the random-effects model was adopted. Subgroup analyses were performed based on the treatment modalities used and the fatigue assessment times.
A flow chart of the study selection process and exclusion criteria is shown in Figure 1. According to the search criteria, a total of 2594 studies were identified; the total number of patients was 4736. We filtered the results by title, abstract and full text. In the end, 14 studies met the inclusion and exclusion criteria. Among them, three studies were about the incidence of CRF after ADT treatment for prostate cancer, six reported the incidence of CRF after RT treatment, and three reported the incidence of CRF after RP. Six studies reported the incidence of acute fatigue, and five reported the incidence of persistent fatigue. The characteristics of the included studies are shown in Table 1.
| Ref. | Country | Time of case inclusion | Study design | Sample size | Number of CRF cases | The incidence of CRF, % | Scale | Assessment time | Treatment |
| Yu and Chen[21] | China | 2015.4-2017.9 | Cross-sectional | 174 | 109 | 62.64 | BFI | NA | ADT |
| Wang et al[22], 2017 | China | 2006.12-2016.12 | Longitudinal | 147 | 89 | 60.54 | BFI | NA | NA |
| Ashton et al[11], 2019 | United Kingdom | 2016.10-2017.3 | Cross-sectional | 62 | 15 | 24.19 | BFI | T1 | RARP/ADT |
| Baden et al[28], 2020 | Ireland | 1995.1-2011.3 | Cross-sectional | 2879 | 556 | 19.31 | EORTC QLQ-C30 | NA | NA |
| Feng et al[23], 2017 | United States | 2009.9-2013.11 | Longitudinal | 34 | 14 | 41.17 | FACT-F | T2 | EBRT |
| Feng et al[8], 2019 | United States | 2009.9-2015.2 | Longitudinal | 47 | 16 | 34.04 | FACT-F | T2 | EBRT |
| Gonzalez et al[10], 2018 | Spain | 2014.7-2014.9 | Longitudinal | 26 | 5 | 19.23 | FACT-F | T1 | EBRT |
| Feng et al[7], 2020 | United States | 2009.9-2015.11 | Longitudinal | 64 | 36 | 56.25 | FACT-F | T1/T2 | ADT + RT/EBRT |
| Maliski et al[24], 2005 | United States | NA | Longitudinal | 147 | 28 | 19.04 | SF-36 | NA | NA |
| Nelson et al[12], 2016 | United States | 2008.9-2013.10 | Case control study | 145 | 35 | 24.13 | BFI | T1/T2 | ADT/RP |
| Saligan et al[25], 2016 | United States | 2009.4-2013.12 | Longitudinal | 47 | 39 | 82.97 | FACT-F | T1 | EBRT |
| Storey et al[13], 2012 | United Kingdom | 2005.8-2005.11 | Cross-sectional | 377 | 216 | 57.29 | BFI | T2 | RT/ RP |
| Jones et al[27], 2016 | Canada | NA | Longitudinal | 529 | 90 | 17.01 | FACT-F | NA | NA |
| Ozdemir et al[26], 2019 | Turkey | 2014.3-2018.9 | Cross-sectional | 58 | 31 | 53.44 | FACT-F | T1 | NA |
Most studies were of fair[8,10,21-26] or high[7,11-13,27,28] quality. Only three of these studies had an adequate sample size[13,27,28].
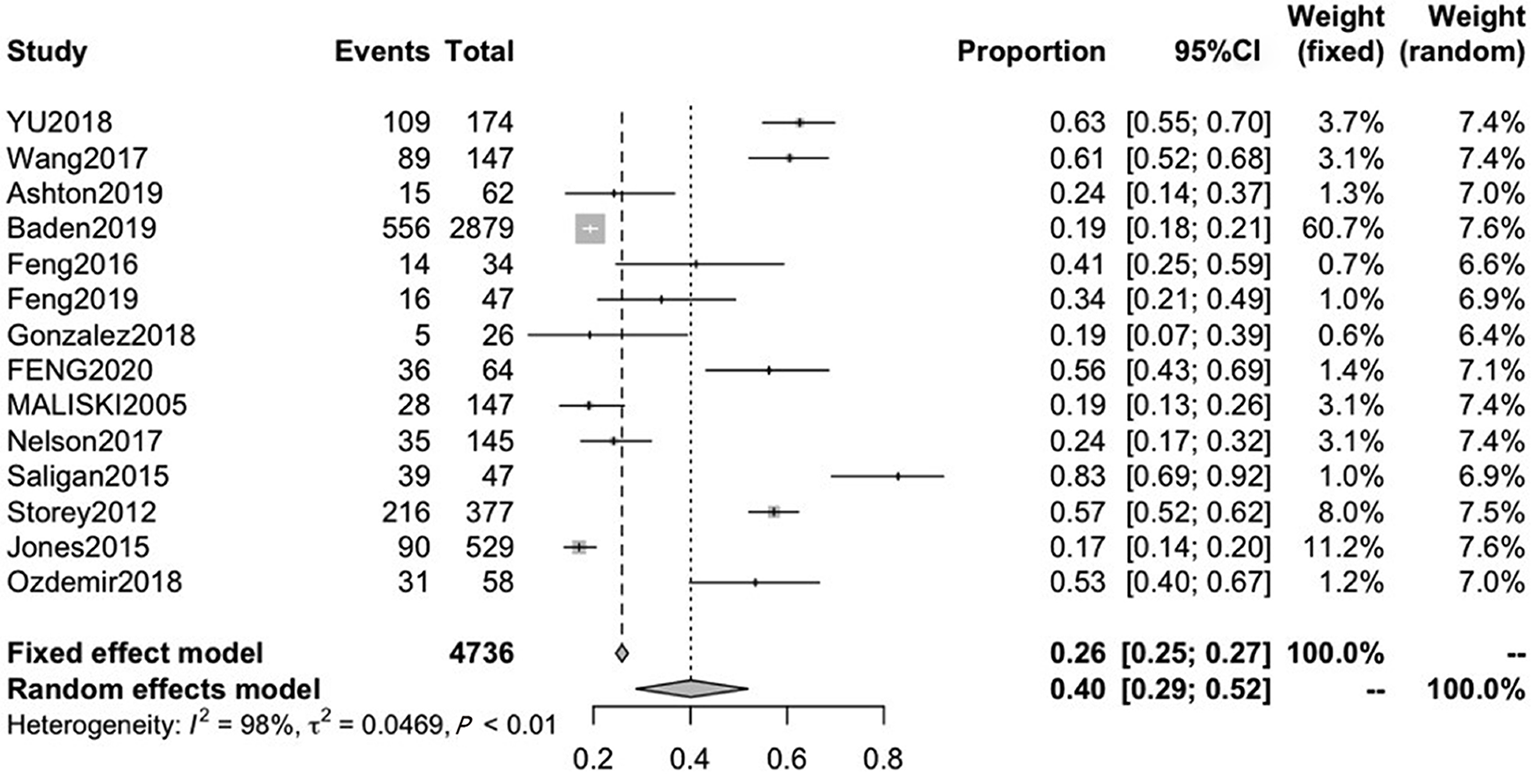
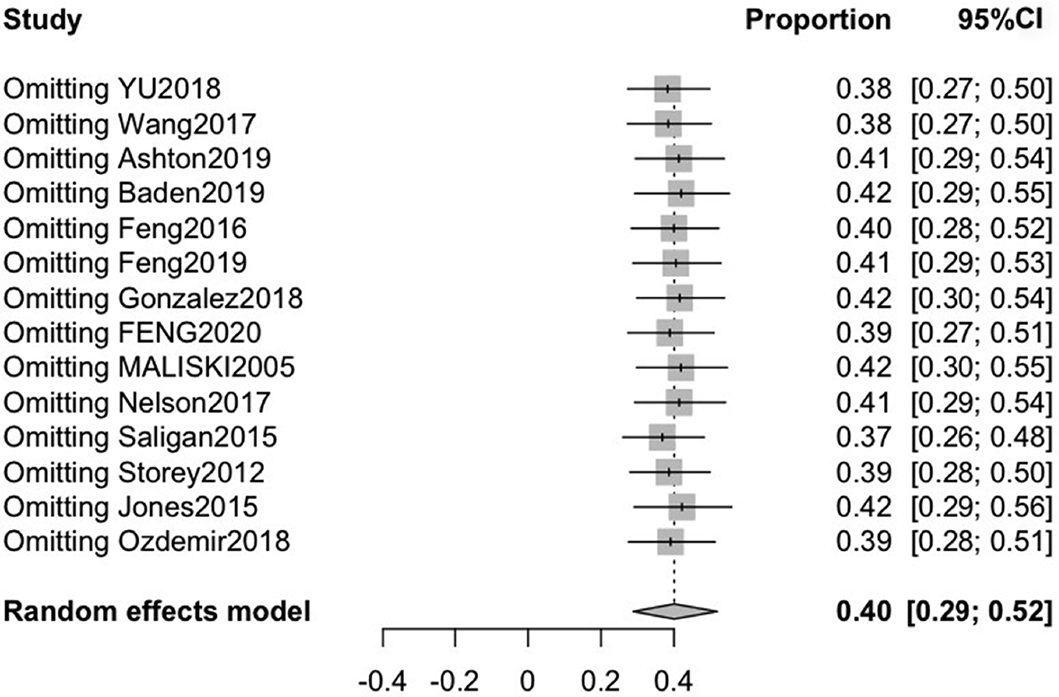
Fourteen studies reported the incidence of fatigue in patients with PCa, with mean ages ranging from 60.0 to 75.3. The pooled CRF prevalence was 40% (95%CI: 29-52), in a total sample of 4736 patients, with a high level of heterogeneity (P < 0.01, I2 = 98%). Therefore, we used a random-effects model (Figure 2).
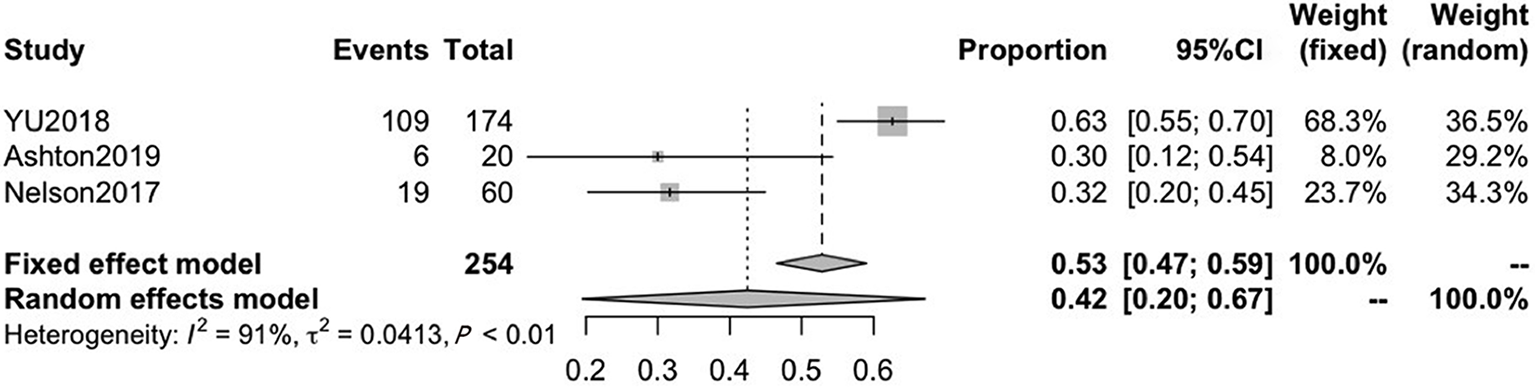
Three of the included studies reported on CRF after ADT treatment, with mean ages ranging from 67.3 to 73.3. The pooled CRF prevalence was 42% (95%CI: 20-67), in a total sample of 254 patients, with a high level of heterogeneity (P < 0.01, I2 = 91%). Therefore, we used a random-effects model (Figure 3).
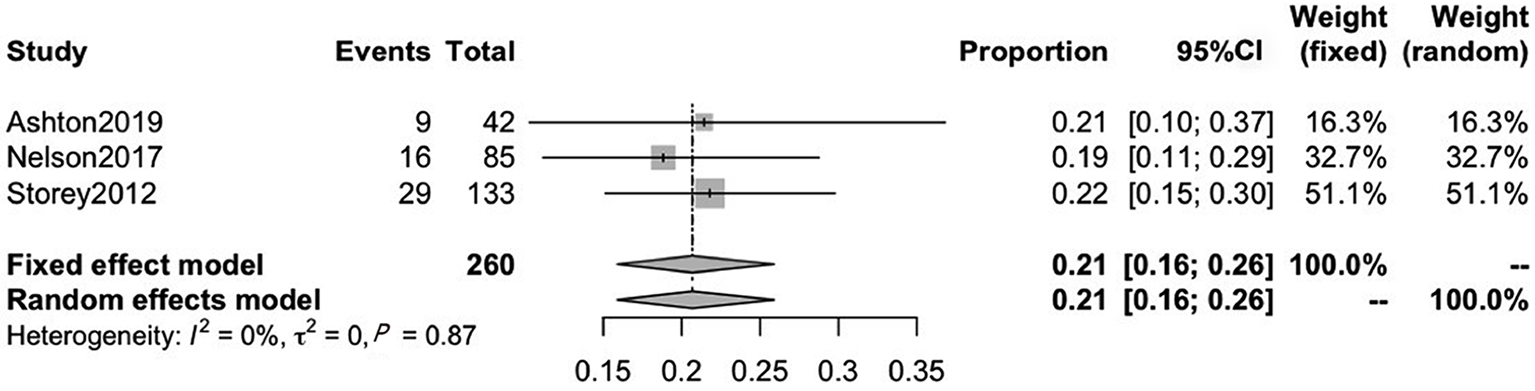
Another three included studies reported on CRF after RP treatment, with mean ages ranging from 63.8 to 67.9. The pooled CRF prevalence was 21% (95%CI: 16–26), in a total sample of 260 patients, with a low level of heterogeneity (P = 0.87, I2 = 0%). Therefore, we used a fixed-effects model (Figure 4).
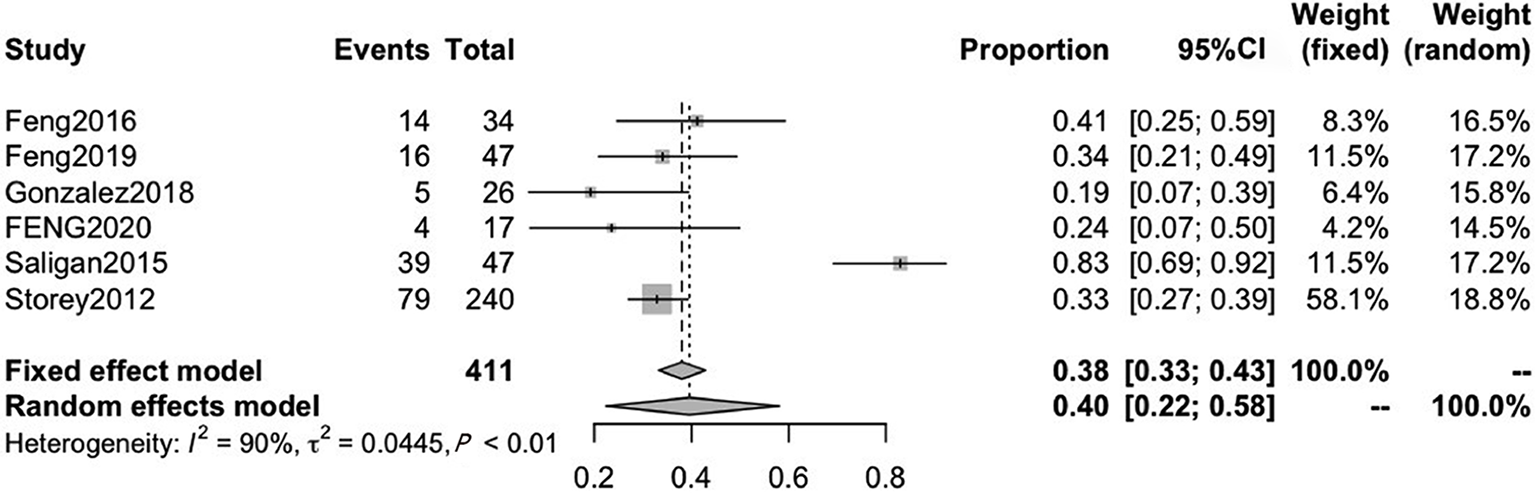
Six studies reported on CRF after RT therapy, with mean ages ranging from 64.1 to 66.0. The pooled CRF prevalence was 40% (95%CI: 22-58) in a total sample of 411 patients, with a high level of heterogeneity (P < 0.01, I2 = 90%). Therefore, we used a random-effects model (Figure 5).
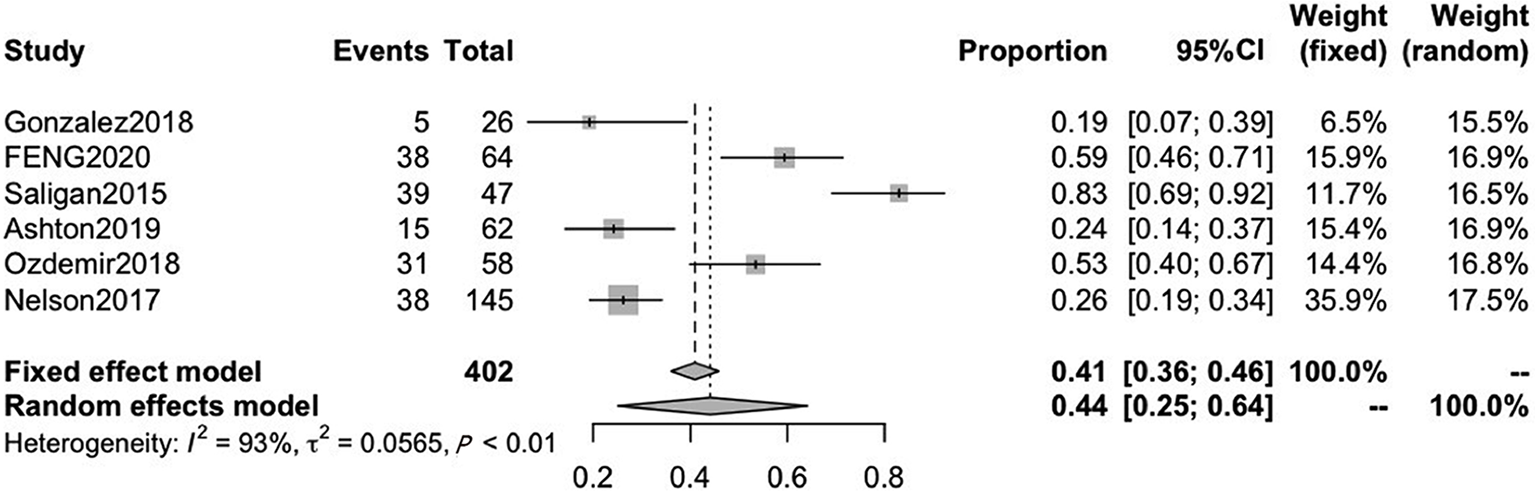
A total of six included studies reported on acute fatigue. The pooled CRF prevalence was 44% (95%CI: 25-64) in a total sample of 402 patients, with a high level of heterogeneity (P < 0.01, I2 = 93%). Therefore, we used a random-effects model (Figure 6).
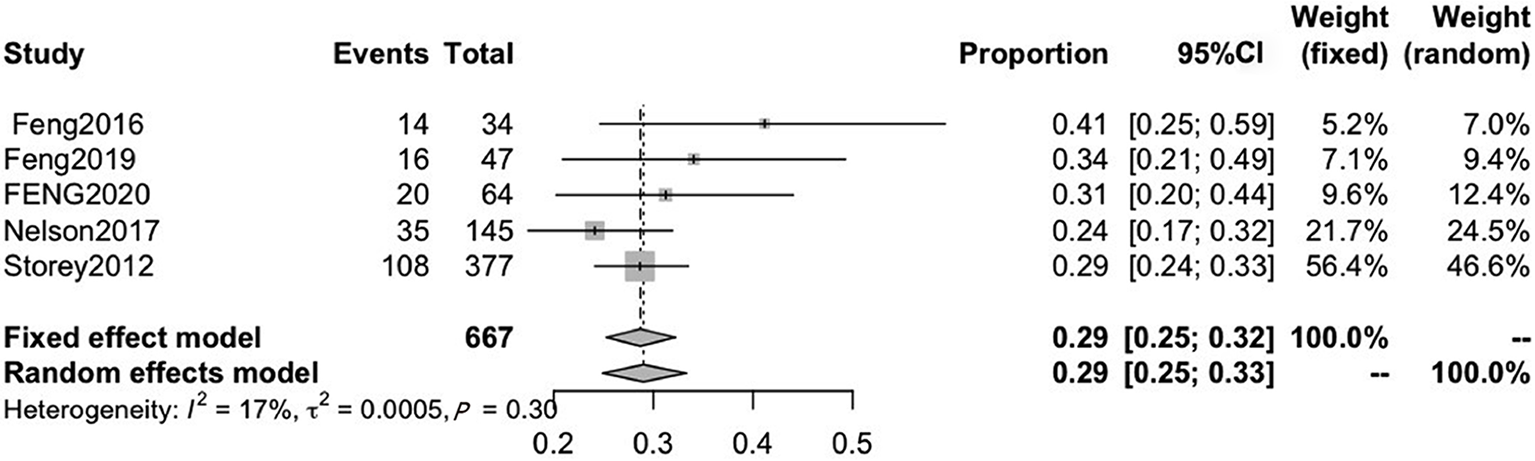
Five included studies reported on persistent fatigue. The pooled CRF prevalence was 29% (95%CI: 25-32), in a total sample of 667 patients, with a high level of heterogeneity (P = 0.30, I2 = 17%). Therefore, a fixed-effects model was used (Figure 7).
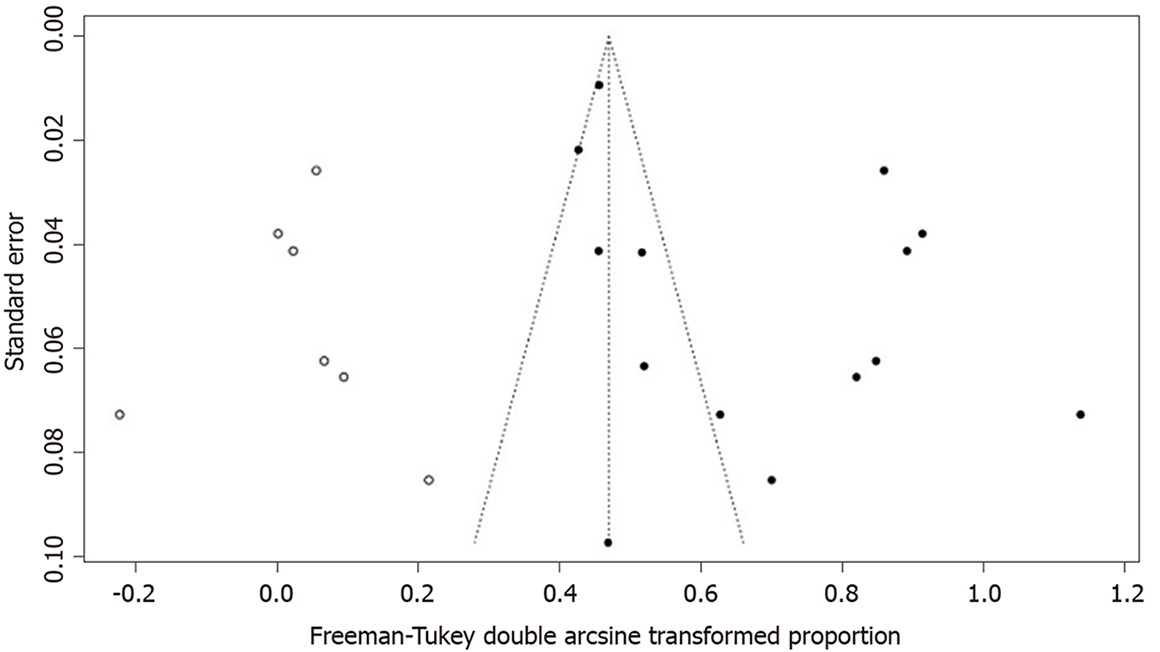
A funnel plot was created to represent the total prevalence of CRF; the plot showed an asymmetric distribution of the study points. Egger’s test result (P = 0.02617) also suggested the possibility of publication bias. A nonparametric shear complement method was used to estimate the number of missing studies and evaluate the influence of publication bias on the results. The results showed significant differences in the results before and after splicing. The prevalence of CRF, calculated before and after trimming, was 40% (95%CI: 29-52) and 20% (95%CI: 11-31), respectively, suggesting that publication bias had a great influence on the stability of the results (Figure 8).
To assess the stability of the results, we performed a sensitivity analysis on the 14 included studies by sequentially excluding individual studies. After arbitrarily excluding one study, the combined conversion rate based on the random-effects model was 40% (95%CI: 29-52), indicating that it had little influence on the combined effect size. Therefore, the results of our meta-analysis are stable and reliable (Figure 9).
CRF is a common side effect of PCa treatment that can negatively affect a patient’s daily life, physiology and psychology[29,30]. Previous studies on the fatigue status of PCa patients have shown that the prevalence of CRF is between 17% and 82%, varying broadly due to various associated factors[25,27]. The present meta-analysis estimated the CRF prevalence to be 40% in a sample of 4736 patients, indicating that a sizeable proportion of men with PCa experience severe fatigue.
This review found that fatigue is associated with all common PCa treatment types. Specifically, our results showed that the prevalence of CRF in patients receiving RP (21%) was lower than that in those receiving ADT (42%) or RT (40%), similar to other published results[11,12], suggesting that RP has little impact on fatigue prevalence. This finding is likely due to the fact that patients receiving ADT and RT are older, have more underlying diseases and are in advanced stages of disease. Conversely, RP is mainly suited to patients with localized, significant disease and having > 10 years of life expectancy and those with the ability to perform activities of daily living. The rate of clinical fatigue associated with ADT treatment was similar to that with RT treatment, as both primarily cause fatigue via their hematologic toxicity.
Only one study[7] included in this meta-analysis reported the incidence of CRF in patients treated with a combination of RT and ADT; thus, only a descriptive analysis was performed. The highest incidence of fatigue (68%) occurred in patients receiving a combination of RT and ADT, which may be associated with the combination treatment aggravating the resultant hemotoxicity and peripheral and central nervous system mitochondrial dysfunction caused by either treatment alone.
Men with PCa are more likely than other cancer patients to report persistent fatigue for more than 6 mo after treatment, with a high incidence of functional impairment due to the fatigue[31]. The subgroup analysis results of this study showed that the prevalence of acute and persistent fatigue was 44% and 29%, respectively. After the initiation of RT or ADT, the fatigue severity increases and continues to increase over time[20]. Although there is evidence that fatigue severity returns to baseline levels 6–8 wk after completing treatment[32,33], this was not the case in the study by Feng et al[34]. Rather, they found a subset of patients in their cohort who continued to experience fatigue for a year after RT, long after the treatment-associated hematologic toxicities had resolved. These findings suggest that acute and persistent fatigue may be independent phenomena that are mechanistically different; each may be driven by distinct underlying pathogenic processes[8]. Storey et al[13] suggested that the presence of post-treatment CRF may be more influenced by the patient’s current medical and psychological comorbidities than by the initial type of treatment received. Most evidence suggests that persistent fatigue is associated with depression, anxiety, urinary symptoms, pain and insomnia[8,23]. Furthermore, while effective treatments for persistent fatigue do not currently exist, targeting each of the fatigue-related symptoms may provide relief for patients suffering from this debilitating condition.
The present meta-analysis is characterized by some limitations. First, there was considerable heterogeneity among the primary studies. This might be attributable to differences in the cultures of the patients, the study settings and the variety of tools used to measure the prevalence of fatigue. Second, only one article analyzed the incidence of CRF associated with a combination therapy, precluding a meaningful analysis of the impacts of combination therapy. Third, the distribution of the funnel plot results was asymmetric, indicating possible publication bias, which might affect the accuracy of the results.
In conclusion, our meta-analysis revealed that patients with PCa have a high prevalence of CRF and that significant treatment-related differences in CRF incidence exist; further, there is a high prevalence of persistent fatigue. Similarly, high levels of symptoms have been reported in patients with breast cancer, and many interventions have been developed and tested to treat these symptoms[35,36]. Unfortunately, limited fatigue management research has been conducted in patients with PCa. Reported PCa research indicates that physical activity interventions, such as aerobic exercise and resistance exercise, are beneficial for reducing fatigue[37,38]. Additional behavioral interventions that have been shown to mitigate fatigue in cancer patients, including energy conservation[39], cognitive-behavioral therapy[40] and nutritional therapy[41], deserve further study to determine effective fatigue management strategies for patients with PCa.
Fatigue is a common symptom in men with prostate cancer, especially those using hormone therapy.
The side effects of prostate cancer (PCa) treatment are very prominent, with cancer-related fatigue (CRF) being the most common. Fatigue is a distressing symptom that interferes with daily functioning and seriously affects patient quality of life during, and for many years after, treatment. However, the exact statistics on the prevalence of CRF in patients with PCa remain unknown.
Recently, there has been an increased interest in investigating the impacts of fatigue in men with PCa. However, they do not adequately reflect the current prevalence of fatigue in PCa patients.
We performed a meta-analysis with two main aims. The first aim was to compute a robust estimate of the prevalence of PCa-related fatigue based on high-quality studies with sufficiently large sample sizes. The second aim was to evaluate the effects of different treatment methods and the fatigue assessment times on the prevalence of CRF in patients.
A systematic search of EMBASE, PubMed, OVID, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, WANFANG DATA, Technology Journal Database and the Chinese Biological Medical Database was conducted up to July 28, 2020. Included studies measured the incidence of prostate CRF and differentiated fatigue outcomes (incidence) between treatment modalities and fatigue assessment times. In our meta-analysis, both fixed and random-effects models were used to estimate the pooled prevalence of prostate CRF. Publication and sensitivity bias analyses were performed to test the robustness of the associations.
Fourteen studies, involving 4736 patients, were eligible for the review. The results showed that the pooled prevalence of cancer treatment-related fatigue was 40%. Interestingly, the prevalence of CRF was associated with the type of treatment that the patients received; those undergoing radical prostatectomy had the lowest prevalence of fatigue. Further, there is a high prevalence of persistent fatigue.
Fatigue is a common symptom in men with prostate cancer, especially those using hormone therapy.
Our meta-analysis revealed that patients with PCa have a high prevalence of CRF. Unfortunately, limited fatigue management research has been conducted in patients with PCa. Many interventions deserve further study to determine effective fatigue management strategies for patients with PCa.
We would like to thank Shen XP from the Department of Epidemiology and Biostatistics of School of Public Health of Lanzhou University for providing the help of biostatistics service.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scaggiante B S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55666] [Article Influence: 7952.3] [Reference Citation Analysis (132)] |
| 2. | Australian Institute of Health and Welfare. Cancer in Australia: actual incidence and mortality data from 1982 to 2007 and projections to 2010. Asia Pac J Clin Oncol. 2011;7:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Australian Institute of Health and Welfare. Cancer in Australia: Actual incidence data from 1991 to 2009 and mortality data from 1991 to 2010 with projections to 2012. Asia Pac J Clin Oncol. 2013;9:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Australian Institute of Health and Welfare. Cancer survival and prevalence in Australia: period estimates from 1982 to 2010. Asia Pac J Clin Oncol. 2013;9:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Ballon-Landa E, Parsons JK. Nutrition, physical activity, and lifestyle factors in prostate cancer prevention. Curr Opin Urol. 2018;28:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Mohler JL, Antonarakis ES. NCCN Guidelines Updates: Management of Prostate Cancer. J Natl Compr Canc Netw. 2019;17:583-586. [PubMed] |
| 7. | Feng LR, Wolff BS, Liwang J, Regan JM, Alshawi S, Raheem S, Saligan LN. Cancerrelated fatigue during combined treatment of androgen deprivation therapy and radiotherapy is associated with mitochondrial dysfunction. Int J Mol Med. 2020;45:485-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Feng LR, Fuss T, Dickinson K, Ross A, Saligan LN. Co-Occurring Symptoms Contribute to Persistent Fatigue in Prostate Cancer. Oncology. 2019;96:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Bandara V, Capp A, Ahmed G, Arm J, Martin J. Assessment and predictors of fatigue in men with prostate cancer receiving radiotherapy and androgen deprivation therapy. J Med Imaging Radiat Oncol. 2019;63:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Gonzalez VJ, Abbas-Aghababazadeh F, Fridley BL, Ghansah T, Saligan LN. Expression of Sestrin Genes in Radiotherapy for Prostate Cancer and Its Association With Fatigue: A Proof-of-Concept Study. Biol Res Nurs. 2018;20:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Ashton RE, Tew GA, Robson WA, Saxton JM, Aning JJ. Cross-sectional study of patient-reported fatigue, physical activity and cardiovascular status in men after robotic-assisted radical prostatectomy. Support Care Cancer. 2019;27:4763-4770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Nelson AM, Gonzalez BD, Jim HS, Cessna JM, Sutton SK, Small BJ, Fishman MN, Zachariah B, Jacobsen PB. Characteristics and predictors of fatigue among men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. 2016;24:4159-4166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Storey DJ, McLaren DB, Atkinson MA, Butcher I, Liggatt S, O'Dea R, Smyth JF, Sharpe M. Clinically relevant fatigue in recurrence-free prostate cancer survivors. Ann Oncol. 2012;23:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Yang B, Wang J. Effects of Exercise on Cancer-related Fatigue and Quality of Life in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Meta-analysis of Randomized Clinical Trials. Chin Med Sci J. 2017;32:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Husson O, Mols F, van de Poll-Franse L, de Vries J, Schep G, Thong MS. Variation in fatigue among 6011 (long-term) cancer survivors and a normative population: a study from the population-based PROFILES registry. Support Care Cancer. 2015;23:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Husson O, Nieuwlaat WA, Oranje WA, Haak HR, van de Poll-Franse LV, Mols F. Fatigue among short- and long-term thyroid cancer survivors: results from the population-based PROFILES registry. Thyroid. 2013;23:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13296] [Article Influence: 831.0] [Reference Citation Analysis (0)] |
| 19. | Pluye P, Gagnon MP, Griffiths F, Johnson-Lafleur J. A scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in Mixed Studies Reviews. Int J Nurs Stud. 2009;46:529-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 669] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 20. | Langston B, Armes J, Levy A, Tidey E, Ream E. The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support Care Cancer. 2013;21:1761-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Yu CY, Chen F. Analysis of Cancer-Related Fatigue in Prostate Cancer Patients with Androgen Deprivation Therapy. Jiefangjun Huli Zazhi. 2018;25:21-24. |
| 22. | Wang L, Luo HC, Fu ZC, Cheng HH, Shen ZY. The clinical study of cancer-related fatigue symptoms in prostate cancer patients. Xiandai Zhongliu Yixue. 2017;25:2938-2941. [DOI] [Full Text] |
| 23. | Feng LR, Wolff BS, Lukkahatai N, Espina A, Saligan LN. Exploratory Investigation of Early Biomarkers for Chronic Fatigue in Prostate Cancer Patients Following Radiation Therapy. Cancer Nurs. 2017;40:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Maliski SL, Kwan L, Orecklin JR, Saigal CS, Litwin MS. Predictors of fatigue after treatment for prostate cancer. Urology. 2005;65:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Saligan LN, Lukkahatai N, Holder G, Walitt B, Machado-Vieira R. Lower brain-derived neurotrophic factor levels associated with worsening fatigue in prostate cancer patients during repeated stress from radiation therapy. World J Biol Psychiatry. 2016;17:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Ozdemir K, Keser I, Sen I, Ozgur Tan M. Investigating the relationships between quality of life, fatigue and leisure time physical activity in prostate cancer patients. J Back Musculoskelet Rehabil. 2019;32:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Jones JM, Olson K, Catton P, Catton CN, Fleshner NE, Krzyzanowska MK, McCready DR, Wong RK, Jiang H, Howell D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 28. | Baden M, Lu L, Drummond FJ, Gavin A, Sharp L. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support Care Cancer. 2020;28:4813-4824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Wang XS, Zhao F, Fisch MJ, O'Mara AM, Cella D, Mendoza TR, Cleeland CS. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 30. | Drummond FJ, Kinnear H, O'Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 31. | Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 32. | Geinitz H, Thamm R, Scholz C, Heinrich C, Prause N, Kerndl S, Keller M, Busch R, Molls M, Zimmermann FB. Longitudinal analysis of quality of life in patients receiving conformal radiation therapy for prostate cancer. Strahlenther Onkol. 2010;186:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Monga U, Kerrigan AJ, Thornby J, Monga TN. Prospective study of fatigue in localized prostate cancer patients undergoing radiotherapy. Radiat Oncol Investig. 1999;7:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Feng LR, Suy S, Collins SP, Saligan LN. The role of TRAIL in fatigue induced by repeated stress from radiotherapy. J Psychiatr Res. 2017;91:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Yates P, Aranda S, Hargraves M, Mirolo B, Clavarino A, McLachlan S, Skerman H. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2005;23:6027-6036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Fillion L, Gagnon P, Leblond F, Gélinas C, Savard J, Dupuis R, Duval K, Larochelle M. A brief intervention for fatigue management in breast cancer survivors. Cancer Nurs. 2008;31:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 491] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 38. | Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud'Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D'Angelo ME. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 380] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 39. | Barsevick AM, Dudley W, Beck S, Sweeney C, Whitmer K, Nail L. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Montgomery GH, Kangas M, David D, Hallquist MN, Green S, Bovbjerg DH, Schnur JB. Fatigue during breast cancer radiotherapy: an initial randomized study of cognitive-behavioral therapy plus hypnosis. Health Psychol. 2009;28:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Baguley BJ, Bolam KA, Wright ORL, Skinner TL. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |