Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5514
Peer-review started: February 9, 2021
First decision: April 19, 2021
Revised: April 27, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 16, 2021
Processing time: 147 Days and 22.9 Hours
The impact of type 2 diabetes mellitus (T2DM) on the prognosis and complications of liver cirrhosis is not fully clarified.
To clarify the mortality and related risk factors as well as complications in cirrhotic patients with T2DM.
We searched PubMed, EMBASE, and the Cochrane Library from their inception to December 1, 2020 for cohort studies comparing liver transplant-free mortality, hepatocellular carcinoma (HCC), ascites, spontaneous bacterial peritonitis (SBP), variceal bleeding, and hepatic encephalopathy (HE) in cirrhotic patients with vs without T2DM. Odds ratios (ORs) were combined by using fixed-effects or random-effects models with RevMan software.
The database search generated a total of 17 cohort studies that met the inclusion criteria. Among these studies, eight reported the risk of mortality, and eight reported the risk of HCC. Three studies provided SBP rates, and two documented ascites rates. Four articles focused on HE rates, and three focused on variceal bleeding rates. Meta-analysis indicated that T2DM was significantly associated with an increased risk of liver transplant-free mortality [OR: 1.28, 95% confidence intervals (CI): 1.16-1.41, P < 0.0001] and HCC incidence (OR: 1.82, 95%CI: 1.32-2.51, P = 0.003). The risk of SBP was not significantly increased (OR: 1.16 95%CI: 0.86-1.57, P = 0.34). Additionally, T2DM did not significantly increase HE (OR: 1.31 95%CI: 0.97-1.77, P = 0.08), ascites (OR: 1.11 95%CI: 0.84-1.46, P = 0.46), and variceal bleeding (OR: 1.34, 95%CI: 0.99-1.82, P = 0.06).
The findings suggest that cirrhotic patients with T2DM have a poor prognosis and high risk of HCC. T2DM may not be associated with an increased risk of SBP, variceal bleeding, ascites, or HE in cirrhotic patients with T2DM.
Core Tip: No consensus is available in the literature about whether type 2 diabetes mellitus (T2DM) can influence the prognosis and complications of liver cirrhosis. This is the first systematic review and meta-analysis comparing the mortality, hepatocellular carcinoma, hepatic encephalopathy, ascites, esophageal varices bleeding, and spontaneous bacterial peritonitis rates between T2DM and non-T2DM cirrhotic patients.
- Citation: Liu ZJ, Yan YJ, Weng HL, Ding HG. Type 2 diabetes mellitus increases liver transplant-free mortality in patients with cirrhosis: A systematic review and meta-analysis. World J Clin Cases 2021; 9(20): 5514-5525
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5514.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5514
Globally, liver cirrhosis is an increasing cause of morbidity and mortality and is the 14th most common cause of death[1]. The liver plays a pivotal role in glucose homeostasis. It stores glycogen in the fed state and produces glucose through glycogenolysis. Former researchers found that 20%-30% of overt type 2 diabetes mellitus (T2DM) cases and 60%-80% of impaired glucose tolerance cases occur in liver cirrhotic patients[2,3]. Whether the presence of T2DM in patients with cirrhosis can increase mortality is also controversial. Some studies proved that cirrhosis patients with T2DM have higher mortality than patients without[4,5], while other studies found opposite results[6,7]. T2DM may increase the risk of infection; however, some research found no difference in the spontaneous bacterial peritonitis (SBP) rate between cirrhosis patients with and without T2DM[8].
We therefore conducted a meta-analysis to evaluate the association between T2DM and mortality as well as its complications in patients with liver cirrhosis.
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[9]. Our primary endpoints were defined as liver transplant-free mortality and hepatocellular carcinoma (HCC) incidence. Secondary endpoints included ascites, SBP, variceal bleeding, and hepatic encephalopathy (HE). All these outcomes were defined by the authors of the primary studies. Two investigators (Liu ZJ and Yan YJ) independently searched PubMed, EMBASE, and the Cochrane Register of Controlled Trials (up to December 1, 2020) using the following MeSH and their free terms: “Liver cirrhosis” AND “type 2 diabetes mellitus” AND (“mortality” OR “spontaneous bacterial peritonitis” OR “ascites” OR “variceal bleeding” OR “hepatic encephalopathies” OR “hepatocellular carcinoma”). The search was further reviewed systematically, and the literature was limited by the language of English.
Studies were included if: (1) They were retrospective or prospective cohort studies comparing mortality and complications of liver cirrhosis patients who had vs did not have T2DM; (2) They were published in full text in a peer-reviewed journal; and (3) They involved 50 or more adult patients and the follow-up period was longer than 6 mo. Studies were excluded if: (1) They were animal or basic studies; (2) They were meta-analyses or reviews; (3) They were cross-sectional studies or case-control studies; (4) They were case reports; (5) They involved patients undergoing liver transplant surgery; (6) They were conference abstracts; or (7) They were not published in English.
Two investigators (Liu ZJ and Yan YJ) independently and separately assessed the trials for eligibility and extracted data. For each individual study, the following study characteristics were collected: First authors’ name, total patients included, year, country, mean age, sex, study design, etiology of the underlying liver diseases, mean follow-up time, endpoint events, liver function, and glucose regulation (T2DM vs non-T2DM).
The included cohort studies were assessed using the Newcastle–Ottawa Scale. Studies were considered high quality if they received 5 or more points, whereas studies were considered low quality if they received 4 or fewer points.
Meta-analysis was performed using Review Manager 5.4 (Cochrane Center, Denmark). Odds ratios (ORs) and 95% confidence intervals (95%CIs) were used as summary estimates, and analysis was performed using the fixed-effects model or random-effects model if heterogeneity was considered significant. Heterogeneity was measured using the I2statistic. High statistical heterogeneity was defined as greater than 70%, medium heterogeneity was defined as 50%-70%, and low heterogeneity was defined as 0%-50%. The Begg and Egger tests were performed using STATA MP16 with P < 0.05 indicating significant publication bias.
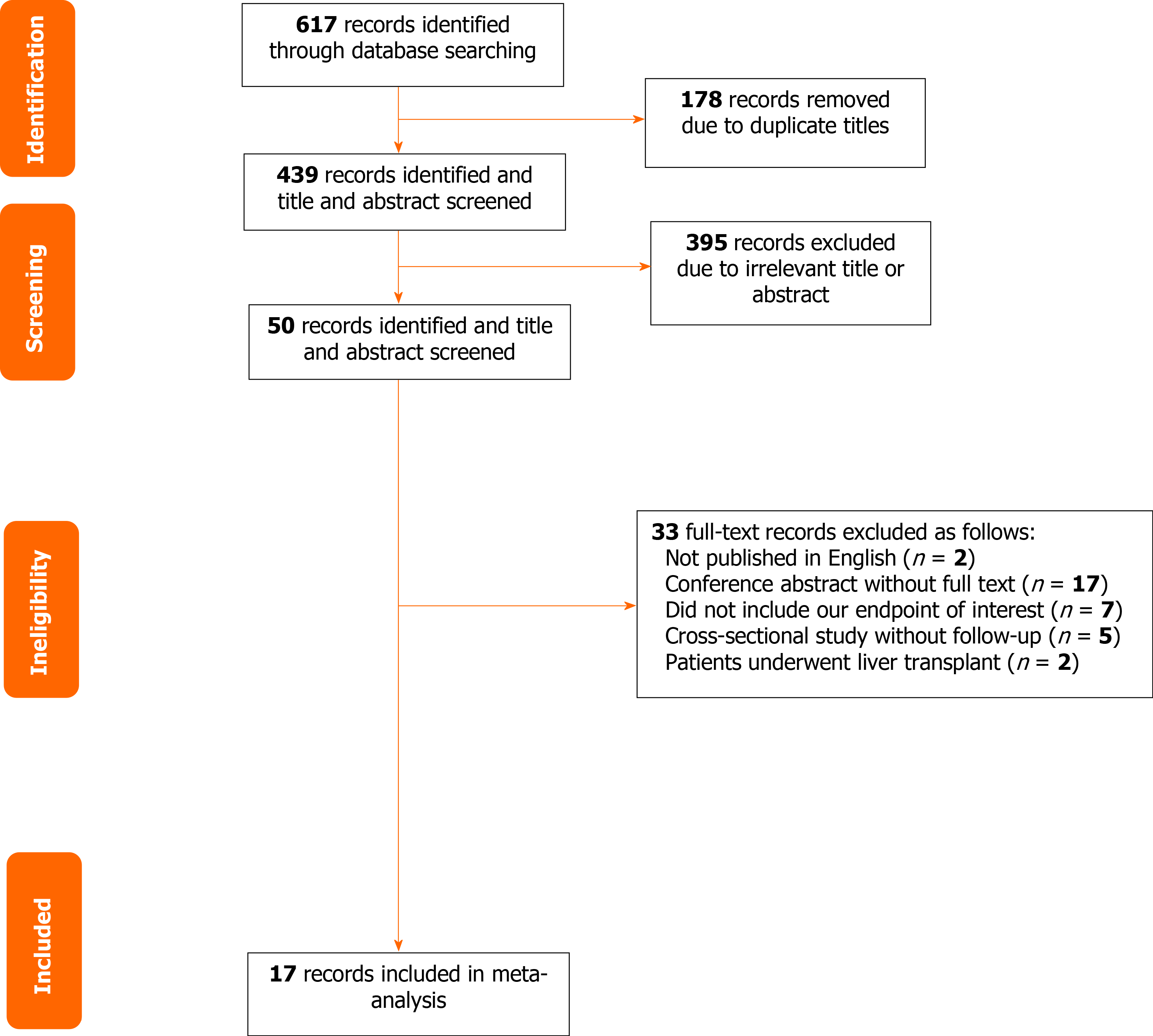
A total of 617 articles were searched from PubMed, EMBASE, and the Cochrane Register of Controlled Trials. After removal of duplicates, 439 articles were screened using the title and abstract. Full text was obtained for 50 articles, which were screened for inclusion eligibility in the study. Overall, 17 articles met the criteria for eligibility and were included in the meta-analysis[4-8,10-21]. A Reviews and Meta-Analyses flow diagram showing the study selection process is shown in Figure 1. Eight articles focused on mortality, eight on HCC, two on ascites, three on variceal bleeding, four on HE, three on HCC, and three on SBP. The characteristics of the included studies are presented in Table 1. The methodologic quality assessment of the included studies is presented in Table 2. All studies were of high quality.
| Ref. | Year | Patients number | Country | Mean age (yr) | Male | Study design | Etiology | Median follow-up time (mo) | Endpoint event | MELD score/Child-Pugh score (Non-T2DM vs T2DM) | Glucose regulation o | |
| Bianchi et al[14] | 1994 | 382 | Italy | 54.6 | 229 | Retrospective cohort | Alcohol, HBV, PBC, autoimmune, and cryptogenic | 37 | Mortality | Child-Pugh score: 7.31 ± 2.28 vs 7.35 ± 2.20 (P > 0.05) | Not described | |
| Quintana et al[5] | 2011 | 110 | Mexico | 56.6 | 57 | Prospective cohort | Alcohol, HBV, HCV, autoimmunity, and cryptogenic | 41 | Mortality | MELD score: 10.3 ± 3.7 vs 11.9 ± 4.7 (P = 0.07) | Not described | |
| Ahn et al[4] | 2020 | 8631 | Australia | Not mentioned | 5813 | Retrospective cohort | Alcohol, cryptogenic, NAFLD, HBV, metabolic liver disease, autoimmune liver disease, inflammatory liver disease, and unspecified | 24 | Mortality | Not described | Not described | |
| Ascites | ||||||||||||
| Gastrointestinal bleeding | ||||||||||||
| Hepatic encephalopathy | ||||||||||||
| SBP | ||||||||||||
| HCC | ||||||||||||
| Wlazlo et al[6] | 2013 | 226 | Netherlands | 59.2 | 129 | Retrospective cohort | Alcoholic, NASH, viral, autoimmune, and others | 74.4 | Mortality | MELD score: 12.2 ± 7.5 vs 11.8 ± 7.3 (P = 0.681) | Median non-fasting glucose of 9.8 mmol/L | |
| SBP | ||||||||||||
| Holstein et al[10] | 2002 | 52 | Germany | 58.3 | Not mentioned | Prospectively cohort | Alcohol, hepatitis C, hepatitis B, cryptogenic, primary biliary cirrhosis, hemosiderosis, and hemochromatosis | 42 | Mortality | Child-Pugh score: 44% of patients had stage A cirrhosis, 37% had stage B, and 19% had stage C | Basal C-peptide of 1.66 ± 0.85 nmol/L | |
| Liu et al[8] | 2016 | 72731 | United States | Not mentioned | 39065 | Retrospective cohort | Alcoholic, nonalcoholic, and biliary | 18 | Ascites | Not described | Not described | |
| Gastrointestinal bleeding | ||||||||||||
| Hepatic encephalopathy | ||||||||||||
| SBP | ||||||||||||
| HCC | ||||||||||||
| Ioannou et al[19] | 2007 | 2120 | United States | Not mentioned | 2069 | Retrospective cohort | HBV, HCV, alcohol, and others | 43.4 | HCC | Not described | Not described | |
| N’kontchou et al[20] | 2006 | 771 | France | 61.4 | 431 | Retrospective cohort | HCV and alcoholic cirrhosis | 50.4 | HCC | Not described | Not described | |
| Wang et al[12] | 2020 | 207 | China | 53.1 | 140 | Retrospective cohort | Not described | 6 | Gastrointestinal rebleeding | MELD score: 7.22 ± 3.98 vs 8.29 ± 2.35 (P = 0.141) | Not described | |
| Nishida et al[15] | 2006 | 56 | Japan | 41 | 34 | Prospective cohort | HBV, HCV, alcohol, and unknown | 44 | Mortality | Child-Pugh score: 6.8 ± 2.4 vs 6.9 ± 2.3 (P > 0.05) | HbA1c (%) of 5.6 ± 1.6% | |
| Yang et al[17] | 2016 | 739 | United States | 57 | 433 | Retrospective cohort | HCV | 38 | Mortality | MELD score: 12.4 ± 5.7 vs 11.6 ± 5.1 (P = 0.04) | HOMA2-IR2: 8.3 ± 4.9 | |
| HCC | ||||||||||||
| Elkrief et al[7] | 2014 | 342 | France | 59 | 236 | Retrospective cohort | HCV | 24 | Mortality | Median MELD score of 10 | Not described | |
| Veldt et al[13] | 2008 | 541 | Netherlands | 50 | 370 | Prospective cohort | HCV | 48 | HCC | Not described | Not described | |
| Yin et al[11] | 2019 | 436 | China | 55 | 278 | Prospective cohort | Alcoholic, viral, AIH, and others | 12 | Hepatic encephalopathy | MELD score: 9.1 ± 2.1 vs 9.2 ± 1.9 (P = 0.537) | Not described | |
| Labenz et al[21] | 2020 | 240 | German | 60 | 137 | Prospective cohort | Alcohol, viral hepatitis, NAFLD, autoimmune, and cryptogenic | 17 | Hepatic encephalopathy | MELD score: 10 (8, 15) vs 9 (7, 13) (P = 0.043); Child-Pugh B/C: 42.3% vs 36.9% (P = 0.453) | HbA1c (%) of 5.1 (4.6, 5.5) | |
| Torisu et al[16] | 2007 | 47 | Japan | 54 | 47 | Retrospective cohort | Alcoholic | 81.6 | HCC | Not described | Not described | |
| Braia et al[18] | 2016 | 2556 | Romania | Not mentioned | Prospective cohort | Not described | 48 | HCC | Not described | Not described | ||
| Ref. | Year | Type of study | Selection | Comparability | Outcome | Score |
| Bianchi et al[14] | 1994 | Retrospective cohort | 4 | 1 | 3 | 8 |
| Quintana et al[5] | 2011 | Prospective cohort | 4 | 2 | 2 | 8 |
| Ahn et al[4] | 2020 | Retrospective cohort | 4 | 0 | 2 | 6 |
| Wlazlo et al[6] | 2013 | Retrospective cohort | 3 | 0 | 3 | 6 |
| Holstein et al[10] | 2002 | Prospective cohort | 4 | 2 | 2 | 8 |
| Elkrief et al[7] | 2014 | Retrospective cohort | 3 | 0 | 3 | 6 |
| Liu et al[8] | 2016 | Retrospective cohort | 3 | 0 | 3 | 6 |
| Veldt et al[13] | 2008 | Prospective cohort | 3 | 0 | 3 | 6 |
| Yin et al[11] | 2019 | Prospective cohort | 3 | 1 | 3 | 7 |
| Wang et al[12] | 2020 | Retrospective cohort | 3 | 1 | 2 | 6 |
| Nishida et al[15] | 2006 | Prospective cohort | 4 | 2 | 2 | 8 |
| Ioannou et al[19] | 2006 | Retrospective cohort | 3 | 2 | 3 | 8 |
| N’kontchou et al[20] | 2006 | Retrospective cohort | 3 | 0 | 3 | 6 |
| Labenz et al[21] | 2020 | Prospective cohort | 3 | 1 | 3 | 7 |
| Torisu et al[16] | 2007 | Retrospective cohort | 3 | 0 | 3 | 6 |
| Braia et al[18] | 2016 | Prospective cohort | 4 | 0 | 2 | 6 |
| Yang et al[17] | 2007 | Retrospective cohort | 4 | 0 | 3 | 7 |
Mortality: Eight studies reported the mortality of cirrhotic patients with vs without T2DM[4-7,10,14,15,17]. There was low heterogeneity across studies, so a fixed-effects model was used. Patients with T2DM were associated with a significantly higher liver transplant-free mortality than patients without T2DM (OR: 1.28, 95%CI: 1.16-1.41, P < 0.0001) (Figure 2). Begg’s test showed no significant publication bias (P = 0.07), while Egger’s test showed slight publication bias (P = 0.03).
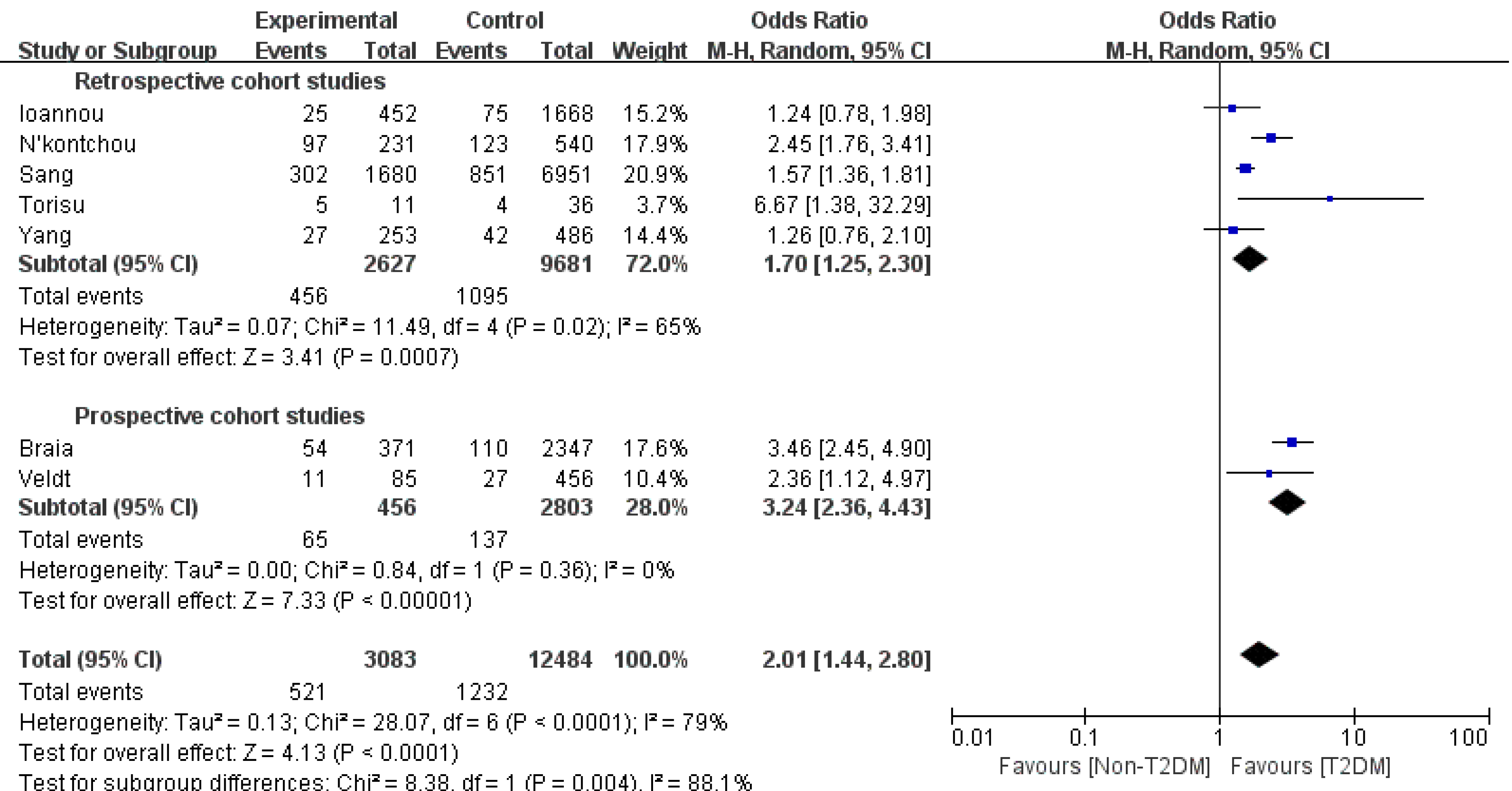
HCC: Eight studies[4,8,11,16-20] assessing the association between T2DM and HCC were enrolled. Compared to the non-T2DM patients, the T2DM patients had a higher incidence of HCC (OR 1.82, 95%CI: 1.32-2.51, P = 0.003, I2= 91%). Sensitivity analysis showed that after removing the study by Liu et al[8], the heterogeneity decreased from 91% to 79%. Subgroup analysis based on the category of the studies was performed to further reduce the heterogeneity among the studies. In the retrospective cohort study subgroup, the OR was 1.7 (95%CI: 1.25- 2.3, P = 0.0007, I2= 65%); however, in the other subgroup, the OR became 3.24 (95%CI: 2.36-4.43, P < 0.0001, I2= 0%) (Figure 3). Publication bias was not detected with a Begg’s test P value of 0.71 and an Egger’s of 0.11.
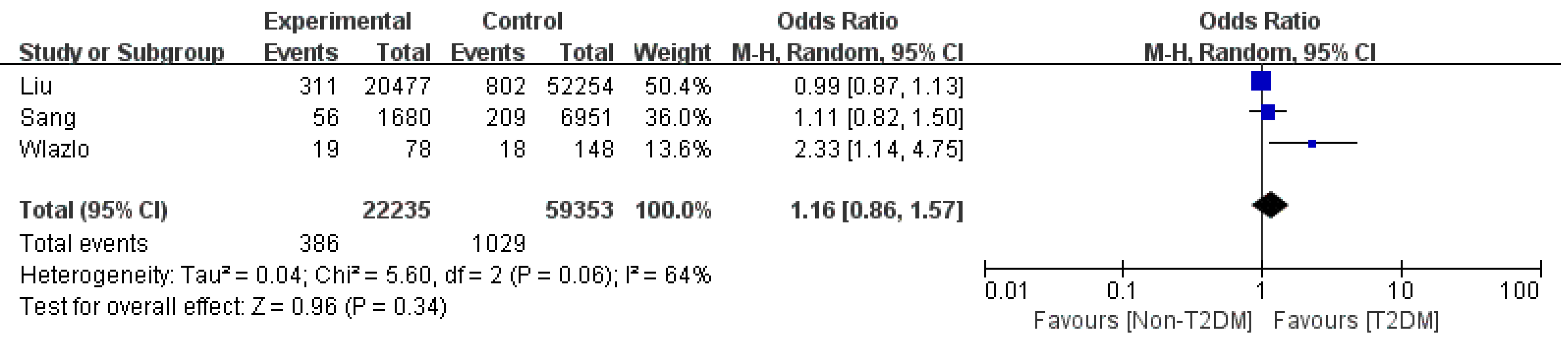
SBP: Three retrospective studies on SBP were included and indicated that cirrhosis patients with T2DM had the same risk of SBP as non-T2DM patients (OR: 1.16 95%CI: 0.86-1.57; Figure 4) with medium heterogeneity[4,6,8]. The sensitivity analysis results showed that Wlazlo et al[6] was the source of the heterogeneity. After removing this study, the heterogeneity decreased to zero (OR: 1.01 95%CI: 0.89-1.54, P = 0.89). The incidence of SBP remained unchanged.
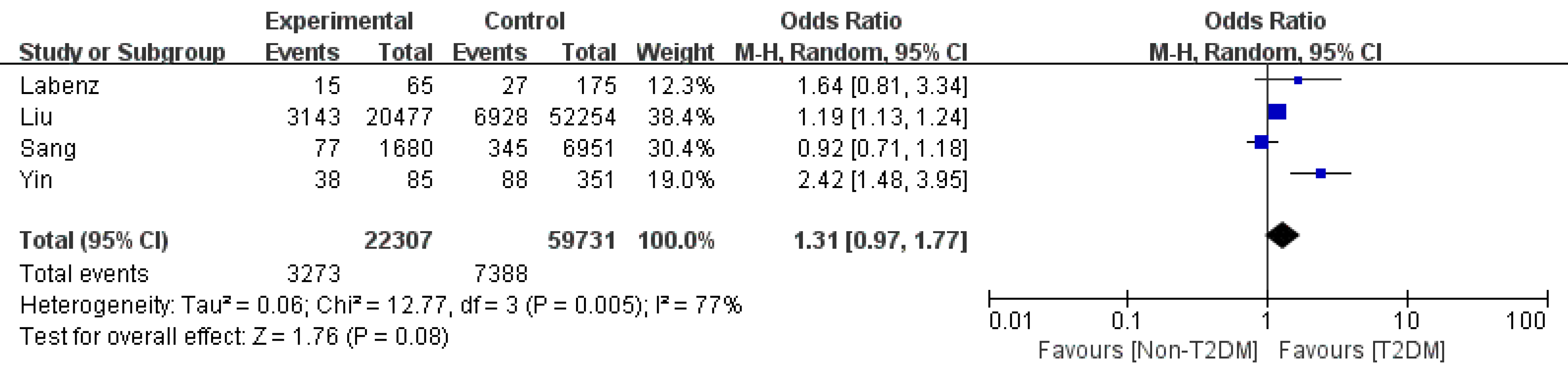
HE: In terms of HE, patients with T2DM did not have a significantly higher rate (OR: 1.31 95%CI: 0.97-1.77, P = 0.08, I2=77%)[4,8,11,21]. We found that Yin et al[11] was the source of the heterogeneity through sensitivity analysis. After combining the other studies, the OR became 1.12 (95%CI: 0.9-1.39, P = 0.3, I2=57%) (Figure 5).
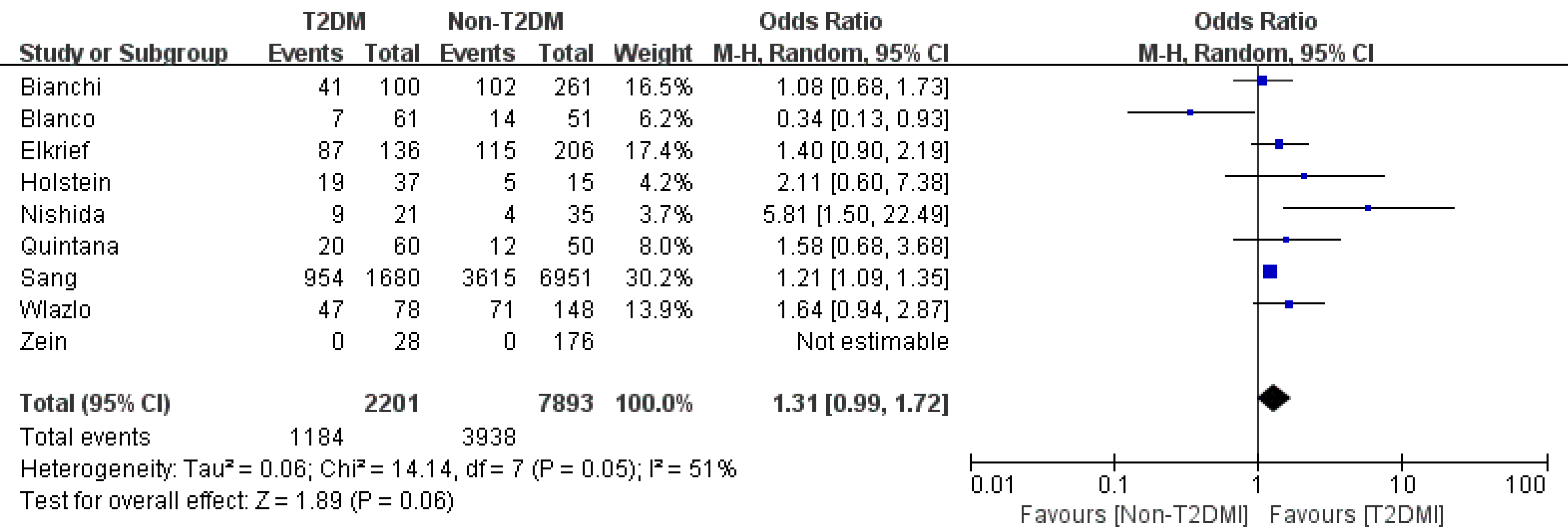
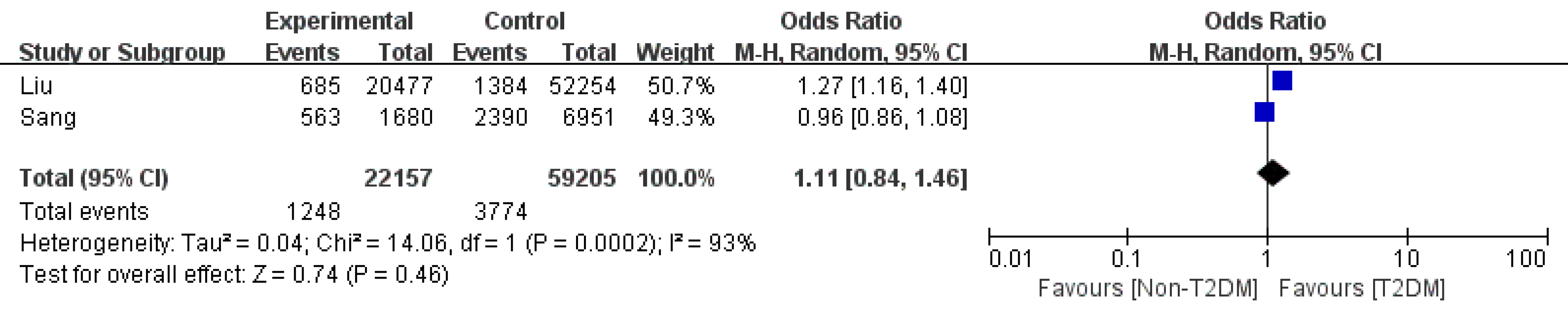
Ascites: Of the two articles[4,8] that reported T2DM and ascites in cirrhosis patients, our meta-analysis identified an OR of 1.11 (95%CI: 0.84-1.46, P = 0.46). Due to the limited study number, subgroup analysis and sensitivity analysis were infeasible. As a result, whether T2DM increases the ascites rate of cirrhosis patients remains controversial (Figure 6).
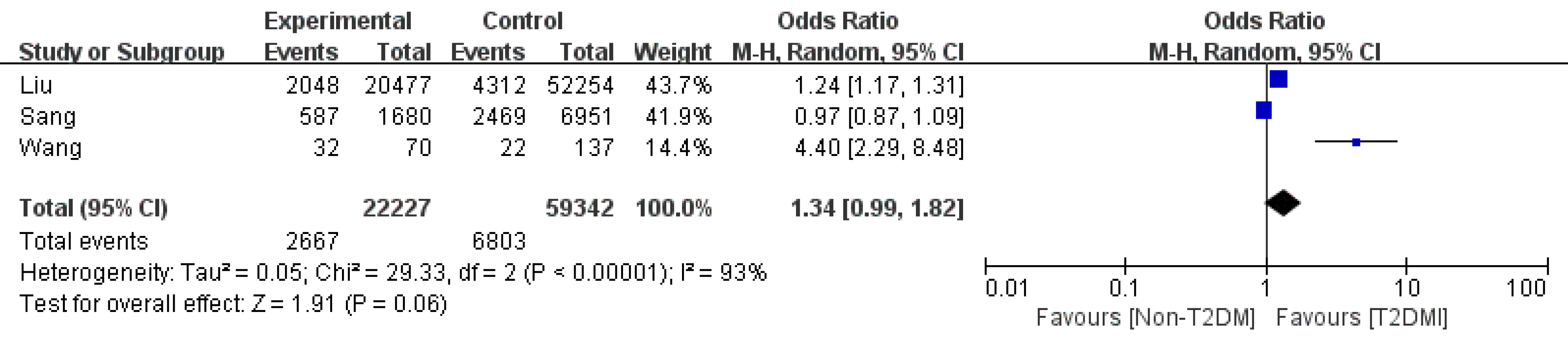
Variceal bleeding: We obtained three studies that focused on variceal bleeding[4,8,12]. They reported an OR of 1.34 (95%CI: 0.99-1.82, P = 0.06, I2=93%). After the sensitivity study or the subgroup study, the heterogeneity was not decreased significantly (Figure 7).
The results of our meta-analysis showed that T2DM was associated with increased liver transplant-free mortality and HCC rates in patients with cirrhosis. The SBP and HE incidence of T2DM vs non-T2DM was not significantly different. Other comparisons of complication rates between T2DM and non-T2DM patients could not be made due to the high heterogeneity and low study numbers.
To the best of our knowledge, this is the first meta-analysis focused on the mortality of cirrhotic patients with T2DM. Because there was no randomized controlled trial study, we only enrolled high-quality cohort studies in this meta-analysis, which we believe did not reduce the credibility of the results. In terms of publication bias, due to the limited number of studies, Begg’s and Egger’s tests might not accurately reflect publication bias, so the contradictory results should be explained cautiously. There are many theories that could explain why T2DM increases the mortality of cirrhosis. As reported recently, inflammation status was enhanced in T2DM, and various cytokines and proinflammatory factors, such as C-reactive protein, tumor necrosis factor-alpha, interleukin-6, interleukin-1β, interleukin-18, and interferon-gamma, were detected in visceral and subcutaneous adipose tissue in patients with diabetes[22,23]. Many of these factors stimulate collagen production by stellate cells, resulting in increased production of connective tissue growth factor and extracellular matrix accumulation and ultimately promoting fibrosis and cirrhosis[24]. Moreover, cirrhotic patients with infections were prone to liver failure, hepatorenal syndrome, and high hospital mortality[25]. In addition, oxidative stress is an upstream event for inflammation, as it induces the activation of monocytes and macrophages and promotes inflammatory responses involved in insulin resistance in T2DM[26]. In the liver, oxidative stress can activate hepatic stellate cells, promote a phenotypic switch, and deposit an excessive amount of extracellular matrix that alters the normal liver architecture and negatively affects liver function. Additionally, oxidative stress can stimulate necrosis and apoptosis of hepatocytes, which can cause liver injury and lead to the progression of end-stage liver disease[27].
As previously reported, our meta-analysis showed that T2DM significantly increased the liver malignancy incidence in liver cirrhotic patients[28,29]. After the sensitivity analysis, we found that Liu et al[8] caused relatively high heterogeneity. This study was a large-number ‘real-world’ study, whose data mainly came from the electrical medical system. Liu et al[8] estimated that the HCC rate in T2DM patients was 5.2%, which was lower than that in other cohort studies. This underestimation was probably caused by missing data and a lack of quality control in the ‘real-world’ study. The subgroup analysis based on the study category further reduced the heterogeneity to a medium level. Compared to retrospective cohort studies, prospective cohort studies could control the bias as well as missing data and make it closer to reality. As a result, we suspected that the OR of HCC in cirrhotic patients with T2DM was approximately 2-3 times that of their non-T2DM counterparts. T2DM might increase the risk of different cancers, and the mechanisms involve the oxidative stress process and activation of the IGF signaling pathway[30,31]. Hyperglycemia could accelerate the formation of reactive oxygen species (ROS). Plasma membrane peroxidation was initiated by ROS and impaired PI-3-kinase signaling pathways. Hyperglycemia-induced cell damage induces ROS production along with cytokine activation, specifically NF-kB and STAT 3, which have an imperative role in inflammatory responses and altered homeostasis in the liver and cause HCC development and progression[32]. Deregulation of the IGF axis signaling pathway may lead to the development of cancer in several tissue types. IGF-I, a major ligand that is extremely expressed in the liver, may be antitumorigenic in HCC but acts as a substrate for HCC development in liver cirrhosis; thus, decreased IGF-I levels could contribute to hepatocarcinogenesis. HCC development commences with a significant decrease in IGF-I levels and the extent of loss of liver function. A reduced IGF-I level is associated with higher tumor intrusiveness and reduced prognosis[33,34].
To our surprise, our meta-analysis did not find a significantly higher prevalence of SBP in T2DM patients. Theoretically, the low-grade inflammatory state of T2DM, as mentioned above, partly came from endotoxemia produced by intestinal microbiota. This might further cause gut permeability, disruption of tight junction proteins in gut epithelial cells, and bacterial translocation, which finally gave rise to SBP. However, Bajaj et al[35] found that although T2DM in the presence of cirrhosis altered the mucosal and stool microbiota, it did not add to the 90-d hospitalization risk or other negative outcomes. Therefore, more research is needed on gut microbiota to determine the relationship between T2DM and SBP. In terms of HE, the present meta-analysis failed to find a clear connection between T2DM and HE. Past research has shown that T2DM can worsen hepatic encephalopathy by increasing glutaminase activity, impairing gut motility, and promoting constipation, intestinal bacterial overgrowth, and bacterial translocation. However, based on the latest guidelines, which kind of HE (minimal or overt HE) that T2DM might lead to is unknown[36]. Because the studies that we enrolled did not define the diagnosis very accurately, future studies should further research this topic. Regarding ascites, some researchers found that perisinusoidal fibrosis, the so-called diabetic hepatosclerosis, most often occurred in subjects with longstanding diabetes and microvascular disease in other organs, especially the kidney, and caused refractory ascites in patients with advanced cirrhosis[37]. Finally, hyperglycemia may aggravate liver function damage and cause hypoalbuminemia, decrease blood coagulation, and finally lead to a series of hemorrhages, including variceal bleeding.
This meta-analysis had some limitations. First, we could only enroll observational studies rather than clinical trials on this topic. Second, the high heterogeneity and low study number disabled us from making quantitative analysis of some secondary outcomes, such as HE, HCC, ascites, and variceal bleeding. Nevertheless, this was the first meta-analysis to research the mortality and major complications of cirrhotic patients with T2DM.
T2DM was associated with increased liver transplant-free mortality in patients with cirrhosis. Future studies should focus on the underlying mechanism and its management.
Type 2 diabetes mellitus (T2DM) and liver cirrhosis have become the major threats to people’s health globally. However, whether the presence of T2DM in patients with cirrhosis can increase mortality and other liver-related complications is also controversial.
A comprehensive systemic review and meta-analysis can help conclude the relative article results and help doctors to make clinical decisions easily.
The aim of this meta-analysis was to clarify the mortality and related risk factors as well as complications in cirrhotic patients with T2DM.
Studies were enrolled following specific criteria. The primary endpoints were defined as liver transplant-free mortality and hepatocellular carcinoma (HCC) incidence. Secondary endpoints included ascites, spontaneous bacterial peritonitis (SBP), variceal bleeding, and hepatic encephalopathy (HE). Studies results were combined using RevMan software.
Meta-analysis indicated that T2DM was significantly associated with an increased risk of liver transplant-free mortality [odds ratios (OR): 1.28, 95% confidence intervals (CI): 1.16-1.41, P < 0.0001] and HCC incidence (OR: 1.82, 95%CI: 1.32-2.51, P = 0.003). The risk of SBP was not significantly increased (OR: 1.16, 95%CI: 0.86-1.57, P = 0.34). Additionally, T2DM did not significantly increase HE (OR: 1.31, 95%CI: 0.97-1.77, P = 0.08), ascites (OR: 1.11, 95%CI: 0.84-1.46, P = 0.46), and variceal bleeding (OR: 1.34, 95%CI: 0.99-1.82, P = 0.06).
T2DM patients have a poor prognosis and high risk of HCC. T2DM may not be associated with an increased risk of SBP, variceal bleeding, ascites, or HE in cirrhotic patients.
More attention should be paid to T2DM in liver cirrhosis patients to improve better prognosis of these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Herold Z, Manesis EK S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1300] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 2. | Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, Reyes-Cabello E, González-González JA, Muñoz-Espinosa LE, Vázquez-Elizondo G, Villarreal-Pérez JZ, Maldonado-Garza HJ. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis. A prospective study. Ann Hepatol. 2012;11:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Ahn SB, Powell EE, Russell A, Hartel G, Irvine KM, Moser C, Valery PC. Type 2 Diabetes: A Risk Factor for Hospital Readmissions and Mortality in Australian Patients With Cirrhosis. Hepatol Commun. 2020;4:1279-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Quintana JO, García-Compean D, González JA, Pérez JZ, González FJ, Espinosa LE, Hernández PL, Cabello ER, Villarreal ER, Rendón RF, Garza HM. The impact of diabetes mellitus in mortality of patients with compensated liver cirrhosis-a prospective study. Ann Hepatol. 2011;10:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Wlazlo N, van Greevenbroek MM, Curvers J, Schoon EJ, Friederich P, Twisk JW, Bravenboer B, Stehouwer CD. Diabetes mellitus at the time of diagnosis of cirrhosis is associated with higher incidence of spontaneous bacterial peritonitis, but not with increased mortality. Clin Sci (Lond). 2013;125:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, Kutala B, Francoz C, Boyer N, Moreau R, Durand F, Marcellin P, Rautou PE, Valla D. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 8. | Liu TL, Trogdon J, Weinberger M, Fried B, Barritt AS 4th. Diabetes Is Associated with Clinical Decompensation Events in Patients with Cirrhosis. Dig Dis Sci. 2016;61:3335-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17371] [Article Influence: 1085.7] [Reference Citation Analysis (1)] |
| 10. | Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Yin X, Zhang F, Xiao J, Wang Y, He Q, Zhu H, Leng X, Zou X, Zhang M, Zhuge Y. Diabetes mellitus increases the risk of hepatic encephalopathy after a transjugular intrahepatic portosystemic shunt in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Wang X, Mei X, Kong D. Effects of diabetes on the rebleeding rate following endoscopic treatment in patients with liver cirrhosis. Exp Ther Med. 2020;20:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 13. | Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, de Knegt RJ, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, Suzuki M, Kanda T, Kawano S, Hiramatsu N, Hayashi N, Hori M. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 16. | Torisu Y, Ikeda K, Kobayashi M, Hosaka T, Sezaki H, Akuta N, Kawamura Y, Yatsuji H, Suzuki F, Suzuki Y, Arase Y, Kumada H. Diabetes mellitus increases the risk of hepatocarcinogenesis in patients with alcoholic cirrhosis: A preliminary report. Hepatol Res. 2007;37:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol. 2016;111:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Braia N, Streba CT, Alexandru DO, Vere CC, Rogoveanu I. Hepatocellular Carcinoma in Diabetic Patients - a Single Center Experience. Curr Health Sci J. 2016;42:180-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2007; 5: 938-945, 945.e1-945. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 20. | N'Kontchou G, Paries J, Htar MT, Ganne-Carrie N, Costentin L, Grando-Lemaire V, Trinchet JC, Beaugrand M. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Labenz C, Nagel M, Kremer WM, Hilscher M, Schilling CA, Toenges G, Kuchen R, Schattenberg JM, Galle PR, Wörns MA. Association between diabetes mellitus and hepatic encephalopathy in patients with cirrhosis. Aliment Pharmacol Ther. 2020;52:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Guest CB, Park MJ, Johnson DR, Freund GG. The implication of proinflammatory cytokines in type 2 diabetes. Front Biosci. 2008;13:5187-5194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Benomar Y, Gertler A, De Lacy P, Crépin D, Ould Hamouda H, Riffault L, Taouis M. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 2013;62:102-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, González-González JA, Maldonado-Garza HJ, Villarreal-Pérez JZ. Plasma cytokine levels imbalance in cirrhotic patients with impaired glucose tolerance and diabetes mellitus. A prospective study. Ann Hepatol. 2014;13:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Sorrentino P, Tarantino G, Conca P, Perrella A, Perrella O. Clinical presentation and prevalence of spontaneous bacterial peritonitis in patients with cryptogenic cirrhosis and features of metabolic syndrome. Can J Gastroenterol. 2004;18:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Bae H, Jeong CH, Cheng WN, Hong K, Seo HG, Han SG. Oxidative stress-induced inflammatory responses and effects of N-acetylcysteine in bovine mammary alveolar cells. J Dairy Res. 2017;84:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Luangmonkong T, Suriguga S, Mutsaers HAM, Groothuis GMM, Olinga P, Boersema M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev Physiol Biochem Pharmacol. 2018;175:71-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 28. | Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 30. | Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol. 2013;47:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Capone F, Guerriero E, Colonna G, Maio P, Mangia A, Marfella R, Paolisso G, Izzo F, Potenza N, Tomeo L, Castello G, Costantini S. The Cytokinome Profile in Patients with Hepatocellular Carcinoma and Type 2 Diabetes. PLoS One. 2015;10:e0134594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Kasprzak A, Adamek A. The insulin-like growth factor (IGF) signaling axis and hepatitis C virus-associated carcinogenesis (review). Int J Oncol. 2012;41:1919-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | de la Garza RG, Morales-Garza LA, Martin-Estal I, Castilla-Cortazar I. Insulin-Like Growth Factor-1 Deficiency and Cirrhosis Establishment. J Clin Med Res. 2017;9:233-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Bajaj JS, Betrapally NS, Hylemon PB, Thacker LR, Daita K, Kang DJ, White MB, Unser AB, Fagan A, Gavis EA, Sikaroodi M, Dalmet S, Heuman DM, Gillevet PM. Gut Microbiota Alterations can predict Hospitalizations in Cirrhosis Independent of Diabetes Mellitus. Sci Rep. 2015;5:18559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Sun Y, Yuan P, Zhang D, Zhong X. [Publication impact of the Chinese Journal of Hepatology from 2006 to 2014]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Elkrief L, Buyse S, Panhard X, Baudry C, Gault N, Moreau R, Rautou PE, Belghiti J, Durand F, Bedossa P, Paradis V, Valla D. Microcirculatory changes in the liver of patients with refractory ascites and their relationship with diabetes and alcohol. Eur J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |