Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5462
Peer-review started: April 6, 2021
First decision: April 28, 2021
Revised: April 30, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: July 16, 2021
Processing time: 91 Days and 22.1 Hours
The World Health Organization reported that 28637952 people worldwide had been infected with severe acute respiratory syndrome coronavirus 2, the causative agent of coronavirus disease 2019 (COVID-19), by September 13.
The aim was to investigate whether long-term use of renin-angiotensin-aldoste
This was a retrospective analysis of lung computed tomography (CT) data and laboratory values of COVID-19 patients with hypertension who were admitted to Huoshenshan Hospital, Wuhan, Hubei Province, between February 18 and March 31, 2020. Patients were divided into two groups. Group A included 19 people who were long-term users of RAAS inhibitors for hypertension; and group B included 28 people who were randomly selected from the database and matched with group A by age, sex, basic diseases, and long-term use of other antihypertensive drugs. All patients underwent a series of CT and laboratory tests. We compared the most severe CT images of the two groups and the laboratory examination results within 2 d of the corresponding CT images.
The time until the most severe CT images from the onset of COVID-19 was 30.37 ± 14.25 d group A and 26.50 ± 11.97 d in group B. The difference between the two groups was not significant (t = 1.01, P = 0.32). There were no significant differences in blood laboratory values, C-reactive protein, markers of cardiac injury, liver function, or kidney function between the two groups. There was no significant difference in the appearance of the CT images between the two groups. The semiquantitative scores of each involved lobe were 11.84 ± 5.88 in group A and 10.36 ± 6.04 group B. The difference was not significantly different (t = 0.84, P = 0.41).
Chest CT is an important imaging tool to monitor the characteristics of COVID-19 and the degree of lung injury. Chronic use of RAAS inhibitors is not related to the severity of COVID-19, and it does not worsen the clinical process.
Core Tip: We investigated whether the use of renin-angiotensin-aldosterone system inhibitors by coronavirus disease 2019 patients with hypertension aggravated the severity of pneumonia by comparing the differences in computed tomography images.
- Citation: Li XL, Li T, Du QC, Yang L, He KL. Effects of angiotensin receptor blockers and angiotensin-converting enzyme inhibitors on COVID-19. World J Clin Cases 2021; 9(20): 5462-5469
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5462.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5462
The World Health Organization reported that 28,637,952 people worldwide had been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), by September 13. Animal experiments have shown that angiotensin-converting enzyme 2 (ACE2) is abundantly expressed in lung, heart and other tissues, and coronaviruses use it as a functional receptor to invade human cells[1-3]. Inhibitors of the renin-angiotensin-aldosterone system (RAAS), namely angiotensin receptor blockers (ARBs) and ACE inhibitors, are first-line drugs for the treatment of hypertension. Some scholars believe that the use of RAAS inhibitors by COVID-19 patients with hypertension will aggravate COVID-19[4-6]. Some investigators also believe that ARBs and ACE inhibitors can inhibit both the activity of the RAAS and the progression of respiratory injury, thus playing a protective role in COVID-19 patients[7-9]. However, those assumptions are mainly based on animal experiments and lack clinical evidence. This study aimed to investi
COVID-19 patients with hypertension who were hospitalized in Huoshenshan Hospital in Wuhan, Hubei Province, between February 18 and March 31, 2020 were analyzed retrospectively. COVID-19’s diagnostic criteria refer to the National Health Commission’s "diagnosis and treatment plan of pneumonia infected by corona virus disease 2019" (trial version 7)[10]. All patients were positive for 2019 novel coronavirus by a laboratory nucleic acid assay (real-time fluorescence polymerase chain reaction, RT-PCR) of pharyngeal swab samples. The standard of cure and discharge required a return of body temperature to normal for more than 3 d, improved respiratory symptoms, significant improvement of the exudative lesions on chest CT, and negative RT-PCR assays of throat swabs with intervals of more than 24 h. COVID-19 patients with hypertension were divided into two groups. Group A included COVID-19 patients with long-term use of ACE inhibitors or/and ARBs recorded in their medical records. Group B included COVID-19 patients with long-term use of other antihypertensive drugs (calcium channel blockers, diuretics, β-adrenoceptor blockers). Group A and group B were matched by age, sex, and underlying diseases, and were randomly selected from the database with a ratio of 1:1.5.
The clinical histories, laboratory test results, and epidemiological histories of the patients were collected. The patients were stratified by mild, normal, severe, and critical disease following the “diagnosis and treatment of pneumonia infected by corona virus disease 2019 (trial version 7)” criteria[10]. All patients underwent a series of CT and laboratory examinations. The most severe CT findings and laboratory results obtained in the two groups within 2 d of the CT examination were compared. Laboratory tests included routine blood counts, C-reactive protein, liver function, renal function, and myocardial injury markers, myoglobin, hypersensitive troponin I and creatine kinase (CK), and the heart failure marker B-type natriuretic peptide. The difference in severity of COVID-19 in patients with hypertension in group A and group B was determined by comparing the chest CT findings.
Chest CT images were read independently by two chest radiology specialists without knowing the clinical laboratory results and patient grouping, and resolved by discussion when there were differences. COVID-19 CT signs included ground glass density (GGD), consolidation, paving stone sign (thickening of the interlobular septum and intralobular septum on the GGD background), fibrous band, air bronchus sign, thickening of small blood vessels, and pleural exudation[11]. We used a semiquantitative scoring system to evaluate the degree of lung involvement based on the number of lobes involved[12], that is, five lobes on both sides. According to the lung parenchyma volume ratio of pneumonia to lobe, the score was 0-5 points in each lobe. The score was 0 if there were no lesions. The subsequent scores, defined as percentages of the lobe volume, were one point if the lesion volume was < 5%, two points if the lesion volume was 5%-25%, three points if the lesion volume was 26%-49%, four points if the lesion volume was 50%-75%, and five points if the lesion volume was > 75%. The total score was the sum of the cumulative scores of each lung lobe. Theoretically, the pulmonary lesions scores could be between 0 and 25 points.
Comparison of the characteristics of COVID-19 patients in the two groups who were treated with different antihypertensive drugs included age, sex, laboratory results, time from chest CT examination to onset, and chest CT score. The Kolmogorov-Smirnov test was used to determine whether the laboratory results and CT scores of the two groups were normally distributed. The values of normally distributed data were reported as means ± SD. Between-group differences were compared by t-tests. Measurement data that were not normally distributed were reported as medians (M) and quartiles (Q1, Q3), and between-group differences were compared by the Wilcoxon signed-rank test. Categorical variables were reported as frequencies and percentage and between-group comparisons were performed by χ2 or Fisher exact tests. A two-tailed P value of < 0.05 was statistically significant. SPSS 21.0 (SPSS Inc., Chicago, IL, United States) was used for the statistical analysis.
Forty-seven COVID-19 patients with hypertension were evaluated and compared, 19 in group A and 28 in group B. The clinical data are shown in Table 1. There were no significant differences in age and sex between the two groups. Initial clinical symptoms included fever, cough, chest tightness, shortness of breath, fatigue, and diarrhea. The average hospital stay was 11.56 ± 4.82 d in group A and 12.83 ± 5.35 d group B. The difference was not significant. In addition, there were no significant differences in the number of patients needing mechanical ventilation, the number admitted to the intensive care unit, or the number of deaths in the two groups.
| Group A (n = 19) | Group B (n = 28) | t- or χ2 value | P value | |
| Age, yr | 64.68 ± 8.48 | 63.46 ± 6.53 | 0.56 | 0.58 |
| Gender | 12 (63.2%) | 18 (64.3%) | 0.006 | 0.937 |
| Mean Hospitalization stay, d | 11.56 ± 4.82 | 12.83 ± 5.35 | -0.80 | 0.429 |
| Mechanical ventilation, n | 1 | 2 | - | 1.0 |
| Intensive care unit, n | 1 | 2 | - | 1.0 |
| Death, n | 1 | 2 | - | 1.0 |
The laboratory results of the two groups are shown in Table 2. There were no significant differences in the routine blood count, C-reactive protein, myoglobin, hypersensitive troponin I, CK, B-type natriuretic peptide, liver function, and renal function between the two groups.
| Group A, n = 19 | Group B, n = 28 | t- or U-value | P value | |
| White blood cells | 6.64 ± 3.65 | 6.55 ± 1.82 | 0.095 | 0.925 |
| Neutrophils | 4.47 ± 3.24 | 4.20 ± 1.61 | 0.329 | 0.744 |
| Lymphocytes | 1.54 ± 0.64 | 1.65 ± 0.40 | −0.649 | 0.522 |
| Neutrophil percentage | 64.16 ± 10.59 | 62.15 ± 8.09 | 0.677 | 0.503 |
| Lymphocyte percentage | 25.64 ± 9.08 | 26.06 ± 6.30 | −0.168 | 0.868 |
| C-reactive protein | 1.67 (0.97, 9.11) | 2.00 (1.00, 2.00) | 187 | 0.316 |
| Markers of myocardial infarction | - | - | - | - |
| Myoglobin | 13.13 ± 15.40 | 9.16 ± 5.96 | 0.657 | 0.532 |
| Hypersensitive troponin | 0.16 ± 0.37 | 0.01 ± 0.001 | 1.107 | 0.311 |
| Creatine kinase | 10.88 ± 6.64 | 12.71 ± 7.86 | −0.742 | 0.463 |
| NT-proBNP | 9.67 ± 13.30 | 20.33 ± 23.29 | −0.958 | 0.352 |
| Glutamic pyruvic transaminase | 34.18 ± 21.89 | 30.36 ± 23.85 | 0.493 | 0.625 |
| Albumin | 38.01 ± 3.97 | 37.12 ± 2.87 | 0.759 | 0.453 |
| γ-glutamyl transpeptidase | 65.01 ± 38.64 | 58.23 ± 51.38 | 0.421 | 0.677 |
| Total bilirubin | 12.63 ± 7.23 | 11.92 ± 4.71 | 0.34 | 0.736 |
| Creatinine | 73.86 ± 28.85 | 69.95 ± 15.50 | 0.504 | 0.618 |
| Urea nitrogen | 4.83 ± 2.41 | 4.75 ± 1.32 | 0.127 | 0.900 |
| Glutamic oxaloacetic transaminase | 24.04 ± 11.63 | 25.21 ± 13.46 | −0.279 | 0.782 |
| Procalcitonin | 0.078 ± 0.081 | 0.047 ± 0.025 | 1.4 | 0.174 |
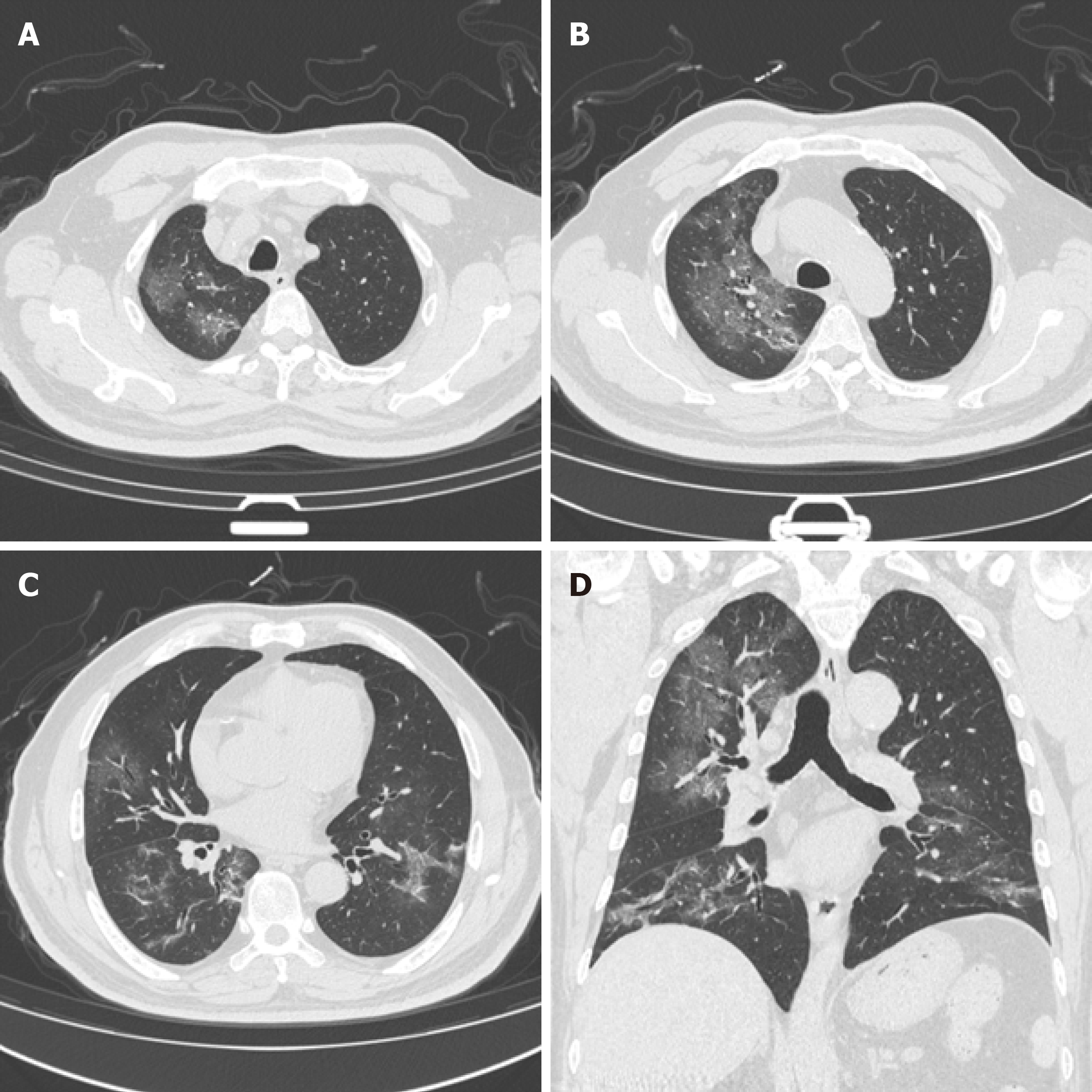
The time between the onset of disease and the CT images were 30.37 ± 14.25 d in group A and 26.50 ± 11.97d in group B. The difference was not significant (t = 1.01, P = 0.32). The peak of the course of disease was characterized in both groups by large GGD shadow, paving stone-like changes, and consolidation accompanied by thickening of small blood vessels, and air bronchial signs. The GGD shadow, paving stone sign, and consolidation gradually decreased and the fibrous band gradually increased (Figure 1) with recovery from the disease. There were no significant between-group differences in the GGD shadow, paving stone change, consolidation, and fibrous band formation. The total cumulative lung scores (Table 3) were 11.84 ± 5.88 in group A and 10.36 ± 6.04 in and group B. The difference was not significant (t = 0.84, P = 0.41).
| Group A, n = 19 | Group B, n = 28 | t or χ2 value | P value | |
| Time between CT to onset of disease in d | 30.37 ± 14.25 | 26.50 ± 11.97 | 1.01 | 0.32 |
| Ground glass density | 13 (68.4) | 21 (75) | 0.25 | 0.62 |
| Solid change | 9 (47.4) | 14 (50) | 0.03 | 0.86 |
| Paving stone sign | 16 (84.2) | 19 (67.9) | 0.85 | 0.36 |
| Fibrous band | 17 (89.5) | 17 (60.7) | 3.35 | 0.07 |
| CT score | 11.84 ± 5.88 | 10.36 ± 6.04 | 0.84 | 0.41 |
The results showed that in COVID-19 patients with hypertension, chronic use of ACE inhibitors and/or ARBs did not worsen the clinical process compared with taking other antihypertensive drugs. In addition, there were no significant differences in the clinical characteristics or the results of routine blood, myocardial enzyme, liver function, renal function testing, or chest CT pneumonia findings.
SARS-CoV-2 enters the cell by binding to a protein called ACE2 followed by virus replication and cell death[13]. The ACE2 protein is widely distributed in the vascular endothelial cells of many tissues of the kidneys, heart, intestine, and liver. Animal experiments show that intravenous administration of ACE inhibitors and/or ARBs upregulates ACE2, thus worsening the clinical process of COVID-19[14]. However, clinical studies have shown that ACE inhibitors and ARBs may play a protective role against pneumonia[15]. This study showed that there were no significant differences in routine blood values, C-reactive protein, myocardial enzyme activity, liver function, renal function, chest CT pneumonia characteristics, or lung CT scores between COVID-19 patients treated with ACE inhibitors and ARBs and COVID-19 patients treated with other antihypertensive drugs. The results are consistent with recent studies showing that the use of ACE inhibitors and ARBs had nothing to do with the severity of COVID-19[16,17].
As a noninvasive and rapid imaging diagnostic tool, chest CT is an important means of diagnosing COVID-19. The sensitivity of chest CT to detect COVID-19 pneumonia is 98%[18]. The main imaging findings of COVID-19 include ground glass opacity, compaction, and paving stone-like changes[19]. Chest CTs can also monitor the progress and prognosis of the disease. Zhang et al[20] divided the course of COVID-19 into early, progressive, peak, and recovery stages. Ground glass opacity was mainly seen in the early stage and progressed to consolidation and paving stone-like changes as the disease evolved. In addition, the ground glass opacity can have a mixed form in the peak stage, and the fibrous band lesions can significantly increase in the recovery stage. Consolidation, paving stone-like lesions, and ground glass lesions decreased gradually, so the stages were not completely separated. This study showed that the most serious chest CT signs and chest CT lung-involvement scores were not significantly different between COVID-19 patients with hypertension treated with ACE inhibitors and ARBs and COVID-19 patients treated with other antihypertensive drugs.
This study had the following shortcomings: (1) The sample size was small and may be biased; and (2) It was a short-term retrospective study. We performed few preli
Overall, this study shows that the clinical process of chronic treatment of hypertensive COVID-19 patients with ACE inhibitors and ARBs did not worsen their pneumonia. Chest CT is an important imaging method to monitor the characteristics of COVID-19 and the degree of lung involvement.
The World Health Organization reported that 28637952 people worldwide had been infected with severe acute respiratory syndrome coronavirus 2, the causative agent of coronavirus disease 2019 (COVID-19), by September 13.
Some investigators believe that the use of RAAS inhibitors by COVID-19 patients with hypertension aggravates COVID-19. Some also believe that angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors can inhibit the activity of the RAAS and as well as the progression of respiratory injury, thus playing a protective role in COVID-19 patients. However, those assumptions are based on animal experiments and lack clinical evidence. This study intended to resolve whether the use of RAAS inhibitors by COVID-19 patients with hypertension aggravated their degree of pneumonia.
The objective was to investigate whether long-term treatment with RAAS inhibitors aggravated the performance of COVID-19 patients with hypertension.
This was a retrospective analysis of lung computed tomography (CT) data and laboratory values of COVID-19 patients with hypertension who were admitted to Huoshenshan Hospital, Wuhan, Hubei Province, between February 18 and March 31, 2020. Patients were divided into two groups. Group A included 19 people who were long-term users of RAAS inhibitors for hypertension and group B included 28 people who were randomly selected from the patient database and matched with group A by age, sex, other diseases, and long-term use of other antihypertensive drugs. All patients underwent a series of CT and laboratory tests. We compared the most severe CT images of the two groups and the laboratory examination results within 2 d of obtaining the corresponding CT images.
Chest CT is an important imaging tool to monitor the characteristics of COVID-19 and the degree of lung injury. Chronic use of RAAS inhibitors was not related to the severity of COVID-19, and they did not worsen the clinical course.
The clinical responses to the long-term treatment of hypertensive COVID-19 patients with ACE inhibitors and ARBs did not worsen their pneumonia. Chest CT is an important imaging method to monitor the characteristics of COVID-19 and the degree of lung involvement. This study showed that the most serious chest CT signs and chest CT scores of lung involvement were not significantly different between COVID-19 patients with hypertension treated with ACE inhibitors and ARBs and COVID-19 patients treated with other antihypertensive drugs. Animal experiments show that intravenous administration of ACE inhibitors and/or ARBs upregulates ACE2, thus worsening the clinical course of COVID-19. Clinical studies have shown that ACE inhibitors and ARBs may play a protective role against pneumonia This study showed that there were no significant differences in routine blood values, C-reactive protein, myocardial enzyme activity, liver function, renal function, chest CT pneumonia characteristics, or lung CT scores between COVID-19 patients treated with ACE inhibitors and ARBs and COVID-19 patients treated with other antihypertensive drugs. The results are consistent with recent studies finding that the use of ACE inhibitors and ARBs had nothing to do with the severity of COVID-19.
This study has two shortcomings: (1) The sample size was small and may be biased; and (2) It was a short-term retrospective study. We performed few preliminary exploratory evaluations. In addition, some patients had residual lung lesions when they met the discharge criteria. The best perspective for future research is to conduct that long-term follow-up of large samples to monitor the outcome of pulmonary lesions.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hirooka Y, Lipsitch M S-Editor: Yan JP L-Editor: Filipodia P-Editor: Li JH
| 1. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14019] [Article Influence: 2803.8] [Reference Citation Analysis (1)] |
| 2. | Chen L, Liu W, Zhang Q, Xu K, Ye G, Wu W, Sun Z, Liu F, Wu K, Zhong B, Mei Y, Zhang W, Chen Y, Li Y, Shi M, Lan K, Liu Y. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 388] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 3. | Yan Y, Shin WI, Pang YX, Meng Y, Lai J, You C, Zhao H, Lester E, Wu T, Pang CH. The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 4. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1944] [Article Influence: 388.8] [Reference Citation Analysis (0)] |
| 5. | Pizzolo F, Rigoni AM, De Marchi S, Friso S, Tinazzi E, Sartori G, Stefanoni F, Nalin F, Montagnana M, Pilotto S, Milella M, Azzini AM, Tacconelli E, Marchi G, Girelli D, Olivieri O, Martinelli N. Deep vein thrombosis in SARS-CoV-2 pneumonia-affected patients within standard care units: Exploring a submerged portion of the iceberg. Thromb Res. 2020;194:216-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Ahamed Mim M, Naznin Rakhi N, Saha O, Rahaman MM. Recommendation of fecal specimen for routine molecular detection of SARS-CoV-2 and for COVID-19 discharge criteria. Pathog Glob Health. 2020;114:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2710] [Cited by in RCA: 2635] [Article Influence: 131.8] [Reference Citation Analysis (0)] |
| 8. | Alsufyani HA, Alrefaie Z. Renin-Angiotensin System Implications to COVID-19 Comorbidities. J Microsc Ultrastruct. 2020;8:148-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1854] [Cited by in RCA: 2009] [Article Influence: 401.8] [Reference Citation Analysis (0)] |
| 10. | National Health Commission of the People’s Republic of China. Notice on the issuance of New Coronavirus pneumonia diagnosis and treatment plan (trial 7th ed). March 3, 2020. [cited 1 April 2021]. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. |
| 11. | Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol. 2020;30:4930-4942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1748] [Article Influence: 349.6] [Reference Citation Analysis (0)] |
| 13. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14143] [Article Influence: 2828.6] [Reference Citation Analysis (0)] |
| 14. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2898] [Article Influence: 579.6] [Reference Citation Analysis (0)] |
| 15. | Tan WSD, Liao W, Zhou S, Mei D, Wong WF. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020;382:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 719] [Cited by in RCA: 717] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 17. | Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, Milinovich A, Svensson LG, Jehi L, Young JB, Chung MK. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 18. | Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115-E117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2088] [Cited by in RCA: 1870] [Article Influence: 374.0] [Reference Citation Analysis (2)] |
| 19. | Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1827] [Cited by in RCA: 1689] [Article Influence: 337.8] [Reference Citation Analysis (0)] |
| 20. | Zhang H, Liu X, Yu P, Cheng M, Wang W, Sun Y, Zeng B, Fan B. Dynamic CT assessment of disease change and prognosis of patients with moderate COVID-19 pneumonia. J Xray Sci Technol. 2020;28:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |